

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332481 ((R)-2-((R)-3-amino-3-(4-((2-methylquinolin-4-yl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332481 ((R)-2-((R)-3-amino-3-(4-((2-methylquinolin-4-yl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332476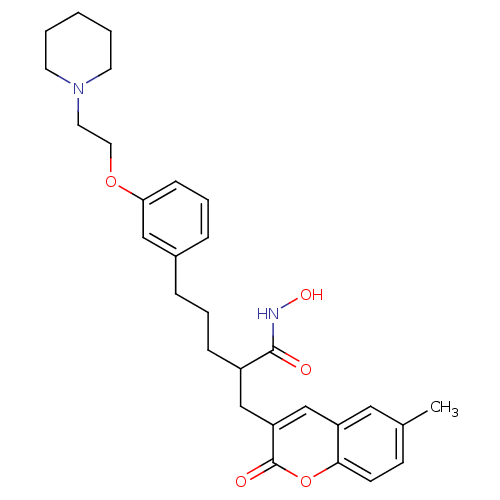 (2-((6-Methyl-2-oxo-2H-chromen-3-yl)methyl)-5-(3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332476 (2-((6-Methyl-2-oxo-2H-chromen-3-yl)methyl)-5-(3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332480 (4-(4-(2-(Diethylamino)ethoxy)phenyl)-2-((7-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332470 (CHEMBL1630100 | N-Hydroxy-4-(4-hydroxyphenyl)-2-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332479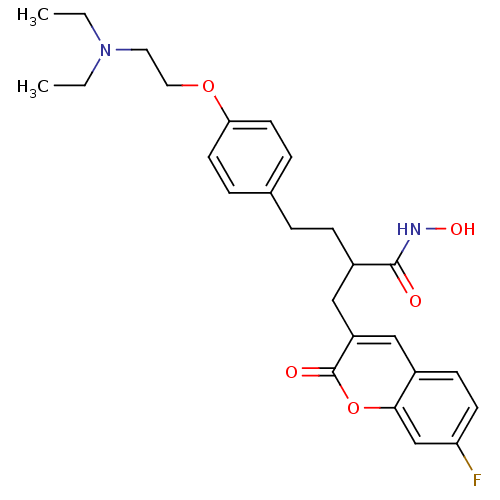 (4-(4-(2-(Diethylamino)ethoxy)phenyl)-2-((7-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50224961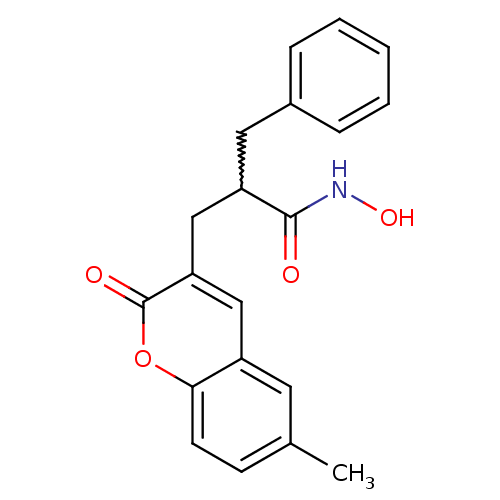 (2-benzyl-N-hydroxy-3-(6-methyl-2-oxo-2H-chromen-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332478 (4-(4-(2-(Diethylamino)ethoxy)phenyl)-2-((6-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332477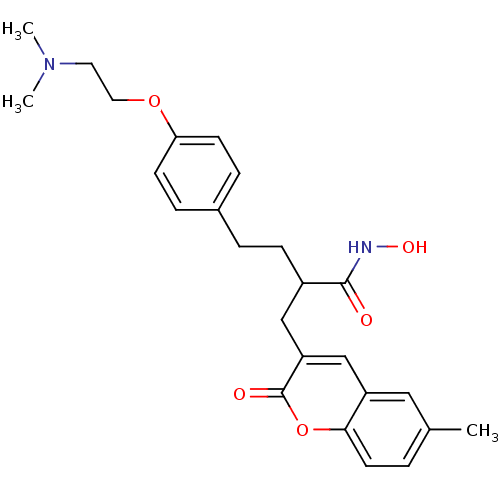 (4-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-((6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332477 (4-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-((6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332474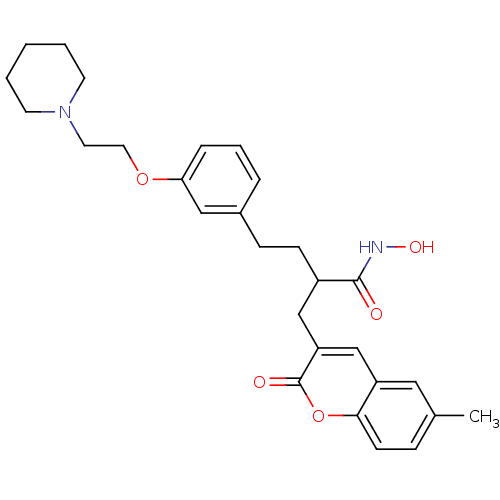 (2-((6-Methyl-2-oxo-2H-chromen-3-yl)methyl)-4-(3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419721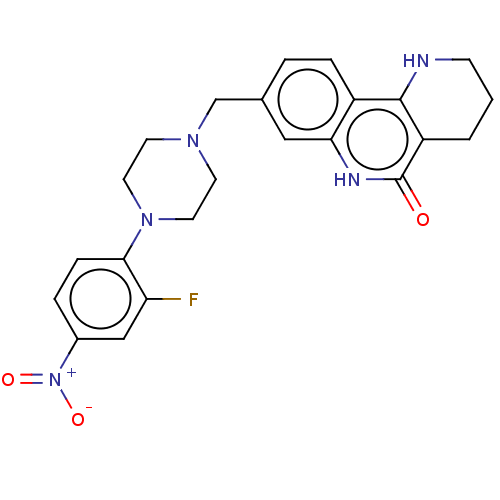 (US10464919, Example 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419799 (US10464919, Example 137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332473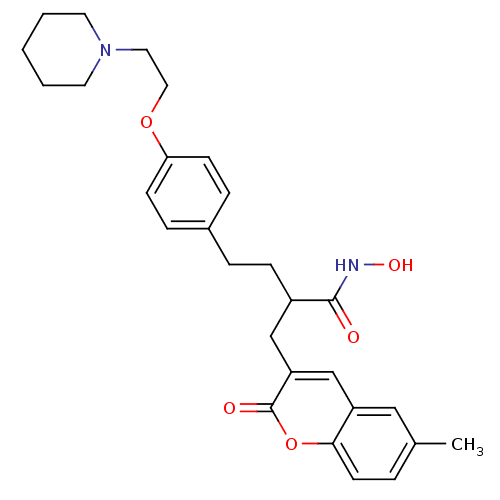 (2-((6-Methyl-2-oxo-2H-chromen-3-yl)methyl)-4-(4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332475 (2-((6-Methyl-2-oxo-2H-chromen-3-yl)methyl)-5-(4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332472 (3-(6-Methyl-2-oxo-2H-chromen-3-yl)-2-(3-(2-(piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE by fluorescent spectroscopy | Bioorg Med Chem 18: 8618-29 (2010) Article DOI: 10.1016/j.bmc.2010.10.006 BindingDB Entry DOI: 10.7270/Q2NV9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419844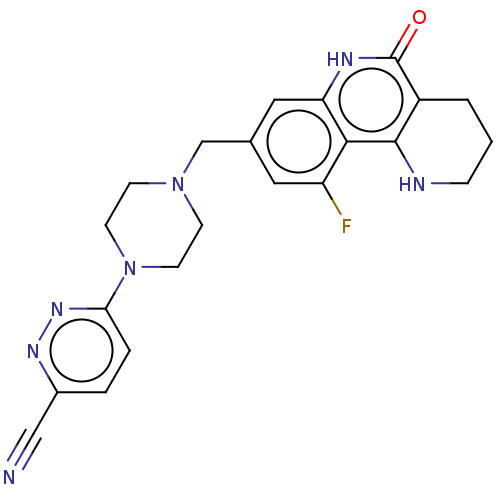 (US10464919, Example 182) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419794 (US10464919, Example 132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419731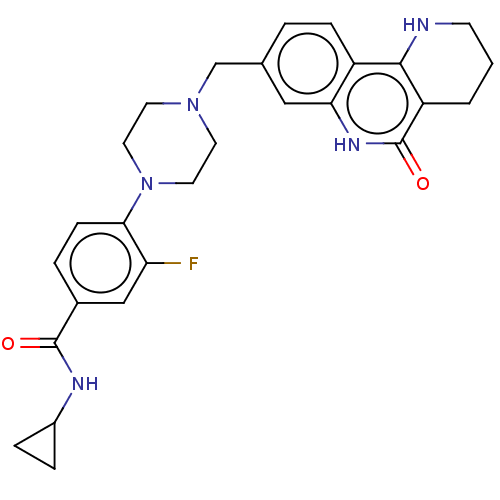 (US10464919, Example 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TNK1 (Homo sapiens (Human)) | BDBM419721 (US10464919, Example 59) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419781 (US10464919, Example 119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419751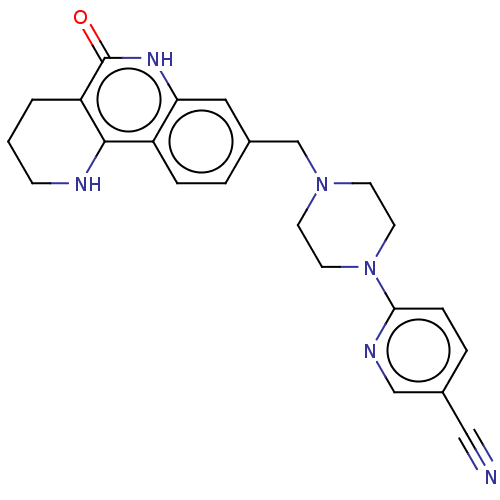 (US10464919, Example 89) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419791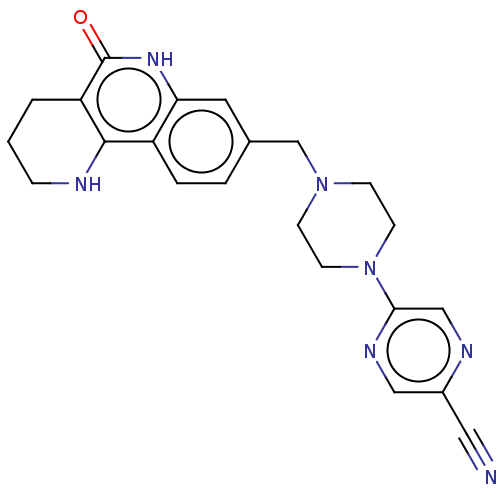 (US10464919, Example 129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419758 (US10464919, Example 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419797 (US10464919, Example 135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419740 (US10464919, Example 78) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM419721 (US10464919, Example 59) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The PARP-1 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of a kit (cat. 80551) p... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419832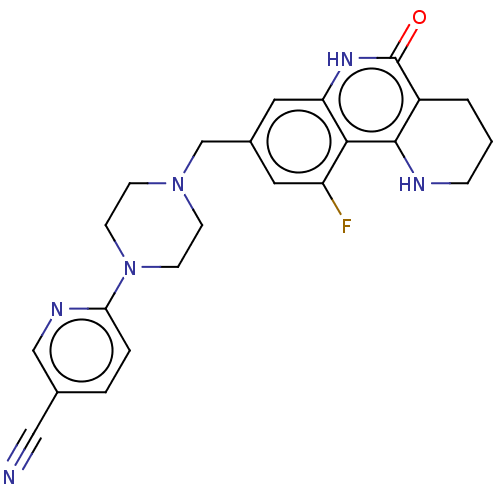 (US10464919, Example 170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419755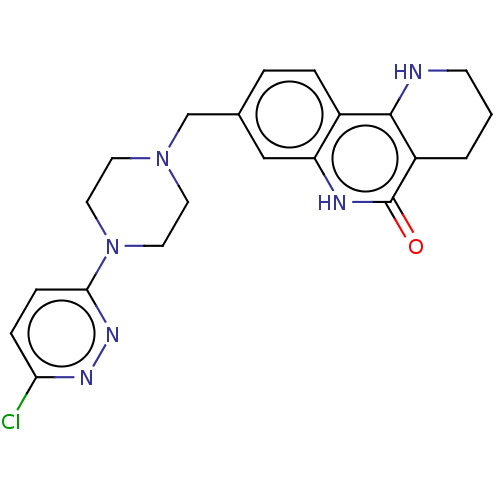 (US10464919, Example 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419761 (US10464919, Example 99) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419765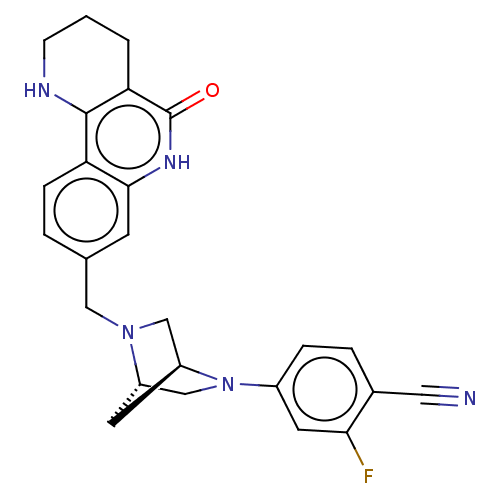 (US10464919, Example 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419842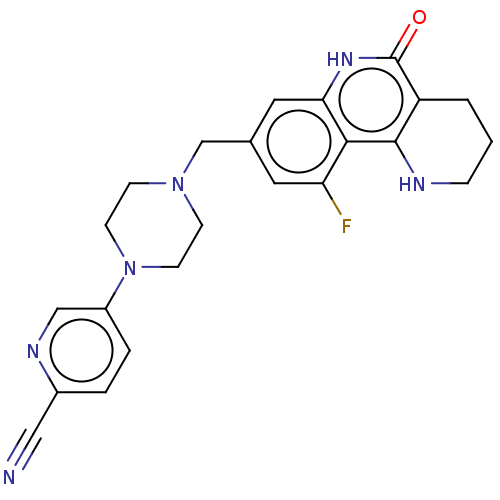 (US10464919, Example 180) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM419751 (US10464919, Example 89) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The PARP-1 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of a kit (cat. 80551) p... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419784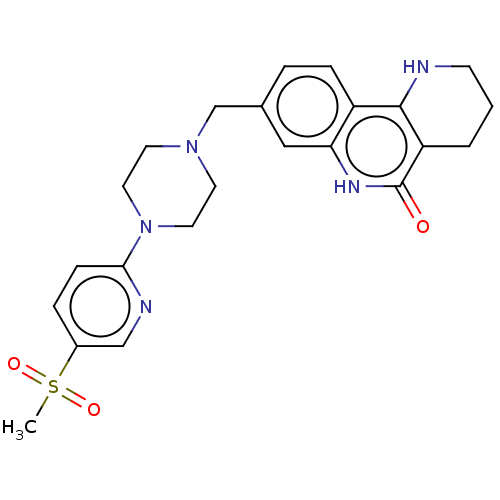 (US10464919, Example 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419785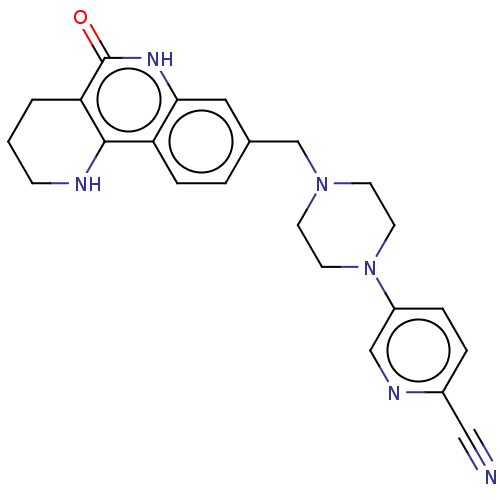 (US10464919, Example 123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419804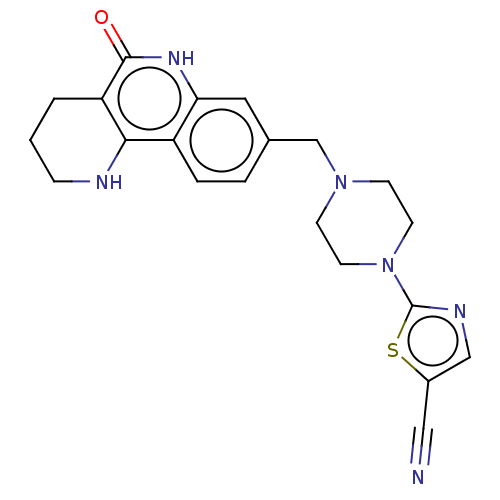 (US10464919, Example 142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM419812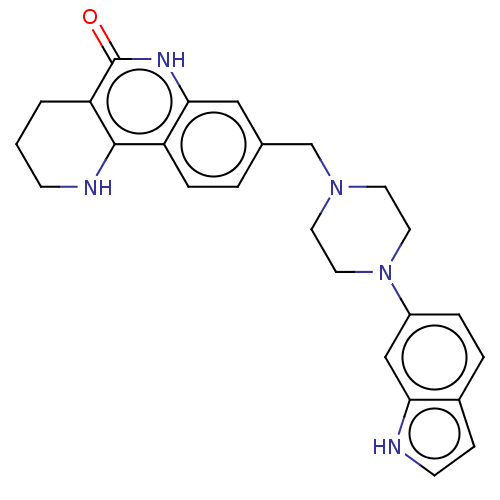 (US10464919, Example 150) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The PARP-1 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of a kit (cat. 80551) p... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419803 (US10464919, Example 141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419796 (US10464919, Example 134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TNK1 (Homo sapiens (Human)) | BDBM419794 (US10464919, Example 132) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419793 (US10464919, Example 131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419738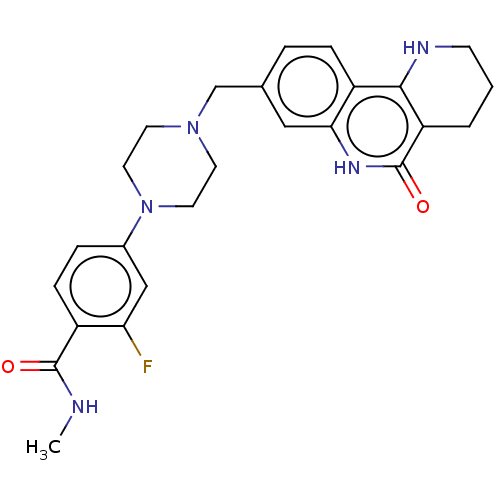 (US10464919, Example 76) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50224956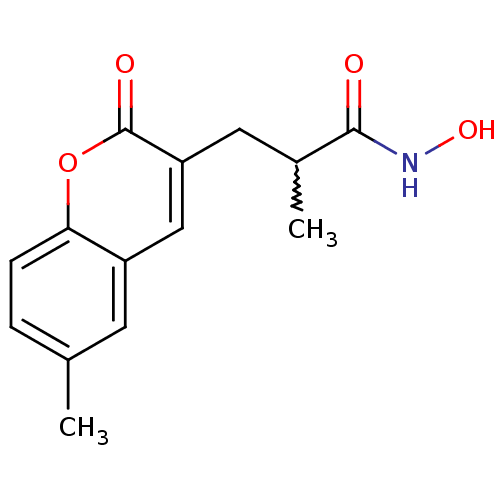 (CHEMBL253710 | N-hydroxy-2-methyl-3-(6-methyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of human TACE | Bioorg Med Chem 16: 530-5 (2008) Article DOI: 10.1016/j.bmc.2007.09.014 BindingDB Entry DOI: 10.7270/Q2QF8SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM419731 (US10464919, Example 69) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The PARP-1 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of a kit (cat. 80551) p... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM419802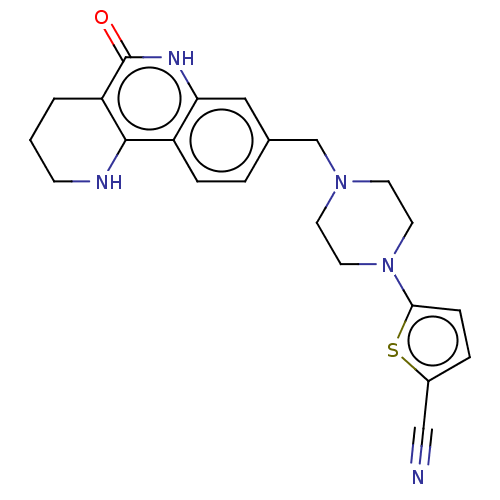 (US10464919, Example 140 | US10464919, Example 186) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The PARP-1 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of a kit (cat. 80551) p... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419787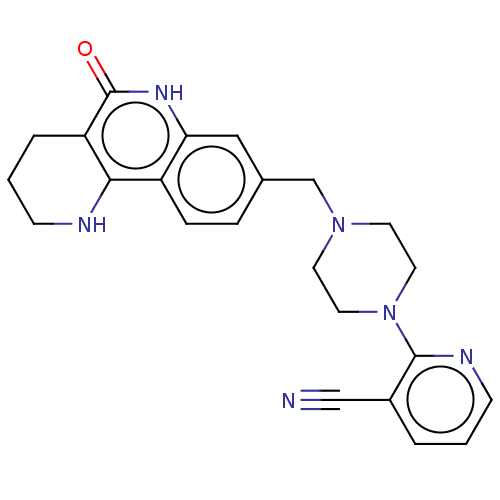 (US10464919, Example 125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419807 (US10464919, Example 145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM419755 (US10464919, Example 93) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The PARP-1 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of a kit (cat. 80551) p... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM419832 (US10464919, Example 170) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The PARP-1 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of a kit (cat. 80551) p... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 557 total ) | Next | Last >> |