

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50160071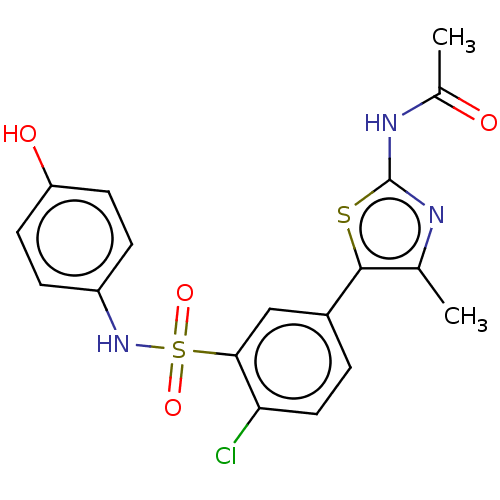 (CHEMBL3787013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50160071 (CHEMBL3787013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kgamma expressed in insect cells preincubated for 15 mins followed by addition of col... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50042924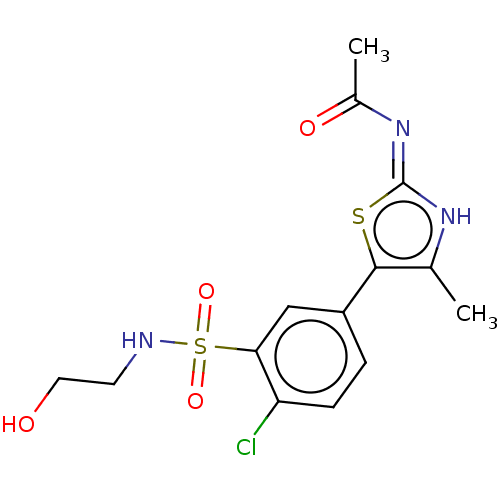 (CHEMBL1229535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kgamma expressed in insect cells preincubated for 15 mins followed by addition of col... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50160066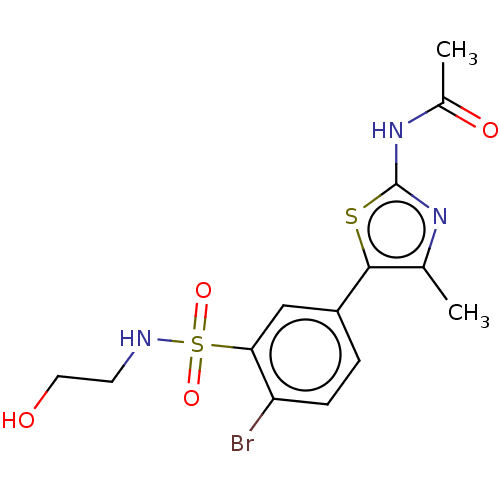 (CHEMBL3787634) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kgamma expressed in insect cells preincubated for 15 mins followed by addition of col... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50160072 (CHEMBL3787054) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50160072 (CHEMBL3787054) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kgamma expressed in insect cells preincubated for 15 mins followed by addition of col... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM50160071 (CHEMBL3787013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human full length VPS34 expressed in baculovirus preincubated for 15 mins followed by addition of cold ATP/gamma... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50160066 (CHEMBL3787634) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50042924 (CHEMBL1229535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM50160066 (CHEMBL3787634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human full length VPS34 expressed in baculovirus preincubated for 15 mins followed by addition of cold ATP/gamma... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50160074 (CHEMBL3785311) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50160067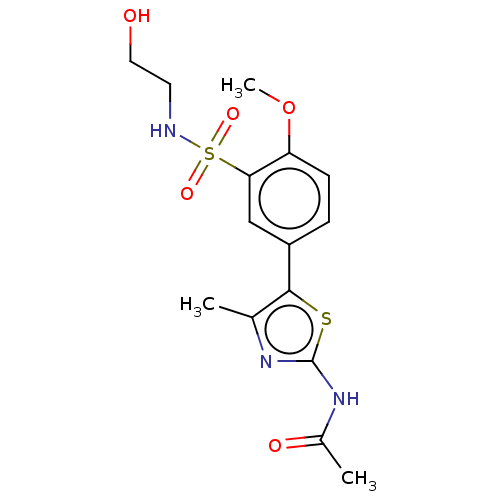 (CHEMBL3786633) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kgamma expressed in insect cells preincubated for 15 mins followed by addition of col... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50160067 (CHEMBL3786633) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM50160072 (CHEMBL3787054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human full length VPS34 expressed in baculovirus preincubated for 15 mins followed by addition of cold ATP/gamma... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM50042924 (CHEMBL1229535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 307 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human full length VPS34 expressed in baculovirus preincubated for 15 mins followed by addition of cold ATP/gamma... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50160070 (CHEMBL3786159) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50160075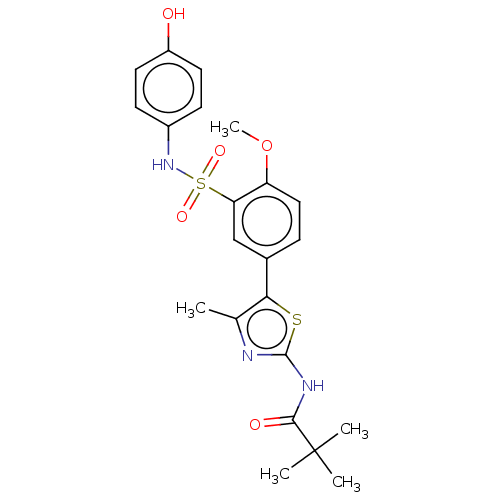 (CHEMBL3786230) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant human full length PI3Kdelta expressed in baculovirus preincubated for 15 mins followed by addition of cold... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513434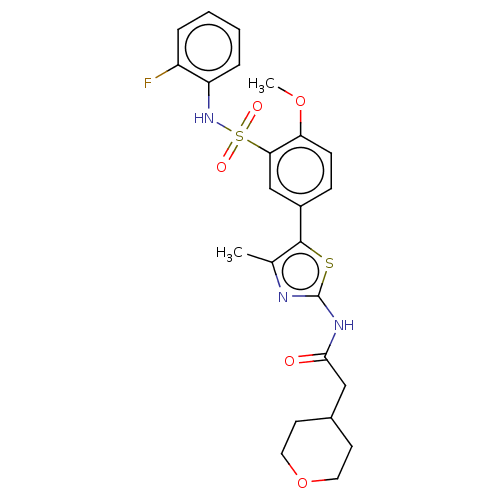 (US11091472, Compound S-230) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513435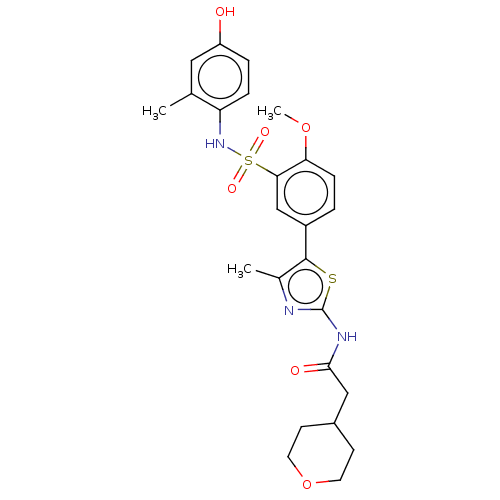 (US11091472, Compound S-231) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase alpha (Homo sapiens (Human)) | BDBM513440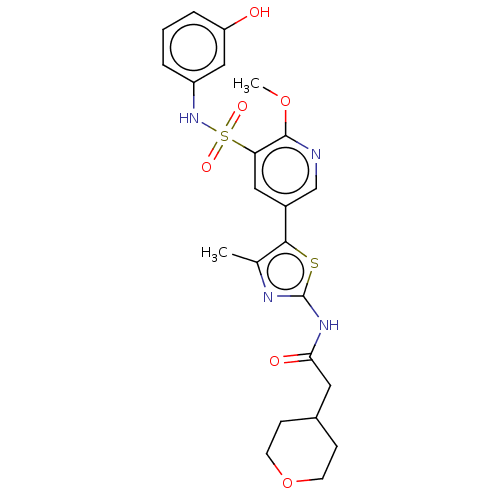 (US11091472, Compound S-259) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513440 (US11091472, Compound S-259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase alpha (Homo sapiens (Human)) | BDBM513439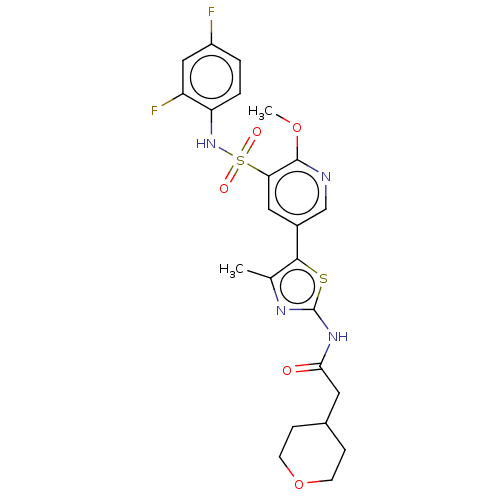 (US11091472, Compound S-258 | US11091472, Compound ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513439 (US11091472, Compound S-258 | US11091472, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase alpha (Homo sapiens (Human)) | BDBM513442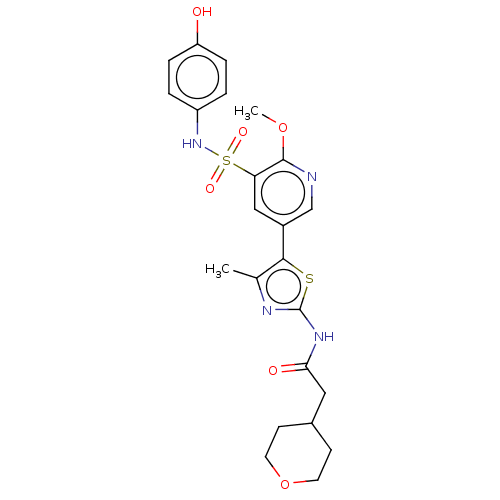 (US11091472, Compound S-261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513442 (US11091472, Compound S-261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase alpha (Homo sapiens (Human)) | BDBM513443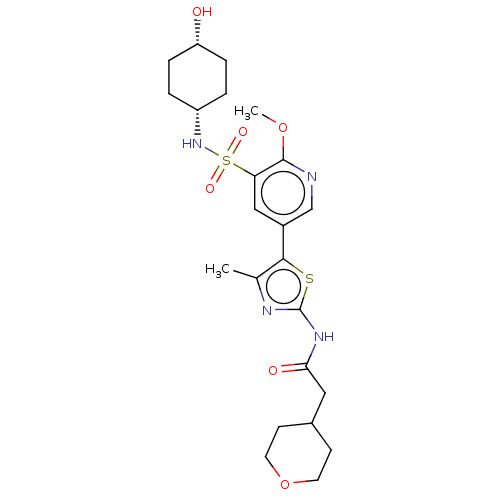 (US11091472, Compound S-262) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513443 (US11091472, Compound S-262) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase alpha (Homo sapiens (Human)) | BDBM513444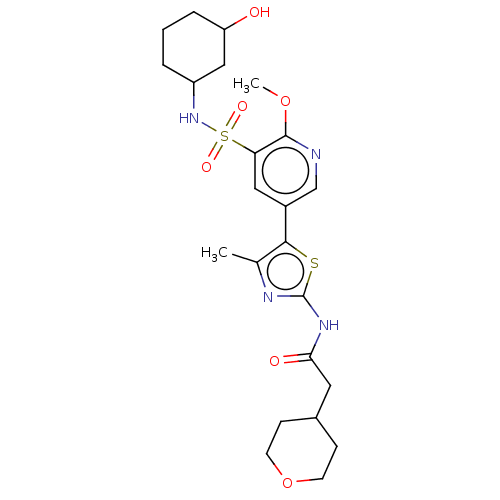 (US11091472, Compound S-263) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513444 (US11091472, Compound S-263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase alpha (Homo sapiens (Human)) | BDBM513445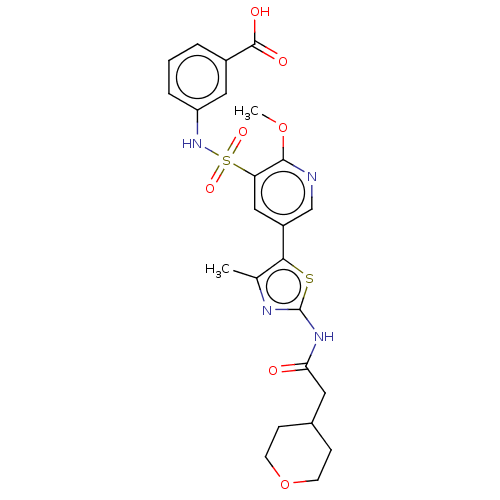 (US11091472, Compound S-265) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513445 (US11091472, Compound S-265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma (Homo sapiens (Human)) | BDBM50160074 (CHEMBL3785311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged recombinant human full length PI3KC2gamma expressed in baculovirus infected Sf21 insect cells preincubated for 15... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma (Homo sapiens (Human)) | BDBM50160075 (CHEMBL3786230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged recombinant human full length PI3KC2gamma expressed in baculovirus infected Sf21 insect cells preincubated for 15... | J Med Chem 59: 1830-9 (2016) Article DOI: 10.1021/acs.jmedchem.5b01311 BindingDB Entry DOI: 10.7270/Q29888W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513328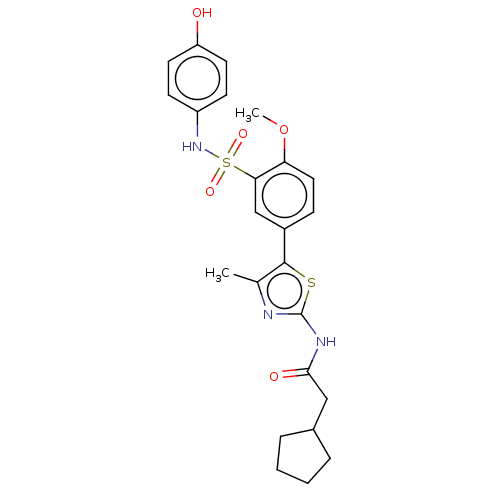 (US11091472, Compound S-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma (Homo sapiens (Human)) | BDBM513328 (US11091472, Compound S-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513330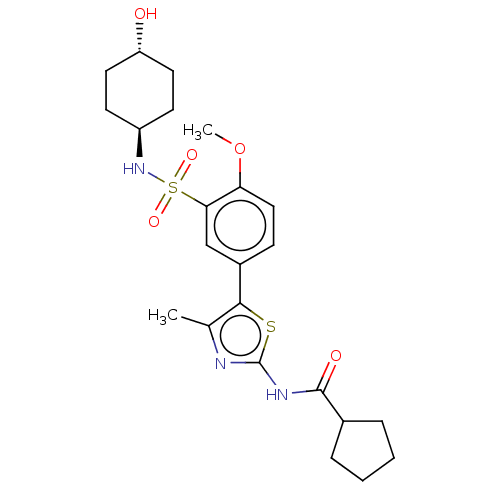 (US11091472, Compound S-9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513338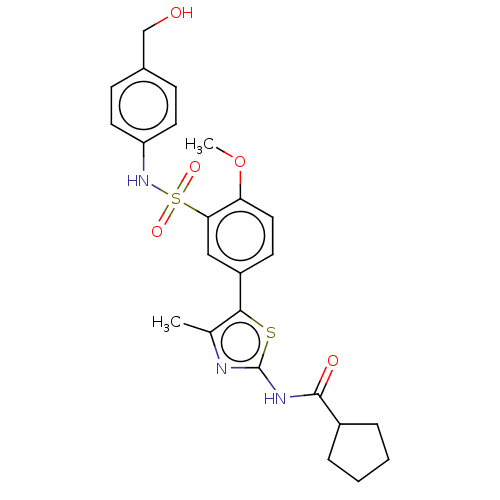 (US11091472, Compound S-41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513341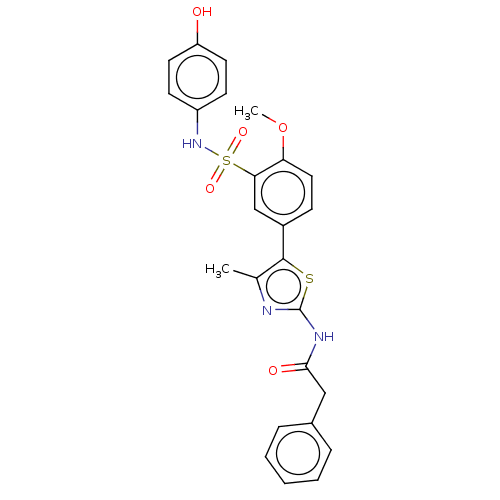 (US11091472, Compound S-49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513352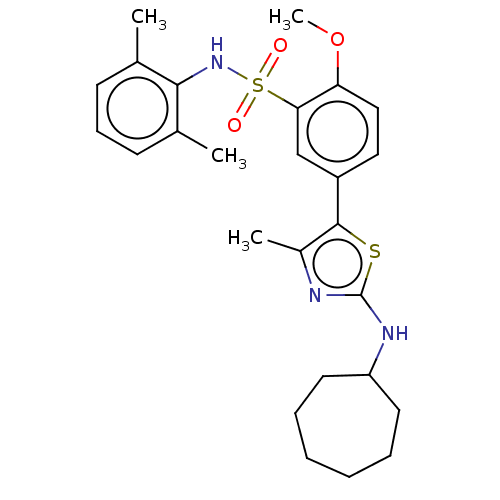 (US11091472, Compound S-98) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513360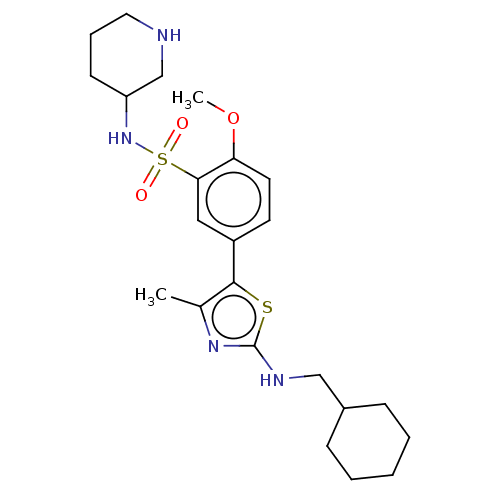 (US11091472, Compound S-115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513365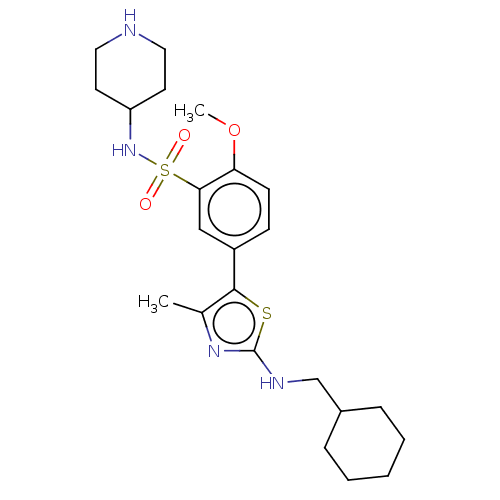 (US11091472, Compound S-123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513370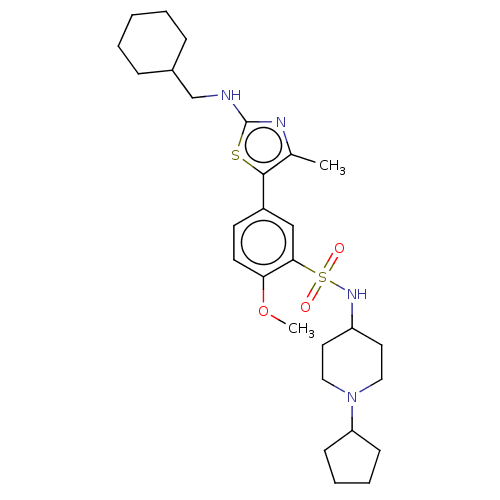 (US11091472, Compound S-133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513371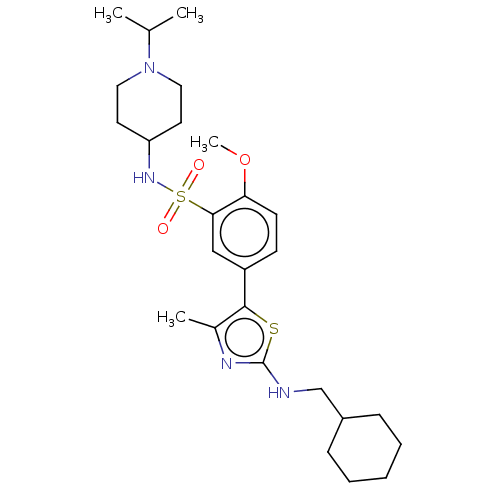 (US11091472, Compound S-134) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513372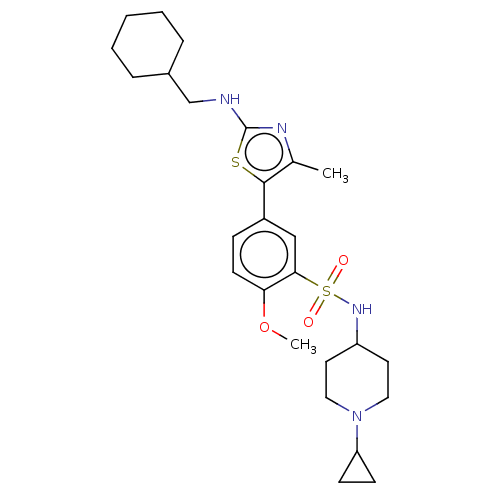 (US11091472, Compound S-135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513373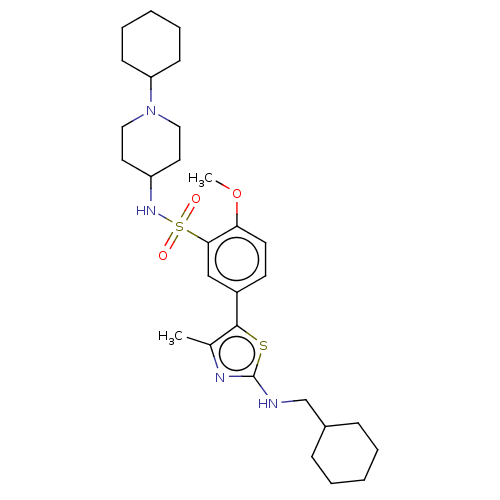 (US11091472, Compound S-136) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513375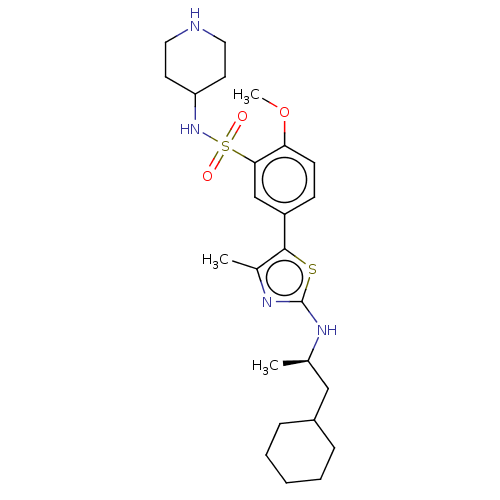 (US11091472, Compound S-145) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513377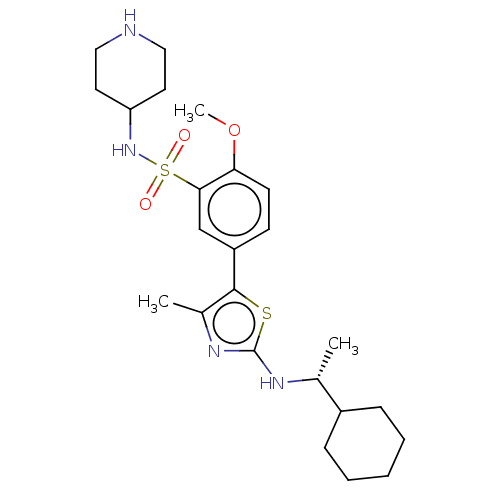 (US11091472, Compound S-147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513378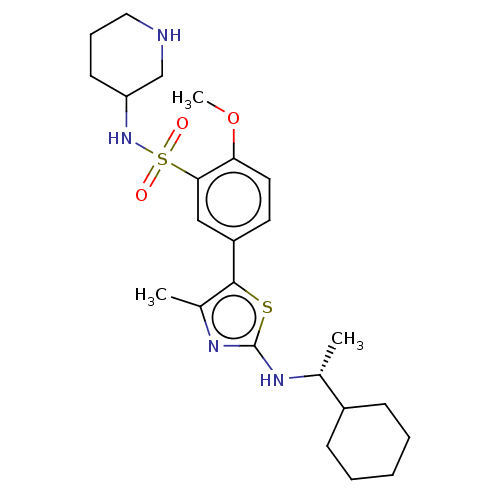 (US11091472, Compound S-148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513379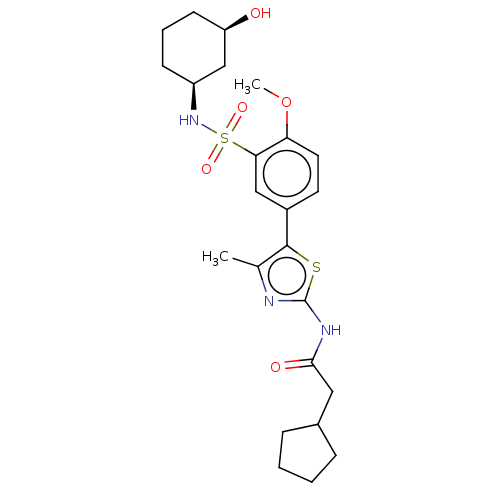 (US11091472, Compound S-149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM513381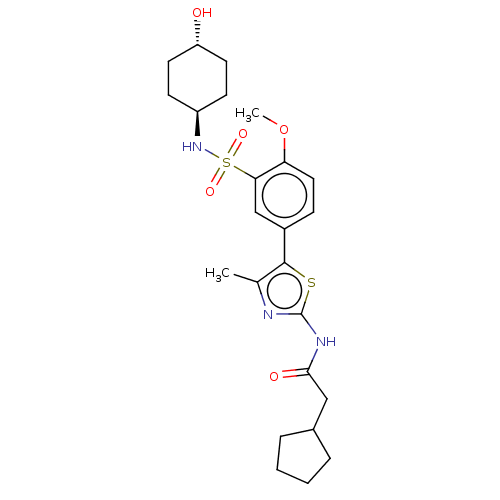 (US11091472, Compound S-151) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In some embodiments, the subject compounds inhibit a P14-kinase, as determined by a kinase activity assay, e.g., by an assay that determines the leve... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5Z5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 234 total ) | Next | Last >> |