

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578360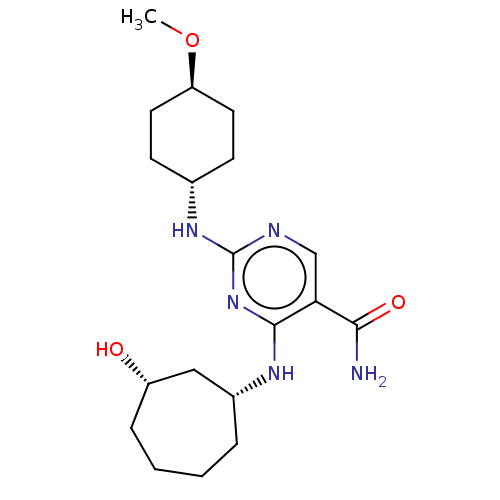 (CHEMBL4849353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578357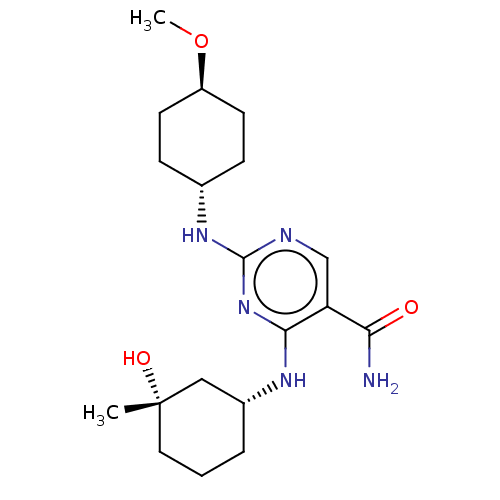 (CHEMBL4856984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578358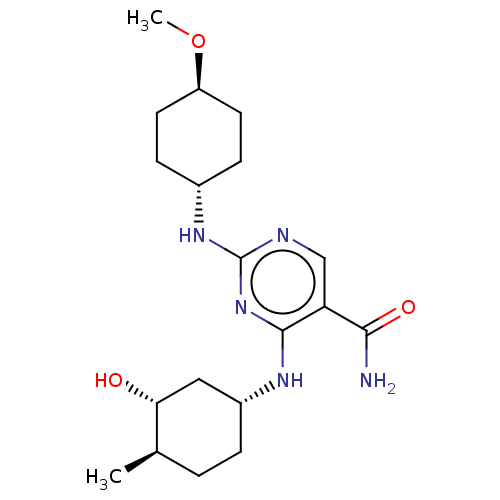 (CHEMBL4878370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578347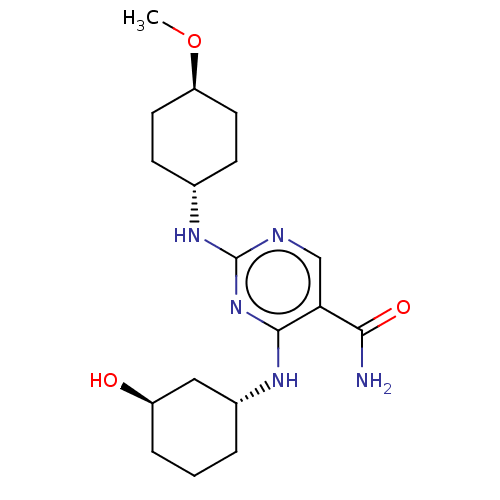 (CHEMBL4853125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578367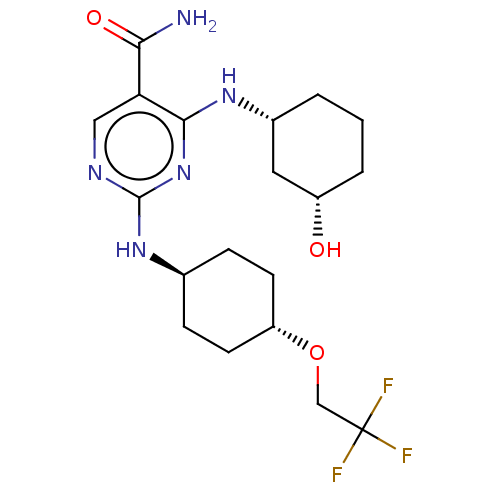 (CHEMBL4864618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578365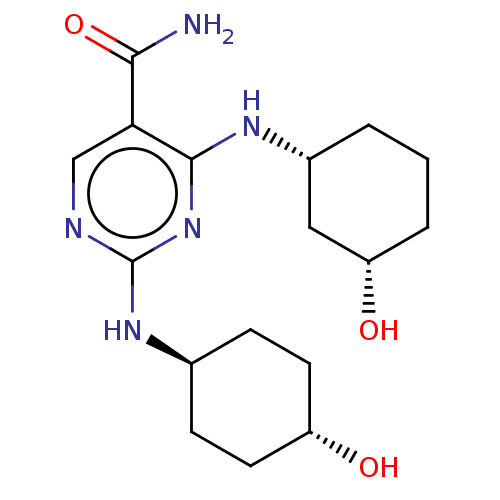 (CHEMBL4853525) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578346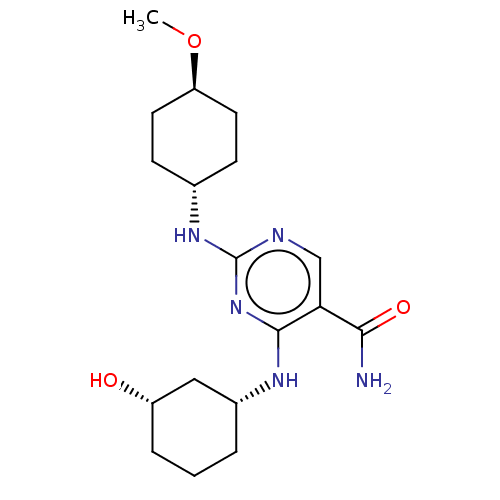 (CHEMBL4847078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578366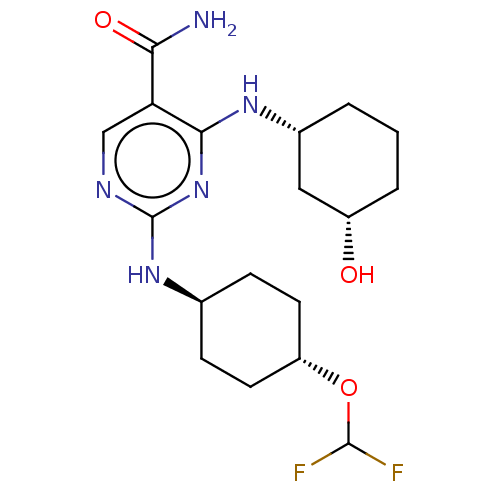 (CHEMBL4845965) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578346 (CHEMBL4847078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578364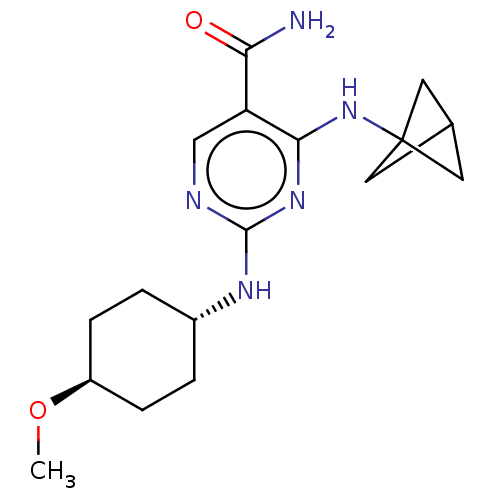 (CHEMBL4875131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50364378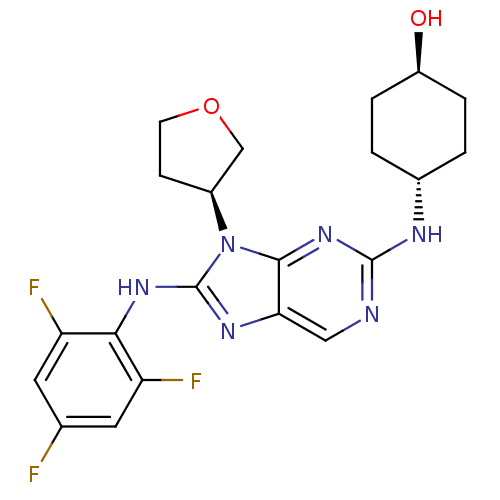 (CHEMBL1950289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50578361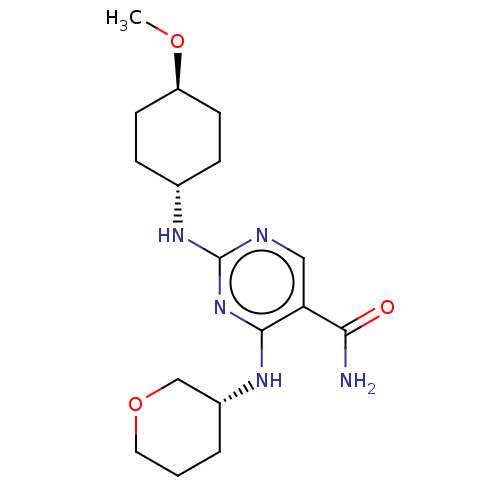 (CHEMBL4877560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50578346 (CHEMBL4847078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50578343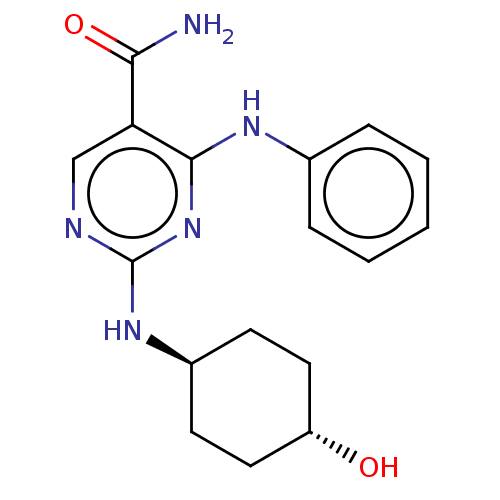 (CHEMBL4878046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578343 (CHEMBL4878046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50578347 (CHEMBL4853125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325499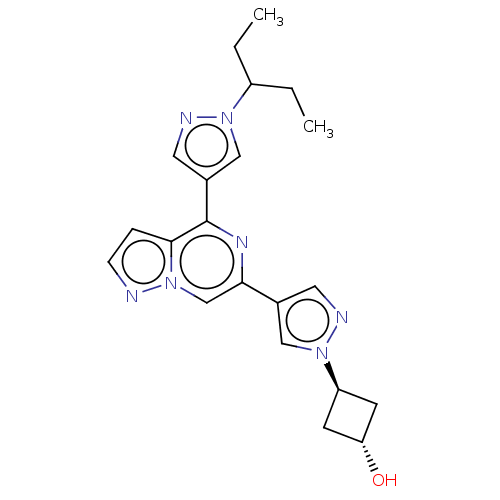 (US10189845, Example 161 | US10730880, Example 161 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10189845 (2019) BindingDB Entry DOI: 10.7270/Q2Z03B7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325500 ((1s,3s)-1-methyl-3-(4-(4-(1-(pentan-3-yl)-1H-pyraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10189845 (2019) BindingDB Entry DOI: 10.7270/Q2Z03B7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325513 ((R)-2-hydroxy-1-(3-(4-(4-(1-(pentan-3-yl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10189845 (2019) BindingDB Entry DOI: 10.7270/Q2Z03B7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325514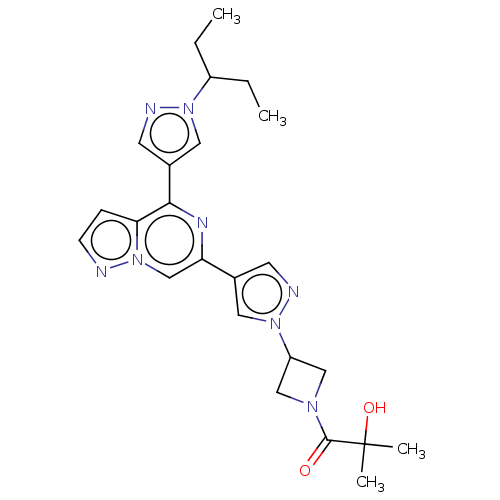 (2-hydroxy-2-methyl-1-(3- (4-(4-(1-(pentan-3-yl)- 1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10189845 (2019) BindingDB Entry DOI: 10.7270/Q2Z03B7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578371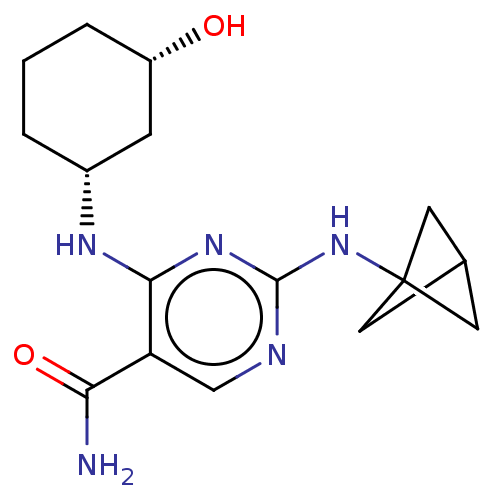 (CHEMBL4877389) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578352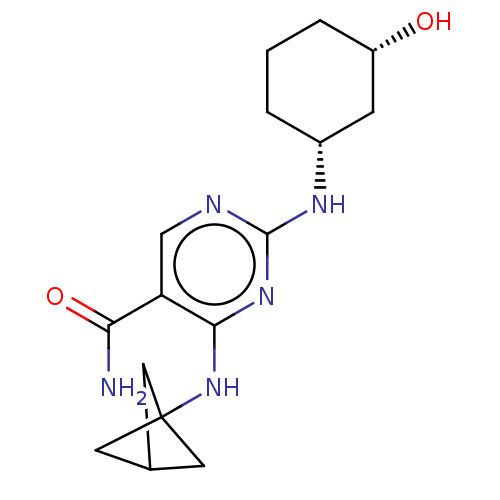 (CHEMBL4877655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325470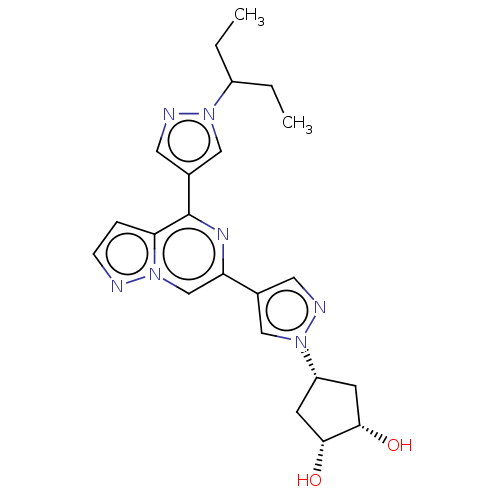 (US10189845, Example 132A | US10189845, Example 133...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325423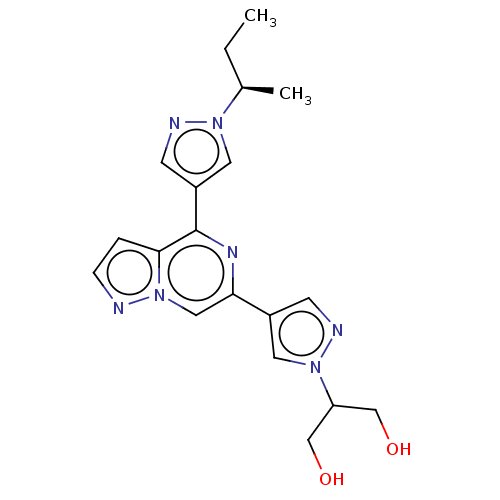 ((R)-2-(4-(4-(1-(sec- butyl)-1H-pyrazol-4- yl)pyraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325424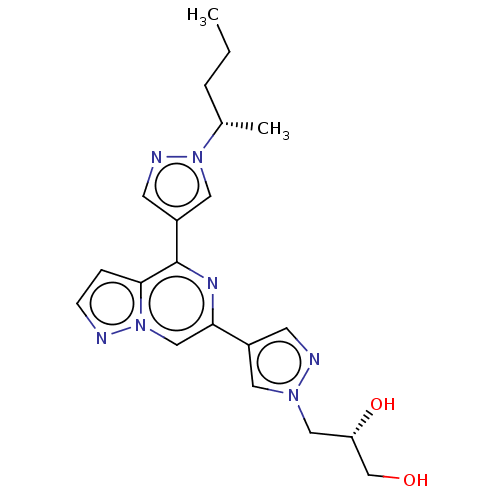 ((S)-3-(4-(4-(1-((S)- pentan-2-yl)-1H-pyrazol- 4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM502821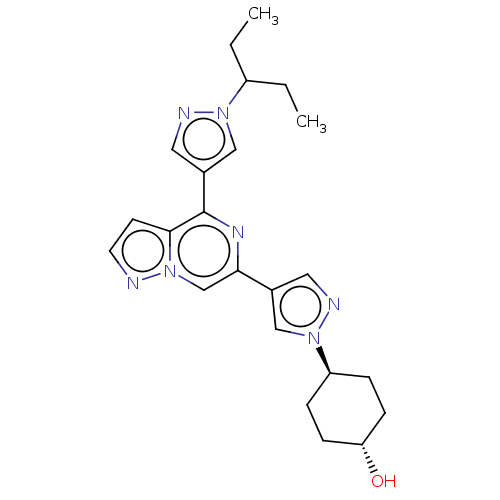 (US11028093, Example 118 | trans-4-(4-(4-(1-(pentan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325457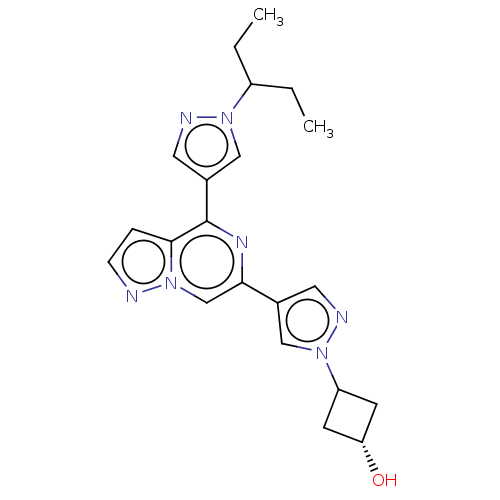 (US10189845, Example 119 | US10730880, Example 119 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325370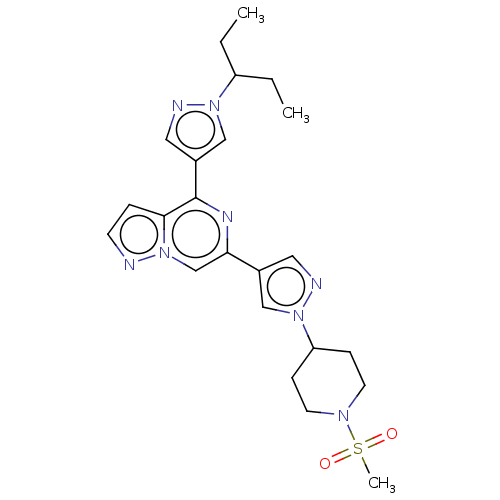 (6-(1-(1- (methylsulfonyl)piperidin- 4-yl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325370 (6-(1-(1- (methylsulfonyl)piperidin- 4-yl)-1H-pyraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325371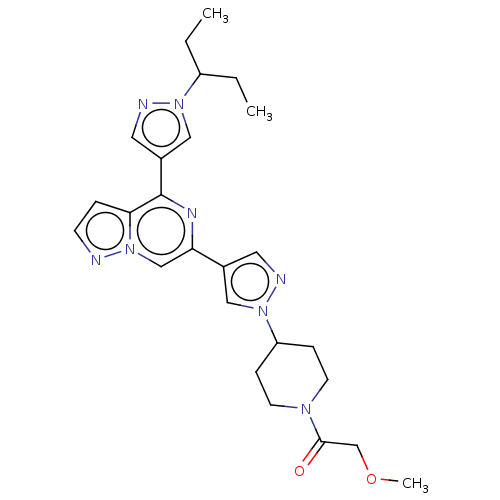 (2-methoxy-1-(4-(4-(4-(1- (pentan-3-yl)-1H-pyrazol-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325371 (2-methoxy-1-(4-(4-(4-(1- (pentan-3-yl)-1H-pyrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325372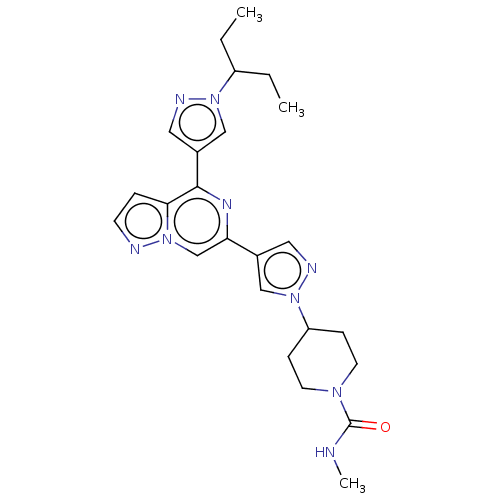 (N-methyl-4-(4-(4-(1- (pentan-3-yl)-1H-pyrazol- 4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325374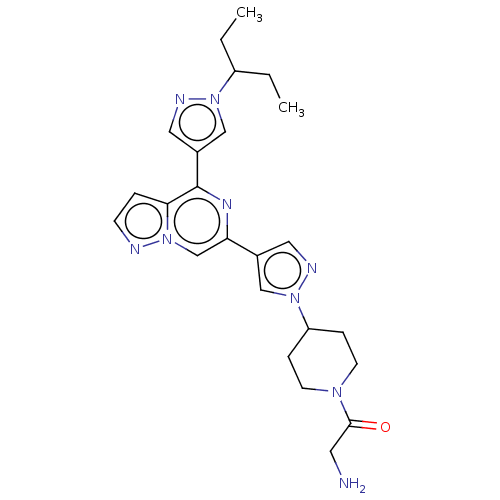 (2-amino-1-(4-(4-(4-(1-(pentan-3-yl)-1H-pyrazol-4-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325374 (2-amino-1-(4-(4-(4-(1-(pentan-3-yl)-1H-pyrazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325375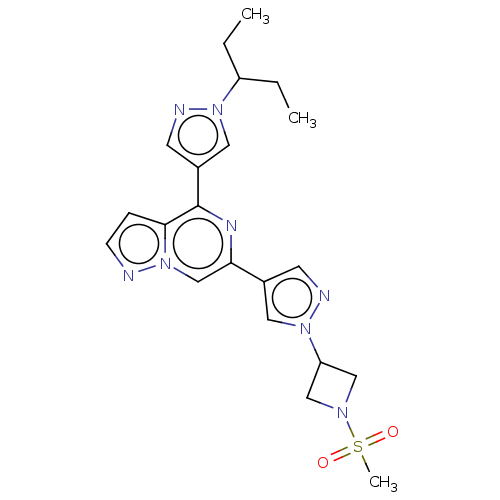 (6-(1-(1-(methylsulfonyl)azetidin-3-yl)-1H-pyrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325386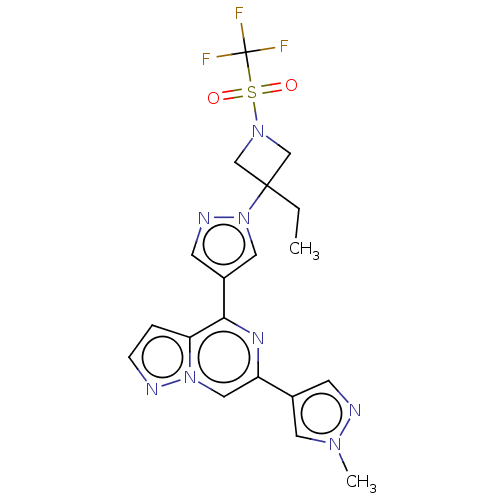 (4-(1-(3-ethyl-1-((trifluoromethyl)sulfonyl)azetidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325386 (4-(1-(3-ethyl-1-((trifluoromethyl)sulfonyl)azetidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325545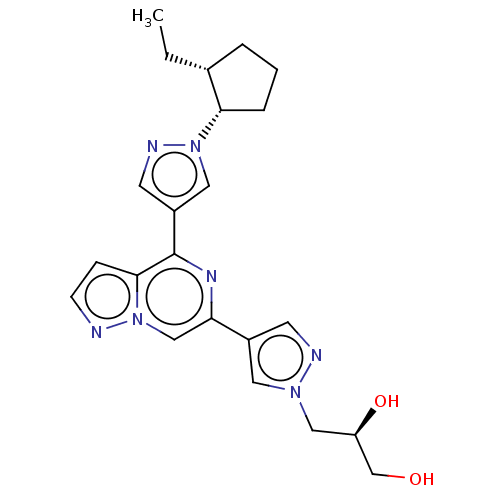 (US10189845, Example 207A | US10730880, Example 207...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325351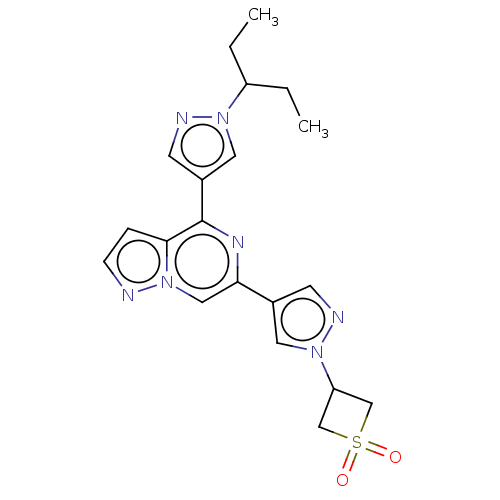 (3-(4-(4-(1-(pentan-3-yl)-1H- pyrazol-4-yl)pyrazolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325369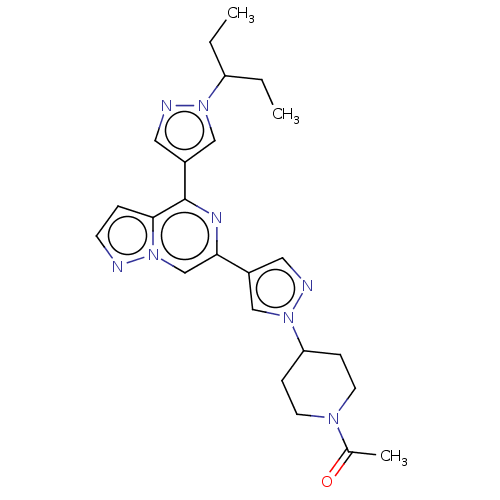 (1-(4-(4-(4-(1-(pentan-3-yl)-1H-pyrazol-4-yl)pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325514 (2-hydroxy-2-methyl-1-(3- (4-(4-(1-(pentan-3-yl)- 1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325515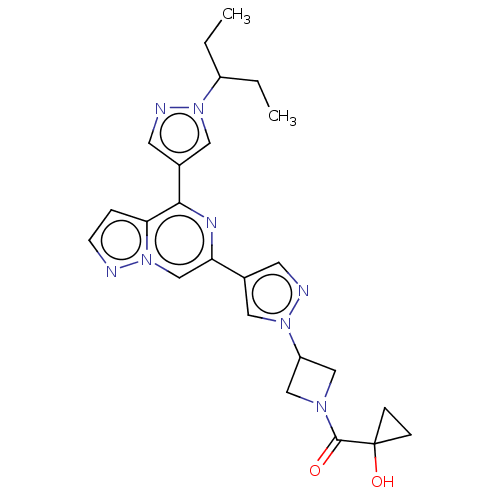 ((1- hydroxycyclopropyl)(3- (4-(4-(1-(pentan-3-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325545 (US10189845, Example 207A | US10730880, Example 207...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM502873 ((1s,3 s)-1-methyl-3-(4-(4-(1-(pentan-3-yl)-1H-pyra...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM502874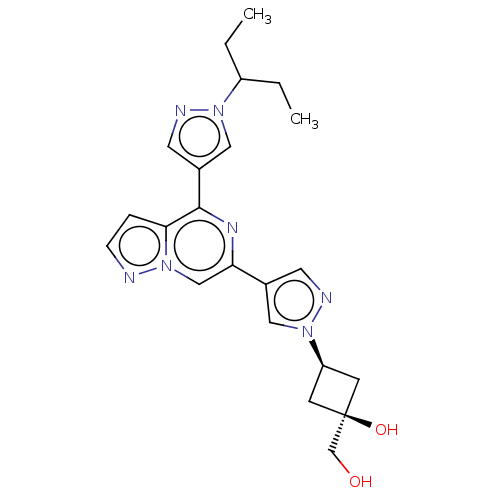 ((1s,3s)-1-(hydroxymethyl)-3-(4-(4-(1-(pentan-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARRAY BIOPHARMA INC.; CELGENE CORPORATION US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US11028093 (2021) BindingDB Entry DOI: 10.7270/Q2T156RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325501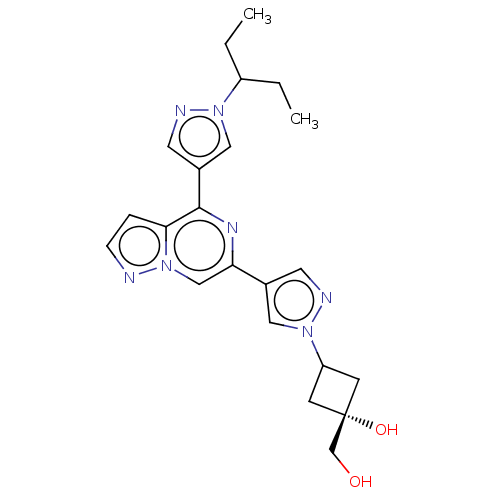 (US10189845, Example 163A | US10189845, Example 164...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325514 (2-hydroxy-2-methyl-1-(3- (4-(4-(1-(pentan-3-yl)- 1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM325515 ((1- hydroxycyclopropyl)(3- (4-(4-(1-(pentan-3-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM325374 (2-amino-1-(4-(4-(4-(1-(pentan-3-yl)-1H-pyrazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM455371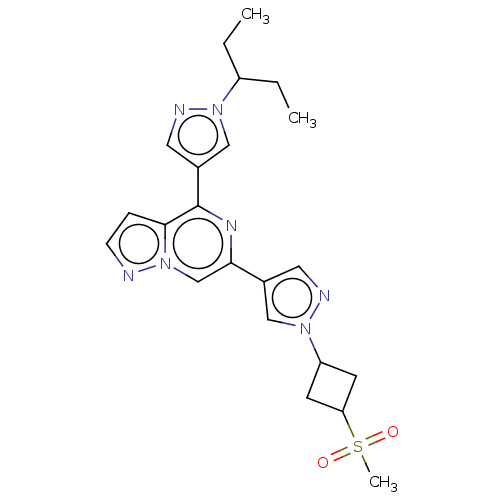 (6-(1-(1-(methylsulfonyl)azetidin-3-yl)-1H-pyrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2691 total ) | Next | Last >> |