 Found 211 hits with Last Name = 'babij' and Initial = 'c'
Found 211 hits with Last Name = 'babij' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165870

(CHEMBL3797403)Show SMILES Fc1ccc(NS(=O)(=O)c2cn[nH]c2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H13F2N9O2S/c20-12-3-4-13(30-33(31,32)10-6-27-28-7-10)14(21)16(12)29-18-11(2-1-5-22-18)15-17-19(25-8-23-15)26-9-24-17/h1-9,30H,(H,22,29)(H,27,28)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM35317
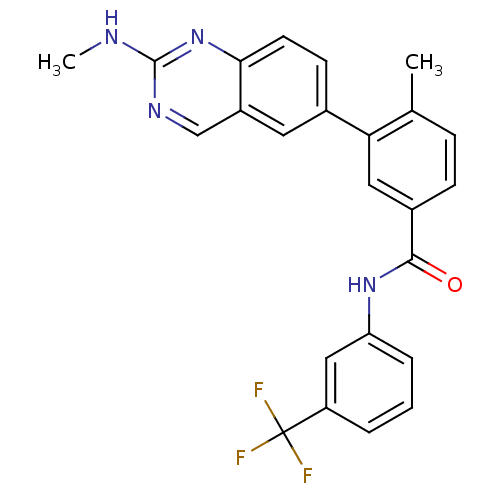
(4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...)Show SMILES CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O/c1-14-6-7-16(22(32)30-19-5-3-4-18(12-19)24(25,26)27)11-20(14)15-8-9-21-17(10-15)13-29-23(28-2)31-21/h3-13H,1-2H3,(H,30,32)(H,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM14971
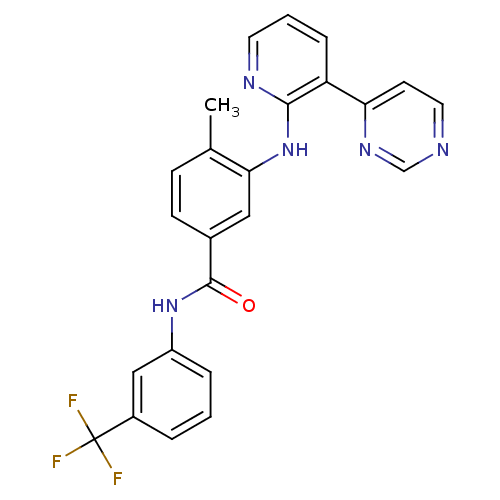
(4-Methyl-3-(3-(pyrimidin-4-yl)pyridin-2-ylamino)-N...)Show SMILES Cc1ccc(cc1Nc1ncccc1-c1ccncn1)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H18F3N5O/c1-15-7-8-16(23(33)31-18-5-2-4-17(13-18)24(25,26)27)12-21(15)32-22-19(6-3-10-29-22)20-9-11-28-14-30-20/h2-14H,1H3,(H,29,32)(H,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | 15 | n/a | n/a | 7.0 | 23 |
Amgen
| Assay Description
The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197676
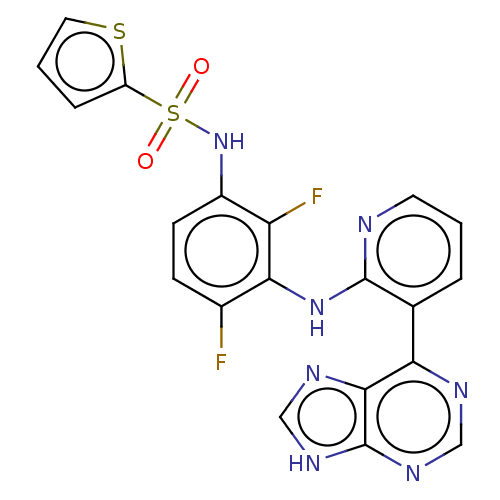
(US9216981, 4)Show SMILES Fc1ccc(NS(=O)(=O)c2cccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H13F2N7O2S2/c21-12-5-6-13(29-33(30,31)14-4-2-8-32-14)15(22)17(12)28-19-11(3-1-7-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-10,29H,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197701
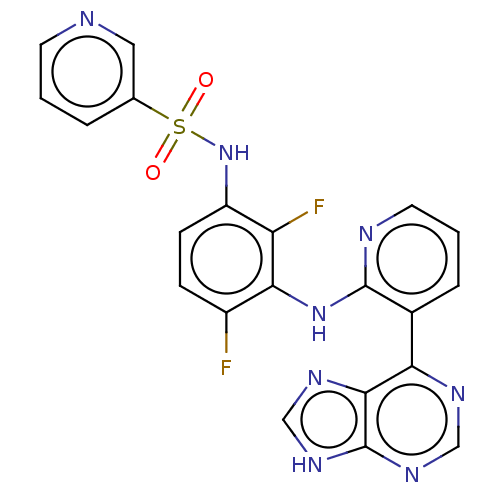
(US9216981, 30)Show SMILES Fc1ccc(NS(=O)(=O)c2cccnc2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C21H14F2N8O2S/c22-14-5-6-15(31-34(32,33)12-3-1-7-24-9-12)16(23)18(14)30-20-13(4-2-8-25-20)17-19-21(28-10-26-17)29-11-27-19/h1-11,31H,(H,25,30)(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197676

(US9216981, 4)Show SMILES Fc1ccc(NS(=O)(=O)c2cccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H13F2N7O2S2/c21-12-5-6-13(29-33(30,31)14-4-2-8-32-14)15(22)17(12)28-19-11(3-1-7-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-10,29H,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197701

(US9216981, 30)Show SMILES Fc1ccc(NS(=O)(=O)c2cccnc2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C21H14F2N8O2S/c22-14-5-6-15(31-34(32,33)12-3-1-7-24-9-12)16(23)18(14)30-20-13(4-2-8-25-20)17-19-21(28-10-26-17)29-11-27-19/h1-11,31H,(H,25,30)(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165870

(CHEMBL3797403)Show SMILES Fc1ccc(NS(=O)(=O)c2cn[nH]c2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H13F2N9O2S/c20-12-3-4-13(30-33(31,32)10-6-27-28-7-10)14(21)16(12)29-18-11(2-1-5-22-18)15-17-19(25-8-23-15)26-9-24-17/h1-9,30H,(H,22,29)(H,27,28)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165868
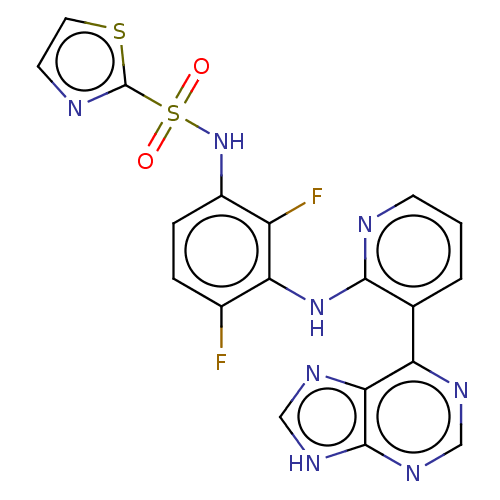
(CHEMBL3800081)Show SMILES Fc1ccc(NS(=O)(=O)c2nccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H12F2N8O2S2/c20-11-3-4-12(29-33(30,31)19-23-6-7-32-19)13(21)15(11)28-17-10(2-1-5-22-17)14-16-18(26-8-24-14)27-9-25-16/h1-9,29H,(H,22,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM35317

(4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...)Show SMILES CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O/c1-14-6-7-16(22(32)30-19-5-3-4-18(12-19)24(25,26)27)11-20(14)15-8-9-21-17(10-15)13-29-23(28-2)31-21/h3-13H,1-2H3,(H,30,32)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM35327
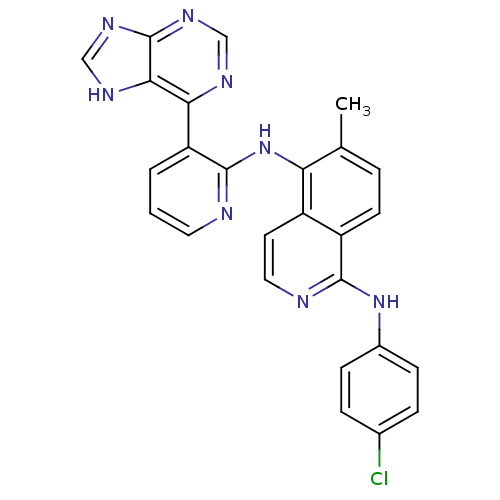
(pyridylpurine aminoisoquinoline, 1)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ncnc2nc[nH]c12 Show InChI InChI=1S/C26H19ClN8/c1-15-4-9-19-18(10-12-29-24(19)34-17-7-5-16(27)6-8-17)21(15)35-25-20(3-2-11-28-25)22-23-26(32-13-30-22)33-14-31-23/h2-14H,1H3,(H,28,35)(H,29,34)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | 1.80 | n/a | n/a | 7.0 | 23 |
Amgen
| Assay Description
The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM35317

(4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...)Show SMILES CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O/c1-14-6-7-16(22(32)30-19-5-3-4-18(12-19)24(25,26)27)11-20(14)15-8-9-21-17(10-15)13-29-23(28-2)31-21/h3-13H,1-2H3,(H,30,32)(H,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | 27 | n/a | n/a | 7.0 | 23 |
Amgen
| Assay Description
The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM35317

(4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...)Show SMILES CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O/c1-14-6-7-16(22(32)30-19-5-3-4-18(12-19)24(25,26)27)11-20(14)15-8-9-21-17(10-15)13-29-23(28-2)31-21/h3-13H,1-2H3,(H,30,32)(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165861
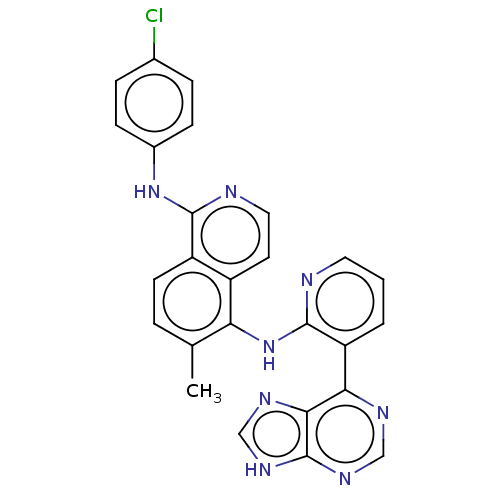
(CHEMBL1197798)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C26H19ClN8/c1-15-4-9-19-18(10-12-29-24(19)34-17-7-5-16(27)6-8-17)21(15)35-25-20(3-2-11-28-25)22-23-26(32-13-30-22)33-14-31-23/h2-14H,1H3,(H,28,35)(H,29,34)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165868

(CHEMBL3800081)Show SMILES Fc1ccc(NS(=O)(=O)c2nccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H12F2N8O2S2/c20-11-3-4-12(29-33(30,31)19-23-6-7-32-19)13(21)15(11)28-17-10(2-1-5-22-17)14-16-18(26-8-24-14)27-9-25-16/h1-9,29H,(H,22,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165861

(CHEMBL1197798)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C26H19ClN8/c1-15-4-9-19-18(10-12-29-24(19)34-17-7-5-16(27)6-8-17)21(15)35-25-20(3-2-11-28-25)22-23-26(32-13-30-22)33-14-31-23/h2-14H,1H3,(H,28,35)(H,29,34)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165861

(CHEMBL1197798)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C26H19ClN8/c1-15-4-9-19-18(10-12-29-24(19)34-17-7-5-16(27)6-8-17)21(15)35-25-20(3-2-11-28-25)22-23-26(32-13-30-22)33-14-31-23/h2-14H,1H3,(H,28,35)(H,29,34)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165884
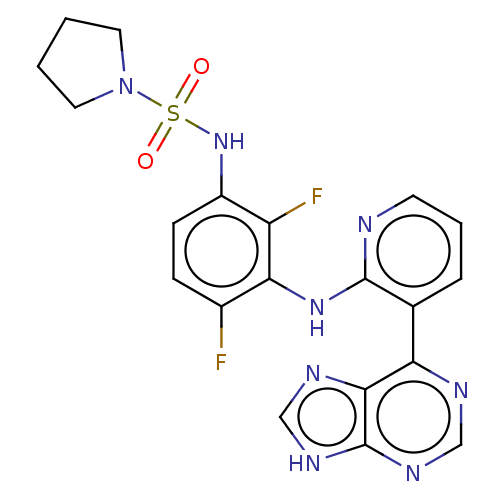
(CHEMBL3799949)Show SMILES Fc1ccc(NS(=O)(=O)N2CCCC2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H18F2N8O2S/c21-13-5-6-14(29-33(31,32)30-8-1-2-9-30)15(22)17(13)28-19-12(4-3-7-23-19)16-18-20(26-10-24-16)27-11-25-18/h3-7,10-11,29H,1-2,8-9H2,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165876
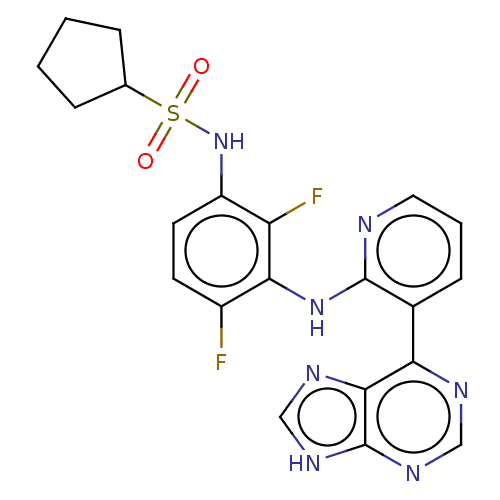
(CHEMBL3800364)Show SMILES Fc1ccc(NS(=O)(=O)C2CCCC2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C21H19F2N7O2S/c22-14-7-8-15(30-33(31,32)12-4-1-2-5-12)16(23)18(14)29-20-13(6-3-9-24-20)17-19-21(27-10-25-17)28-11-26-19/h3,6-12,30H,1-2,4-5H2,(H,24,29)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165873
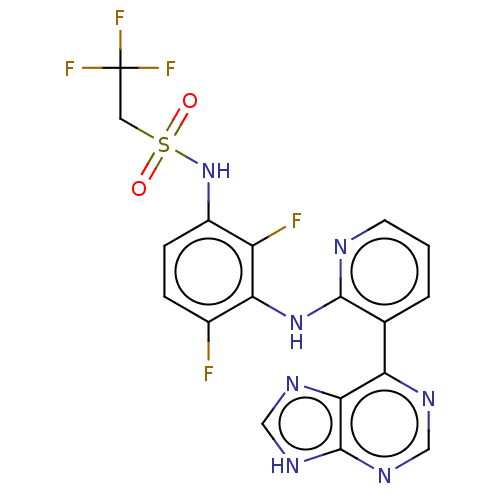
(CHEMBL3800014 | US9550781, 19)Show SMILES Fc1ccc(NS(=O)(=O)CC(F)(F)F)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C18H12F5N7O2S/c19-10-3-4-11(30-33(31,32)6-18(21,22)23)12(20)14(10)29-16-9(2-1-5-24-16)13-15-17(27-7-25-13)28-8-26-15/h1-5,7-8,30H,6H2,(H,24,29)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165864

(CHEMBL3799014)Show SMILES Fc1cccc(c1)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C22H14F3N7O2S/c23-12-3-1-4-13(9-12)35(33,34)32-16-7-6-15(24)19(17(16)25)31-21-14(5-2-8-26-21)18-20-22(29-10-27-18)30-11-28-20/h1-11,32H,(H,26,31)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165863

(CHEMBL3800226)Show SMILES Fc1ccc(NS(=O)(=O)c2ccccc2F)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C22H14F3N7O2S/c23-13-5-1-2-6-16(13)35(33,34)32-15-8-7-14(24)19(17(15)25)31-21-12(4-3-9-26-21)18-20-22(29-10-27-18)30-11-28-20/h1-11,32H,(H,26,31)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM35319
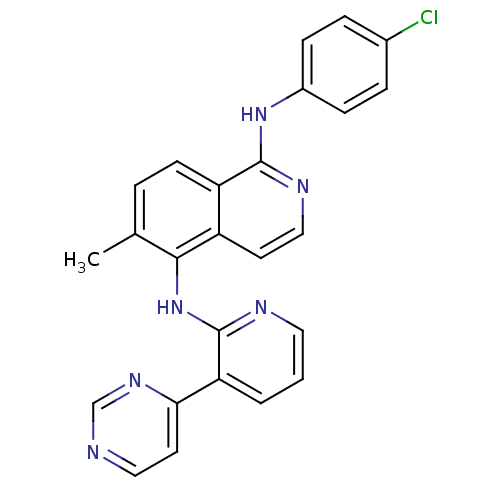
(aminoisoquinoline, 12b)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ccncn1 Show InChI InChI=1S/C25H19ClN6/c1-16-4-9-20-19(10-14-29-24(20)31-18-7-5-17(26)6-8-18)23(16)32-25-21(3-2-12-28-25)22-11-13-27-15-30-22/h2-15H,1H3,(H,28,32)(H,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | 34 | n/a | n/a | 7.0 | 23 |
Amgen
| Assay Description
The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM35317

(4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...)Show SMILES CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O/c1-14-6-7-16(22(32)30-19-5-3-4-18(12-19)24(25,26)27)11-20(14)15-8-9-21-17(10-15)13-29-23(28-2)31-21/h3-13H,1-2H3,(H,30,32)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM14971

(4-Methyl-3-(3-(pyrimidin-4-yl)pyridin-2-ylamino)-N...)Show SMILES Cc1ccc(cc1Nc1ncccc1-c1ccncn1)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H18F3N5O/c1-15-7-8-16(23(33)31-18-5-2-4-17(13-18)24(25,26)27)12-21(15)32-22-19(6-3-10-29-22)20-9-11-28-14-30-20/h2-14H,1H3,(H,29,32)(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM14971

(4-Methyl-3-(3-(pyrimidin-4-yl)pyridin-2-ylamino)-N...)Show SMILES Cc1ccc(cc1Nc1ncccc1-c1ccncn1)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H18F3N5O/c1-15-7-8-16(23(33)31-18-5-2-4-17(13-18)24(25,26)27)12-21(15)32-22-19(6-3-10-29-22)20-9-11-28-14-30-20/h2-14H,1H3,(H,29,32)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165860

(CHEMBL3798630)Show SMILES Fc1ccc(NS(=O)(=O)C2CCC2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H17F2N7O2S/c21-13-6-7-14(29-32(30,31)11-3-1-4-11)15(22)17(13)28-19-12(5-2-8-23-19)16-18-20(26-9-24-16)27-10-25-18/h2,5-11,29H,1,3-4H2,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165864

(CHEMBL3799014)Show SMILES Fc1cccc(c1)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C22H14F3N7O2S/c23-12-3-1-4-13(9-12)35(33,34)32-16-7-6-15(24)19(17(16)25)31-21-14(5-2-8-26-21)18-20-22(29-10-27-18)30-11-28-20/h1-11,32H,(H,26,31)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50396483
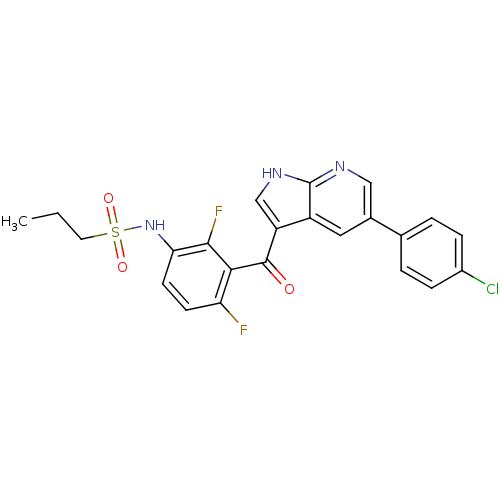
(PLX-4032 | RG 7204 | Ro 5185426 | US10570155, Vemu...)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(cc23)-c2ccc(Cl)cc2)c1F Show InChI InChI=1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM14971

(4-Methyl-3-(3-(pyrimidin-4-yl)pyridin-2-ylamino)-N...)Show SMILES Cc1ccc(cc1Nc1ncccc1-c1ccncn1)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H18F3N5O/c1-15-7-8-16(23(33)31-18-5-2-4-17(13-18)24(25,26)27)12-21(15)32-22-19(6-3-10-29-22)20-9-11-28-14-30-20/h2-14H,1H3,(H,29,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165856
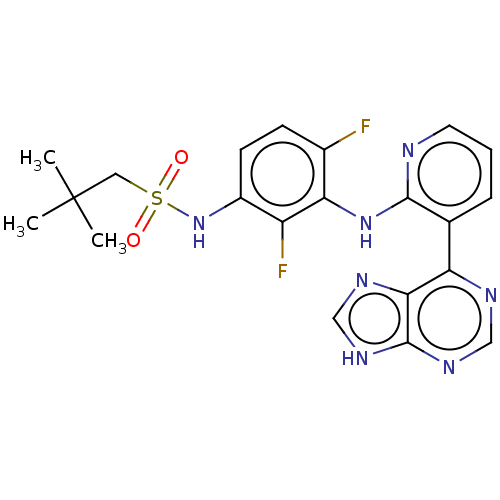
(CHEMBL3799342)Show SMILES CC(C)(C)CS(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C21H21F2N7O2S/c1-21(2,3)9-33(31,32)30-14-7-6-13(22)17(15(14)23)29-19-12(5-4-8-24-19)16-18-20(27-10-25-16)28-11-26-18/h4-8,10-11,30H,9H2,1-3H3,(H,24,29)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165878
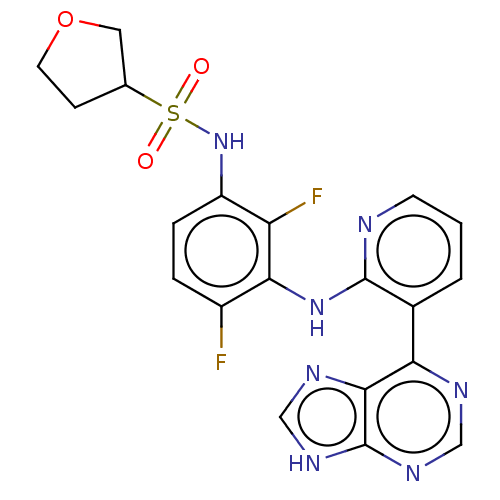
(CHEMBL3798235)Show SMILES Fc1ccc(NS(=O)(=O)C2CCOC2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H17F2N7O3S/c21-13-3-4-14(29-33(30,31)11-5-7-32-8-11)15(22)17(13)28-19-12(2-1-6-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-4,6,9-11,29H,5,7-8H2,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM35324
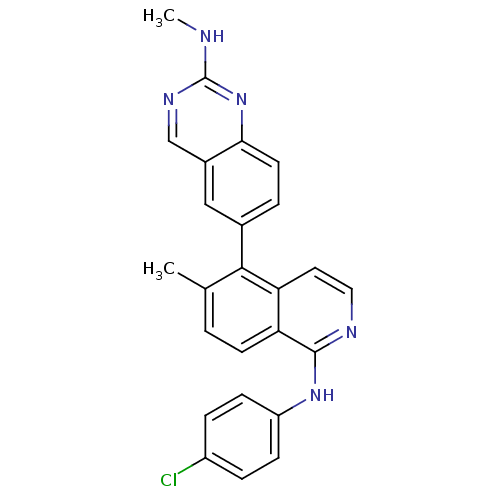
(aminoquinazoline, 18)Show SMILES CNc1ncc2cc(ccc2n1)-c1c(C)ccc2c(Nc3ccc(Cl)cc3)nccc12 Show InChI InChI=1S/C25H20ClN5/c1-15-3-9-21-20(11-12-28-24(21)30-19-7-5-18(26)6-8-19)23(15)16-4-10-22-17(13-16)14-29-25(27-2)31-22/h3-14H,1-2H3,(H,28,30)(H,27,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | 270 | n/a | n/a | 7.0 | 23 |
Amgen
| Assay Description
The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM35323
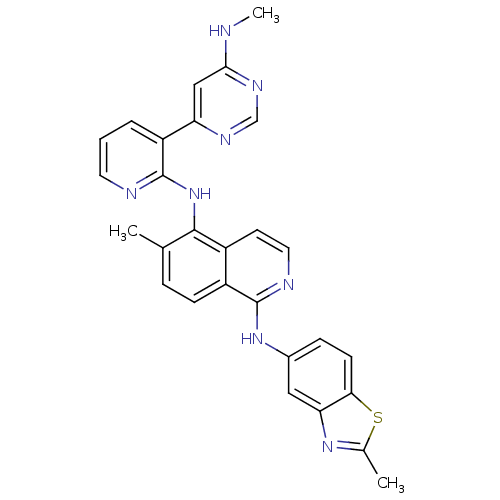
(aminoisoquinoline, 16)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1c(C)ccc2c(Nc3ccc4sc(C)nc4c3)nccc12 Show InChI InChI=1S/C28H24N8S/c1-16-6-8-20-19(10-12-31-27(20)35-18-7-9-24-23(13-18)34-17(2)37-24)26(16)36-28-21(5-4-11-30-28)22-14-25(29-3)33-15-32-22/h4-15H,1-3H3,(H,30,36)(H,31,35)(H,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165870

(CHEMBL3797403)Show SMILES Fc1ccc(NS(=O)(=O)c2cn[nH]c2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H13F2N9O2S/c20-12-3-4-13(30-33(31,32)10-6-27-28-7-10)14(21)16(12)29-18-11(2-1-5-22-18)15-17-19(25-8-23-15)26-9-24-17/h1-9,30H,(H,22,29)(H,27,28)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165874
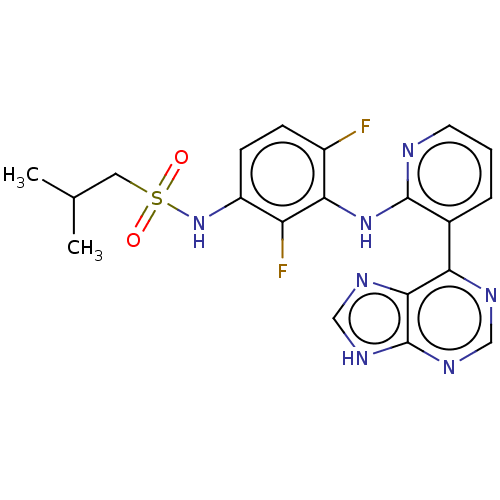
(CHEMBL3799488)Show SMILES CC(C)CS(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C20H19F2N7O2S/c1-11(2)8-32(30,31)29-14-6-5-13(21)17(15(14)22)28-19-12(4-3-7-23-19)16-18-20(26-9-24-16)27-10-25-18/h3-7,9-11,29H,8H2,1-2H3,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165856

(CHEMBL3799342)Show SMILES CC(C)(C)CS(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C21H21F2N7O2S/c1-21(2,3)9-33(31,32)30-14-7-6-13(22)17(15(14)23)29-19-12(5-4-8-24-19)16-18-20(27-10-25-16)28-11-26-18/h4-8,10-11,30H,9H2,1-3H3,(H,24,29)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197676

(US9216981, 4)Show SMILES Fc1ccc(NS(=O)(=O)c2cccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H13F2N7O2S2/c21-12-5-6-13(29-33(30,31)14-4-2-8-32-14)15(22)17(12)28-19-11(3-1-7-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-10,29H,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
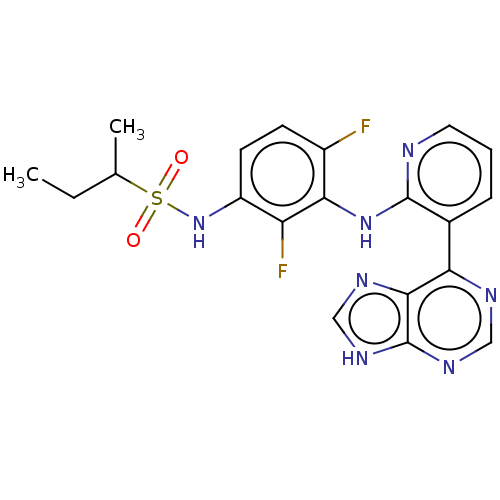
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165858
(CHEMBL3800227)Show SMILES CCC(C)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C20H19F2N7O2S/c1-3-11(2)32(30,31)29-14-7-6-13(21)17(15(14)22)28-19-12(5-4-8-23-19)16-18-20(26-9-24-16)27-10-25-18/h4-11,29H,3H2,1-2H3,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197706

(US9216981, 35 | US9550781, 15)Show SMILES Fc1ccc(NS(=O)(=O)c2ccccc2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C22H15F2N7O2S/c23-15-8-9-16(31-34(32,33)13-5-2-1-3-6-13)17(24)19(15)30-21-14(7-4-10-25-21)18-20-22(28-11-26-18)29-12-27-20/h1-12,31H,(H,25,30)(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
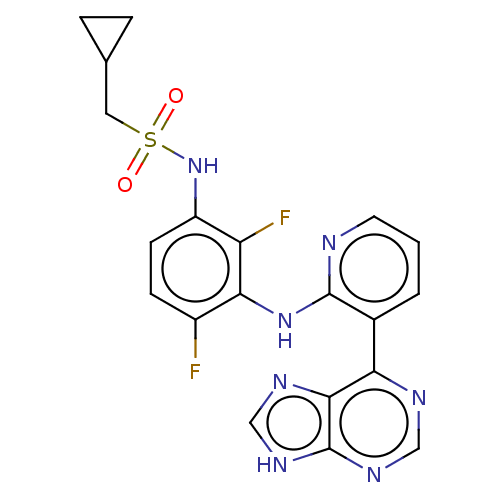
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165875
(CHEMBL3798570)Show SMILES Fc1ccc(NS(=O)(=O)CC2CC2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H17F2N7O2S/c21-13-5-6-14(29-32(30,31)8-11-3-4-11)15(22)17(13)28-19-12(2-1-7-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-2,5-7,9-11,29H,3-4,8H2,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
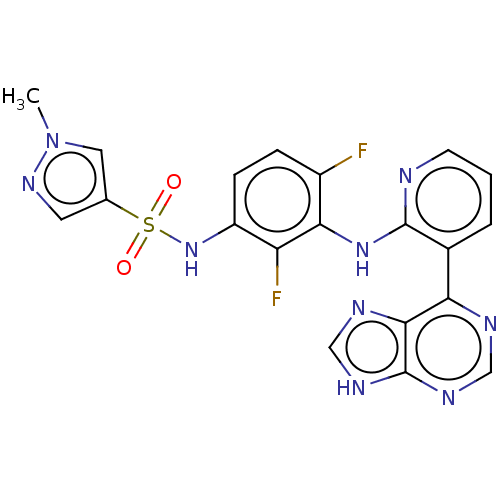
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197714
(US9216981, 43)Show SMILES Cn1cc(cn1)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C20H15F2N9O2S/c1-31-8-11(7-28-31)34(32,33)30-14-5-4-13(21)17(15(14)22)29-19-12(3-2-6-23-19)16-18-20(26-9-24-16)27-10-25-18/h2-10,30H,1H3,(H,23,29)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165880
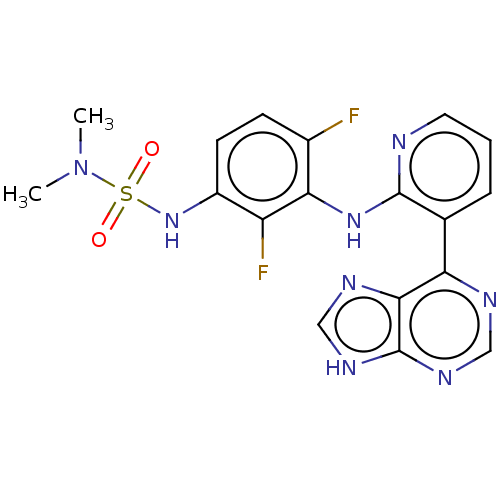
(CHEMBL3798256)Show SMILES CN(C)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C18H16F2N8O2S/c1-28(2)31(29,30)27-12-6-5-11(19)15(13(12)20)26-17-10(4-3-7-21-17)14-16-18(24-8-22-14)25-9-23-16/h3-9,27H,1-2H3,(H,21,26)(H,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM35322
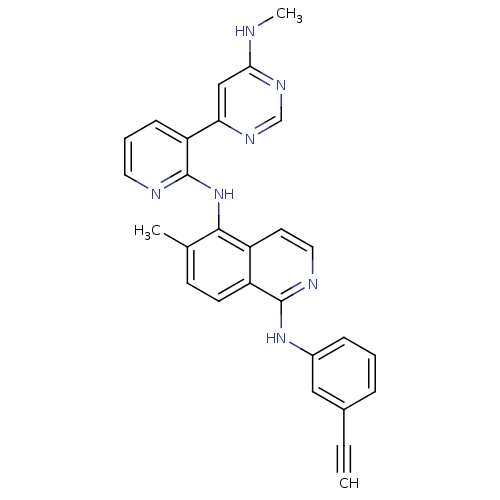
(aminoisoquinoline, 15)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1c(C)ccc2c(Nc3cccc(c3)C#C)nccc12 Show InChI InChI=1S/C28H23N7/c1-4-19-7-5-8-20(15-19)34-27-22-11-10-18(2)26(21(22)12-14-31-27)35-28-23(9-6-13-30-28)24-16-25(29-3)33-17-32-24/h1,5-17H,2-3H3,(H,30,35)(H,31,34)(H,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197677
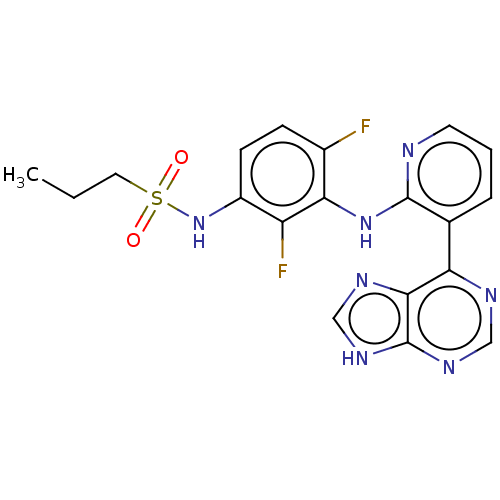
(US9216981, 5 | US9550781, 1)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C19H17F2N7O2S/c1-2-8-31(29,30)28-13-6-5-12(20)16(14(13)21)27-18-11(4-3-7-22-18)15-17-19(25-9-23-15)26-10-24-17/h3-7,9-10,28H,2,8H2,1H3,(H,22,27)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165886
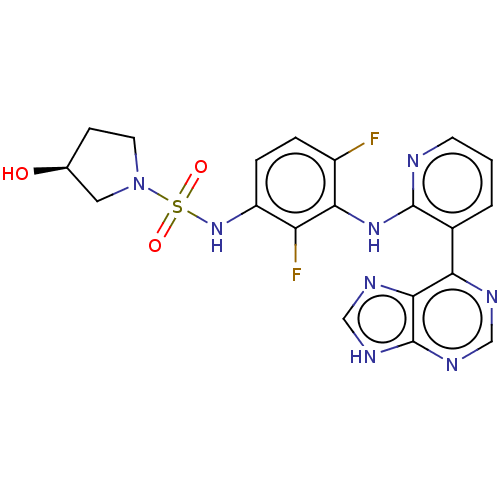
(CHEMBL3800002)Show SMILES O[C@H]1CCN(C1)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F |r| Show InChI InChI=1S/C20H18F2N8O3S/c21-13-3-4-14(29-34(32,33)30-7-5-11(31)8-30)15(22)17(13)28-19-12(2-1-6-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-4,6,9-11,29,31H,5,7-8H2,(H,23,28)(H,24,25,26,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197701

(US9216981, 30)Show SMILES Fc1ccc(NS(=O)(=O)c2cccnc2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C21H14F2N8O2S/c22-14-5-6-15(31-34(32,33)12-3-1-7-24-9-12)16(23)18(14)30-20-13(4-2-8-25-20)17-19-21(28-10-26-17)29-11-27-19/h1-11,31H,(H,25,30)(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data