

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50301066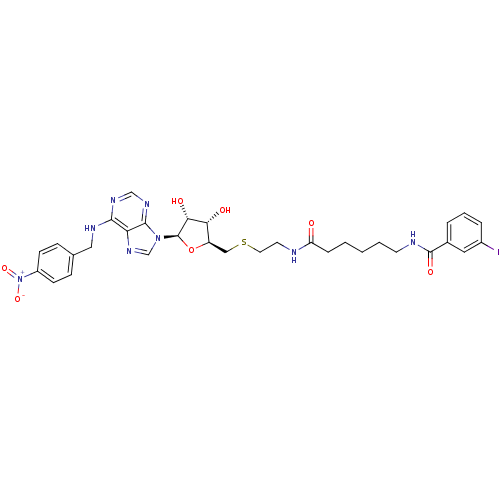 (CHEMBL571939 | N-(6-(2-(((2S,3S,4R,5R)-3,4-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ulm University Curated by ChEMBL | Assay Description Displacement of SAENTA-fluorescein from human ENT1 in HL60 cells by flow cytometry | Bioorg Med Chem Lett 19: 5151-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.017 BindingDB Entry DOI: 10.7270/Q2QJ7HCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM94503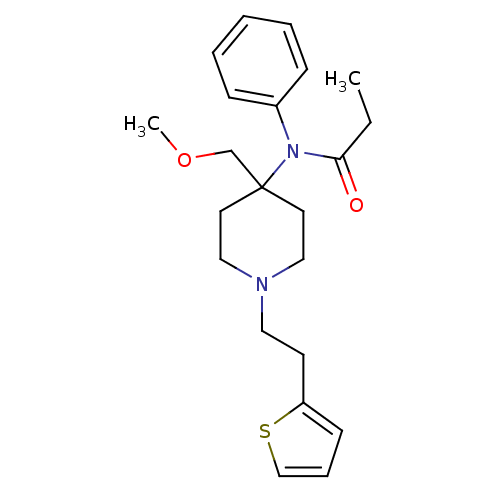 (2-hydroxypropane-1,2,3-tricarboxylic acid;N-[4-(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenates | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Homo sapiens (Human)) | BDBM50301067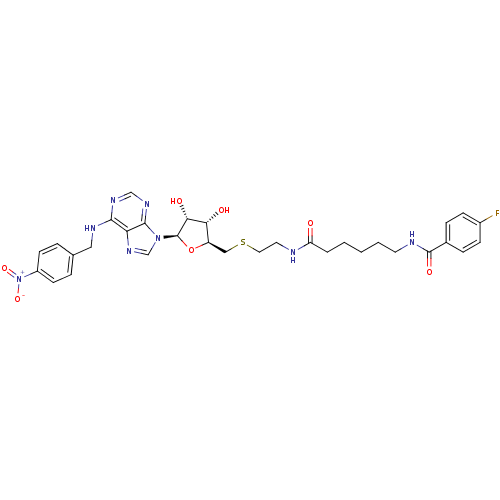 (CHEMBL569223 | N-(6-(2-(((2S,3S,4R,5R)-3,4-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ulm University Curated by ChEMBL | Assay Description Displacement of SAENTA-fluorescein from human ENT1 in HL60 cells by flow cytometry | Bioorg Med Chem Lett 19: 5151-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.017 BindingDB Entry DOI: 10.7270/Q2QJ7HCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212293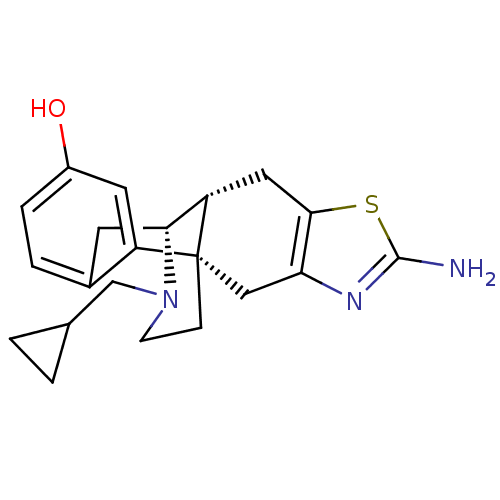 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013943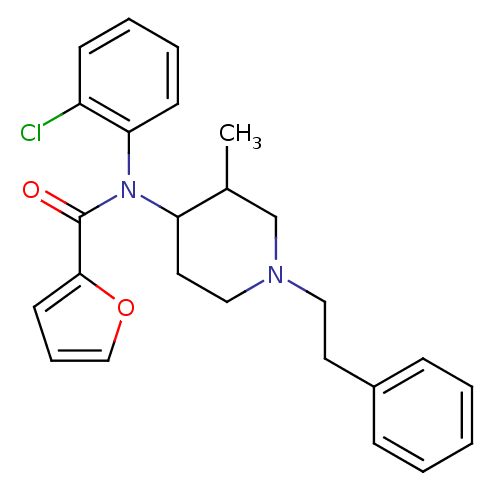 (CHEMBL327270 | Furan-2-carboxylic acid (2-chloro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212297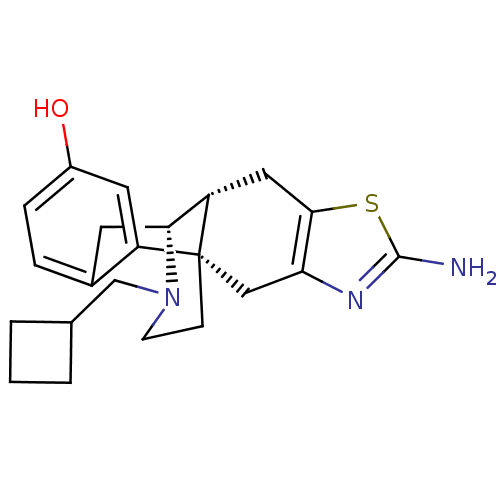 ((1S,9R,10R)-5-amino-20-(cyclobutylmethyl)-6-thia-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013945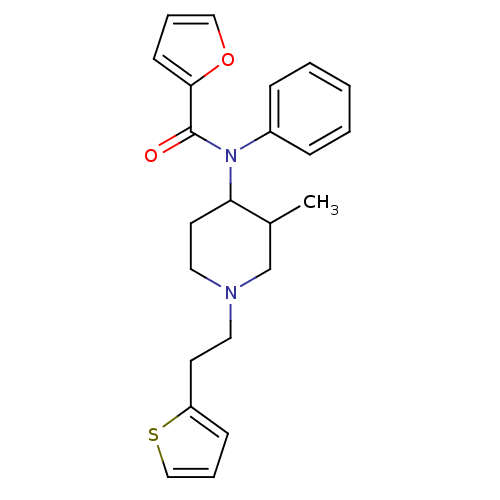 (CHEMBL431047 | Furan-2-carboxylic acid [3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212291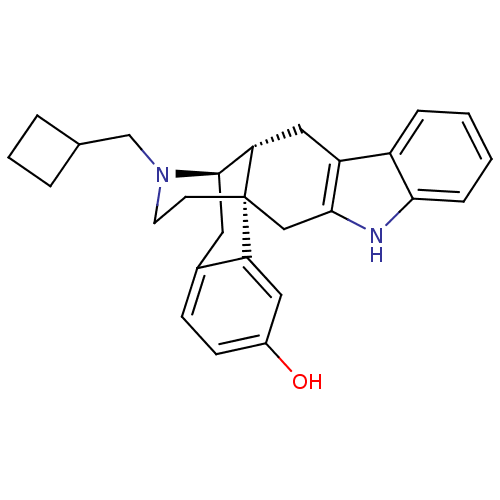 ((1S,13R,14R)-24-(cyclobutylmethyl)-4,24-diazahexac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934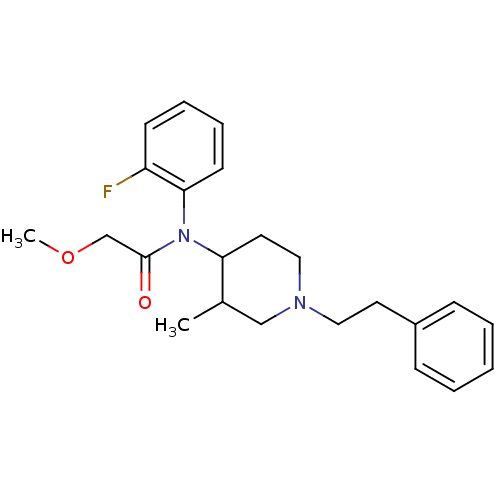 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212296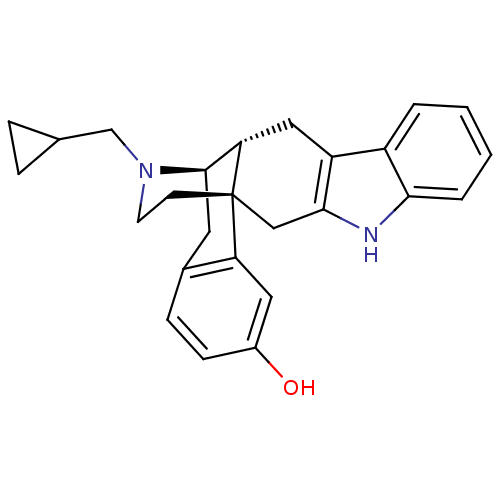 ((1S,13R,14R)-24-(cyclopropylmethyl)-4,24-diazahexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM94503 (2-hydroxypropane-1,2,3-tricarboxylic acid;N-[4-(me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013939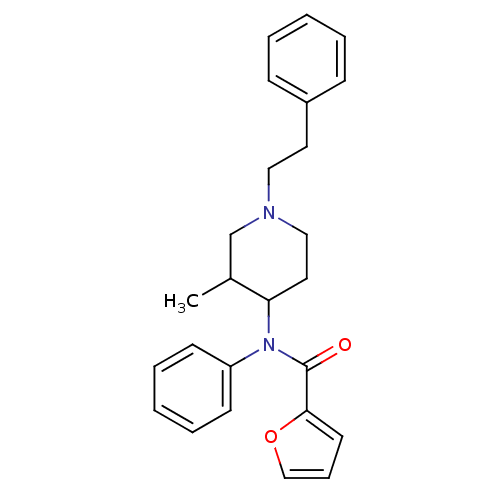 (CHEMBL95247 | Furan-2-carboxylic acid (3-methyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013939 (CHEMBL95247 | Furan-2-carboxylic acid (3-methyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013938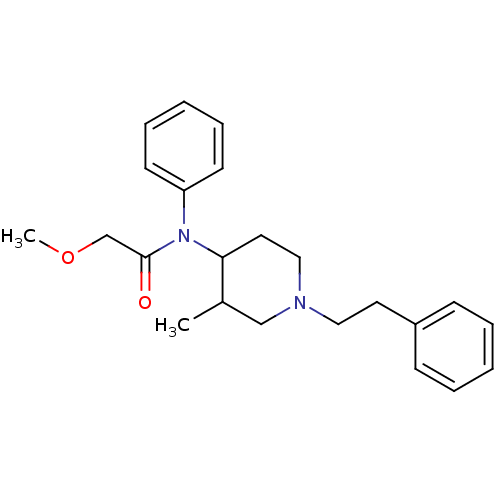 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013938 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212295 ((1S,13R,14R)-24-methyl-4,24-diazahexacyclo[12.7.3....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212297 ((1S,9R,10R)-5-amino-20-(cyclobutylmethyl)-6-thia-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212299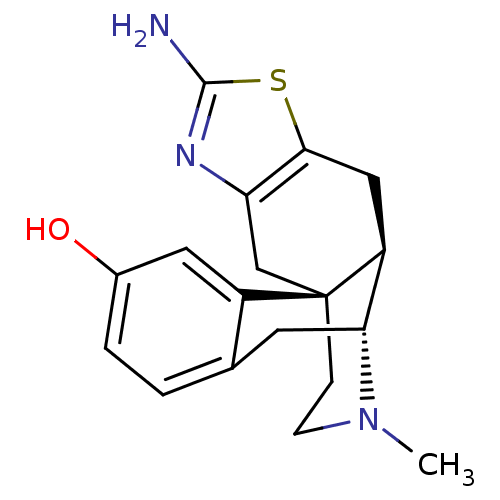 ((1S,9R,10R)-5-amino-20-methyl-6-thia-4,20-diazapen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212296 ((1S,13R,14R)-24-(cyclopropylmethyl)-4,24-diazahexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013949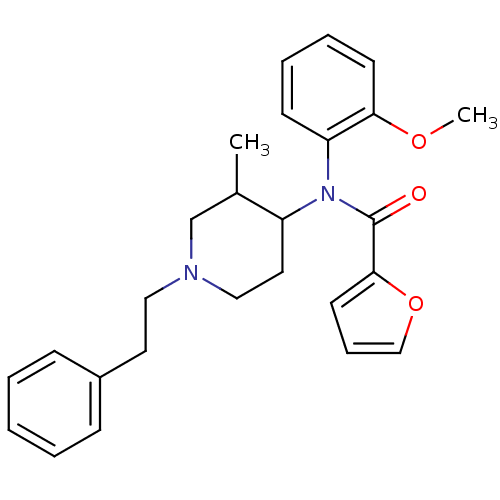 (CHEMBL95248 | Furan-2-carboxylic acid (2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212291 ((1S,13R,14R)-24-(cyclobutylmethyl)-4,24-diazahexac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212296 ((1S,13R,14R)-24-(cyclopropylmethyl)-4,24-diazahexa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013948 (CHEMBL95909 | N-(2-Chloro-phenyl)-2-methoxy-N-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212297 ((1S,9R,10R)-5-amino-20-(cyclobutylmethyl)-6-thia-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212292 ((1S,13R,14R)-24-(cyclopropylmethyl)-19-methoxy-4,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212292 ((1S,13R,14R)-24-(cyclopropylmethyl)-19-methoxy-4,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013947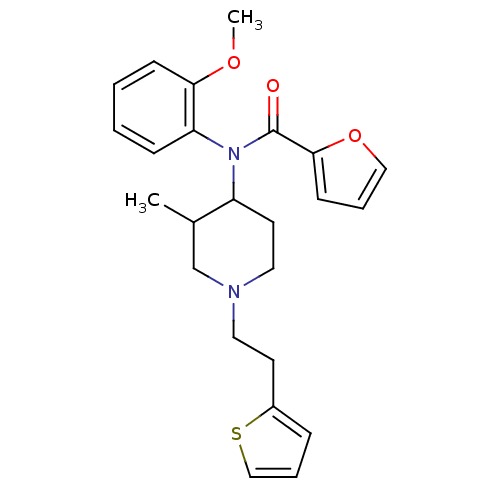 (CHEMBL95786 | Furan-2-carboxylic acid (2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013935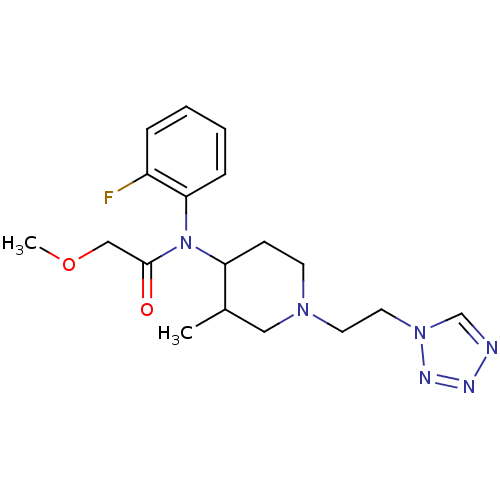 (CHEMBL95390 | N-(2-Fluoro-phenyl)-2-methoxy-N-[3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013940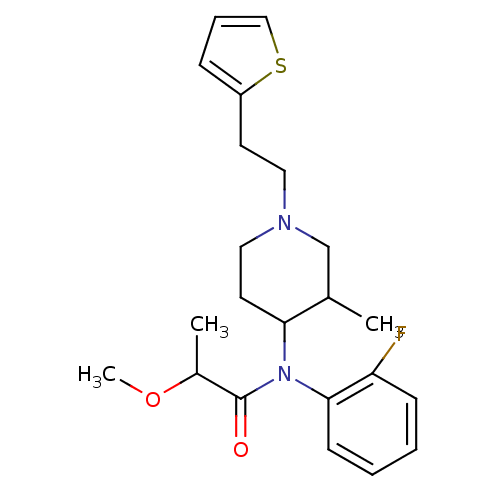 (CHEMBL319343 | N-(2-Fluoro-phenyl)-2-methoxy-N-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenates | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212299 ((1S,9R,10R)-5-amino-20-methyl-6-thia-4,20-diazapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013942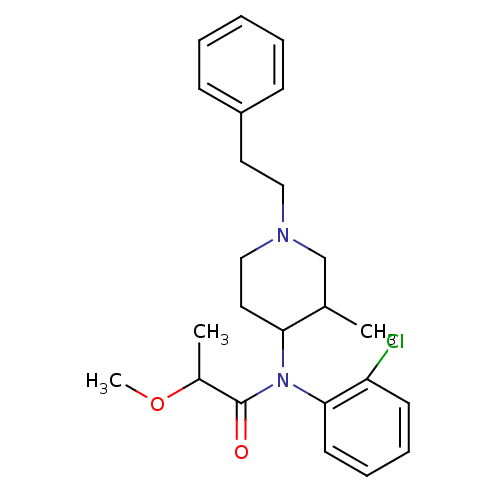 (CHEMBL318895 | N-(2-Chloro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50228269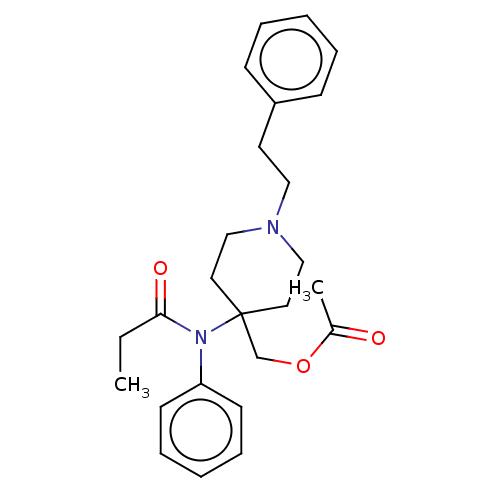 (CHEMBL178035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 sub... | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212291 ((1S,13R,14R)-24-(cyclobutylmethyl)-4,24-diazahexac...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013936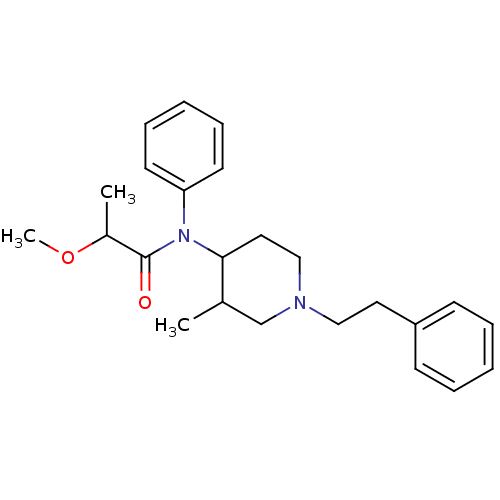 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013936 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212299 ((1S,9R,10R)-5-amino-20-methyl-6-thia-4,20-diazapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212292 ((1S,13R,14R)-24-(cyclopropylmethyl)-19-methoxy-4,2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013944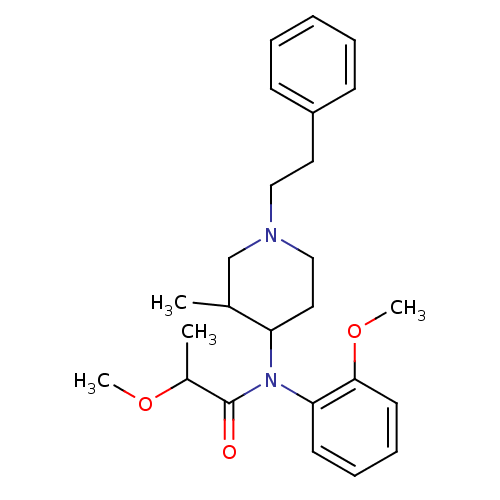 (2-Methoxy-N-(2-methoxy-phenyl)-N-(3-methyl-1-phene...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50228255 (CHEMBL362439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenates | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013941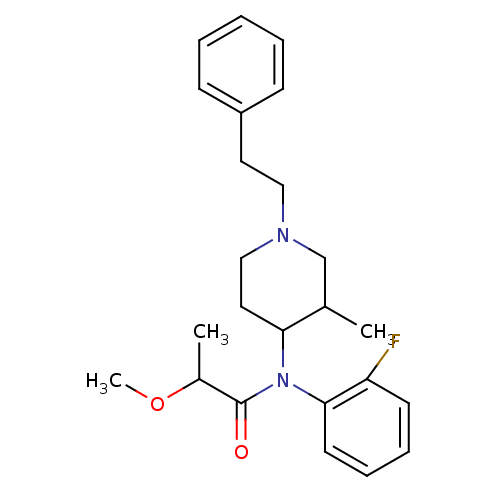 (CHEMBL99596 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM83450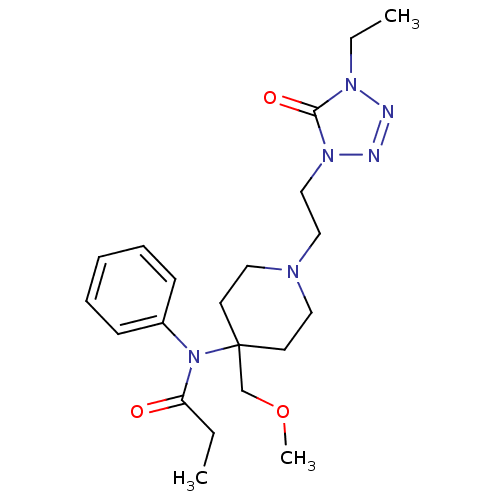 (ALFENTANIL | Alfentanil hydrochloride | MLS0023206...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50228260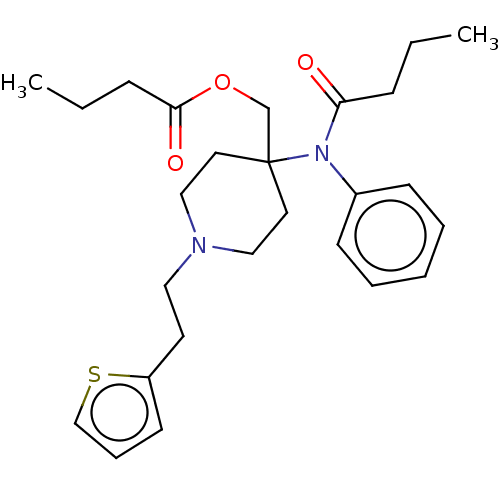 (CHEMBL367286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenates | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM83450 (ALFENTANIL | Alfentanil hydrochloride | MLS0023206...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 sub... | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212298 ((1S,13R,14R)-24-(cyclobutylmethyl)-19-methoxy-4,24...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3623 total ) | Next | Last >> |