 Found 454 hits with Last Name = 'durand' and Initial = 'c'
Found 454 hits with Last Name = 'durand' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50056445
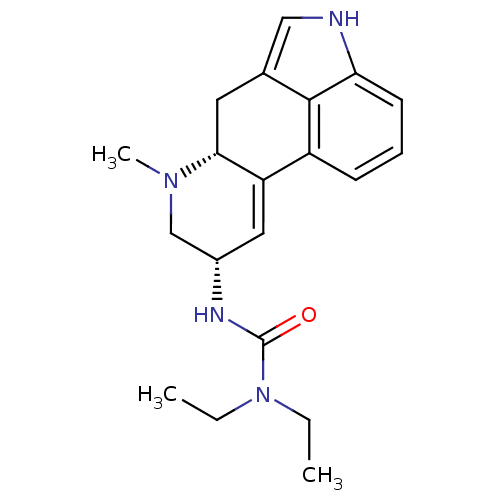
(1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...)Show SMILES CCN(CC)C(=O)N[C@@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |c:23| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50067719

((6aR,9R)-5-Bromo-9-carbamoyl-7-methyl-4,6,6a,7,8,9...)Show SMILES C[N@@H+]1C[C@@H](C=C2[C@H]1Cc1c(Br)[nH]c3cccc2c13)C(N)=O |c:4| Show InChI InChI=1S/C16H16BrN3O/c1-20-7-8(16(18)21)5-10-9-3-2-4-12-14(9)11(6-13(10)20)15(17)19-12/h2-5,8,13,19H,6-7H2,1H3,(H2,18,21)/p+1/t8-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004921
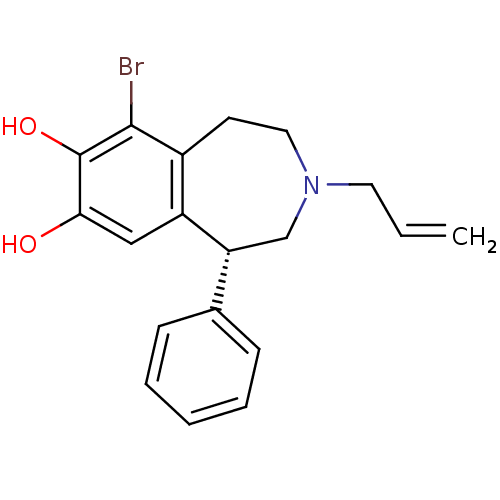
((R)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5...)Show InChI InChI=1S/C19H20BrNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50010289
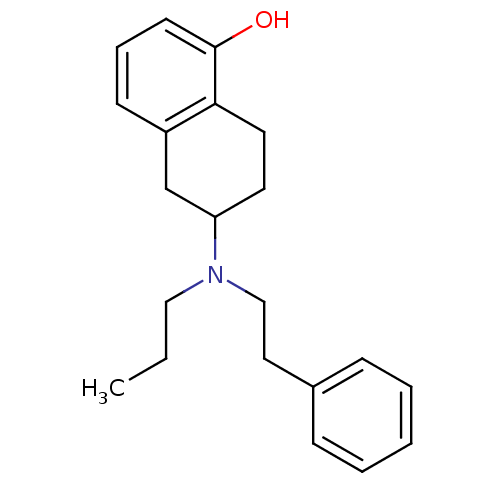
((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C21H27NO/c1-2-14-22(15-13-17-7-4-3-5-8-17)19-11-12-20-18(16-19)9-6-10-21(20)23/h3-10,19,23H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50049048

((R)-3-Allyl-6-chloro-1-phenyl-2,3,4,5-tetrahydro-1...)Show InChI InChI=1S/C19H20ClNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50067726
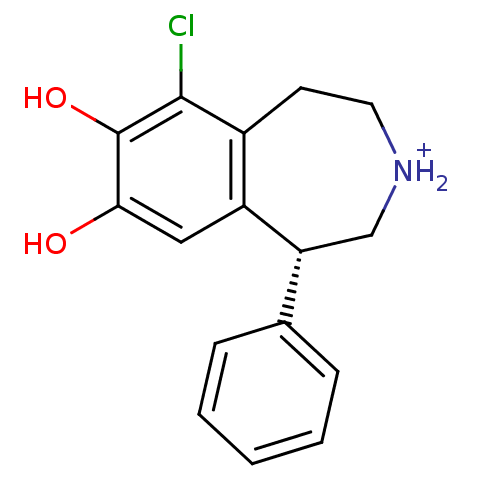
((R)-6-Chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrah...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2/p+1/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50007422
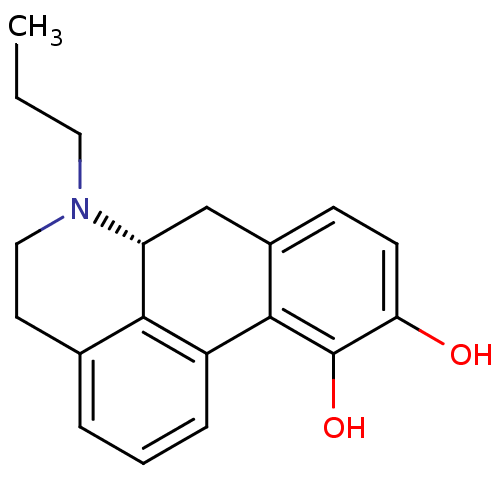
((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...)Show SMILES CCCN1CCc2cccc-3c2[C@H]1Cc1ccc(O)c(O)c-31 |r| Show InChI InChI=1S/C19H21NO2/c1-2-9-20-10-8-12-4-3-5-14-17(12)15(20)11-13-6-7-16(21)19(22)18(13)14/h3-7,15,21-22H,2,8-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50017543
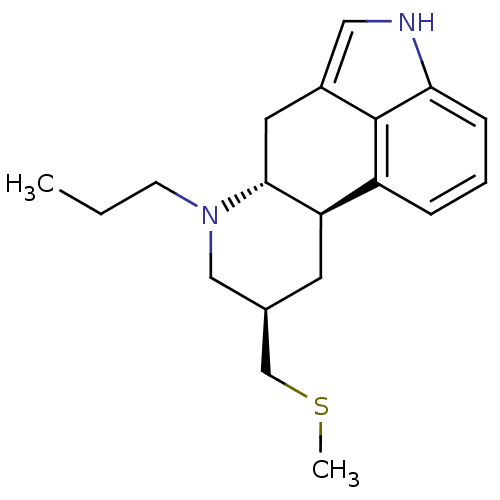
((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...)Show SMILES CCCN1C[C@H](CSC)C[C@H]2[C@H]1Cc1c[nH]c3cccc2c13 |r| Show InChI InChI=1S/C19H26N2S/c1-3-7-21-11-13(12-22-2)8-16-15-5-4-6-17-19(15)14(10-20-17)9-18(16)21/h4-6,10,13,16,18,20H,3,7-9,11-12H2,1-2H3/t13-,16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50056445

(1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...)Show SMILES CCN(CC)C(=O)N[C@@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |c:23| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822
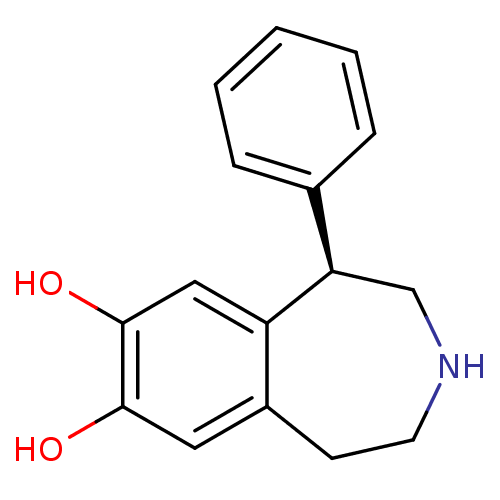
((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50025206
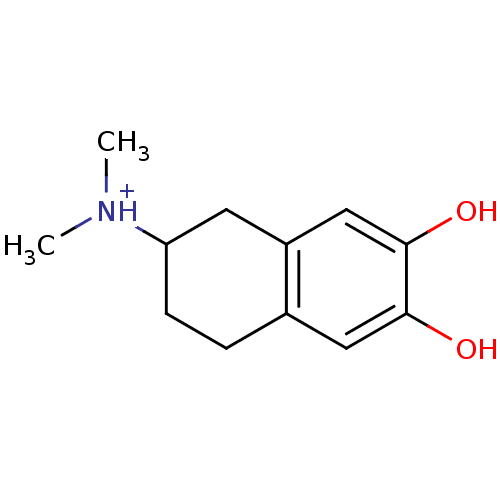
((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C12H17NO2/c1-13(2)10-4-3-8-6-11(14)12(15)7-9(8)5-10/h6-7,10,14-15H,3-5H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50004921

((R)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5...)Show InChI InChI=1S/C19H20BrNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50049048

((R)-3-Allyl-6-chloro-1-phenyl-2,3,4,5-tetrahydro-1...)Show InChI InChI=1S/C19H20ClNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50010289

((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C21H27NO/c1-2-14-22(15-13-17-7-4-3-5-8-17)19-11-12-20-18(16-19)9-6-10-21(20)23/h3-10,19,23H,2,11-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50010686
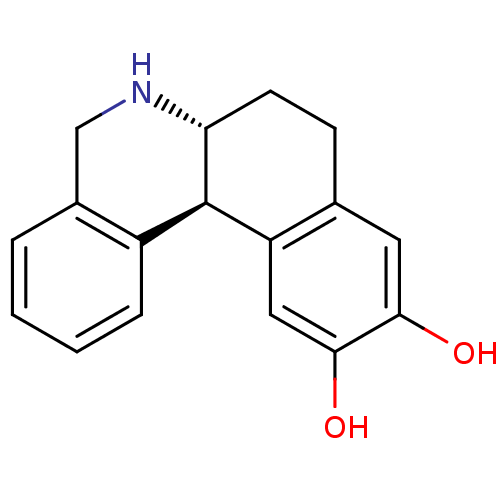
((6aR,12bS)-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydr...)Show InChI InChI=1S/C17H17NO2/c19-15-7-10-5-6-14-17(13(10)8-16(15)20)12-4-2-1-3-11(12)9-18-14/h1-4,7-8,14,17-20H,5-6,9H2/t14-,17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50067719

((6aR,9R)-5-Bromo-9-carbamoyl-7-methyl-4,6,6a,7,8,9...)Show SMILES C[N@@H+]1C[C@@H](C=C2[C@H]1Cc1c(Br)[nH]c3cccc2c13)C(N)=O |c:4| Show InChI InChI=1S/C16H16BrN3O/c1-20-7-8(16(18)21)5-10-9-3-2-4-12-14(9)11(6-13(10)20)15(17)19-12/h2-5,8,13,19H,6-7H2,1H3,(H2,18,21)/p+1/t8-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50007422

((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...)Show SMILES CCCN1CCc2cccc-3c2[C@H]1Cc1ccc(O)c(O)c-31 |r| Show InChI InChI=1S/C19H21NO2/c1-2-9-20-10-8-12-4-3-5-14-17(12)15(20)11-13-6-7-16(21)19(22)18(13)14/h3-7,15,21-22H,2,8-11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50010686

((6aR,12bS)-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydr...)Show InChI InChI=1S/C17H17NO2/c19-15-7-10-5-6-14-17(13(10)8-16(15)20)12-4-2-1-3-11(12)9-18-14/h1-4,7-8,14,17-20H,5-6,9H2/t14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50017543

((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...)Show SMILES CCCN1C[C@H](CSC)C[C@H]2[C@H]1Cc1c[nH]c3cccc2c13 |r| Show InChI InChI=1S/C19H26N2S/c1-3-7-21-11-13(12-22-2)8-16-15-5-4-6-17-19(15)14(10-20-17)9-18(16)21/h4-6,10,13,16,18,20H,3,7-9,11-12H2,1-2H3/t13-,16-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM84637
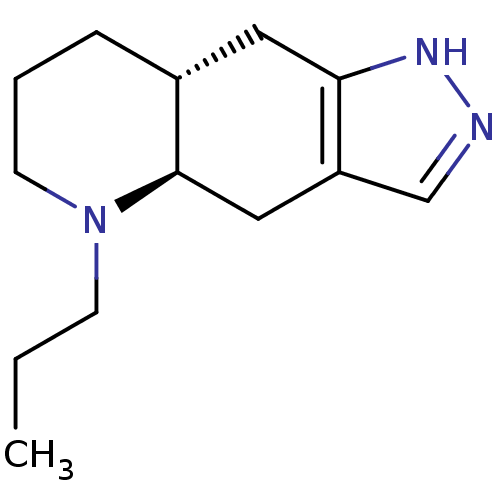
(CAS_85760-74-3 | CHEMBL240773 | NSC_54562 | QUINPI...)Show InChI InChI=1S/C13H21N3/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12/h9-10,13H,2-8H2,1H3,(H,14,15)/t10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50025206

((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C12H17NO2/c1-13(2)10-4-3-8-6-11(14)12(15)7-9(8)5-10/h6-7,10,14-15H,3-5H2,1-2H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM55121
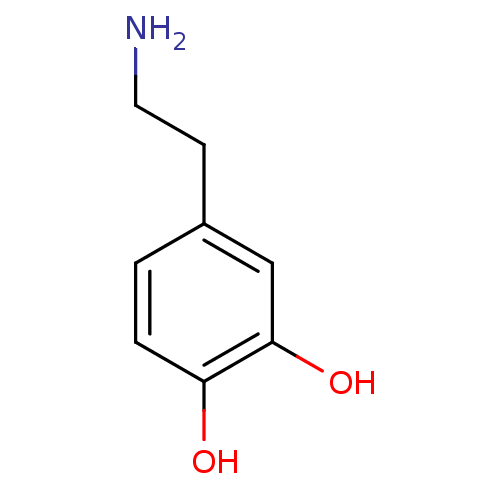
(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50067726

((R)-6-Chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrah...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2/p+1/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822

((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50020502
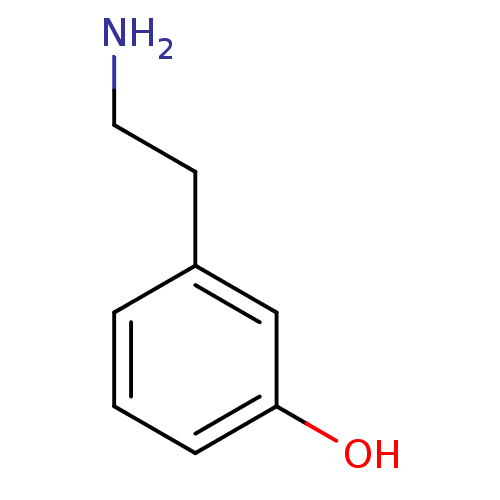
(2-(3-Hydroxy-phenyl)-ethyl-ammonium | 2-(3-hydroxy...)Show InChI InChI=1S/C8H11NO/c9-5-4-7-2-1-3-8(10)6-7/h1-3,6,10H,4-5,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50020502

(2-(3-Hydroxy-phenyl)-ethyl-ammonium | 2-(3-hydroxy...)Show InChI InChI=1S/C8H11NO/c9-5-4-7-2-1-3-8(10)6-7/h1-3,6,10H,4-5,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM84637

(CAS_85760-74-3 | CHEMBL240773 | NSC_54562 | QUINPI...)Show InChI InChI=1S/C13H21N3/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12/h9-10,13H,2-8H2,1H3,(H,14,15)/t10-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542738
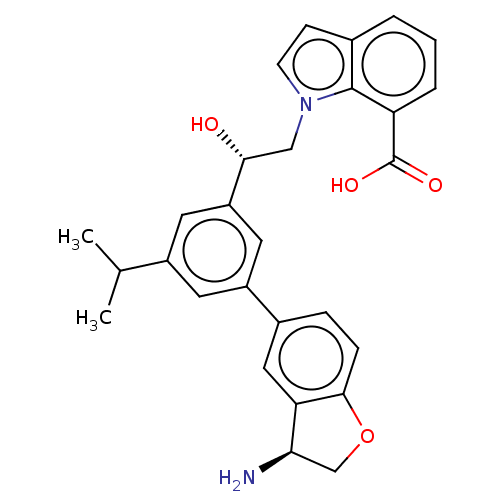
(CHEMBL4637027)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@H](O)Cn1ccc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H28N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-13,16,24-25,31H,14-15,29H2,1-2H3,(H,32,33)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542731
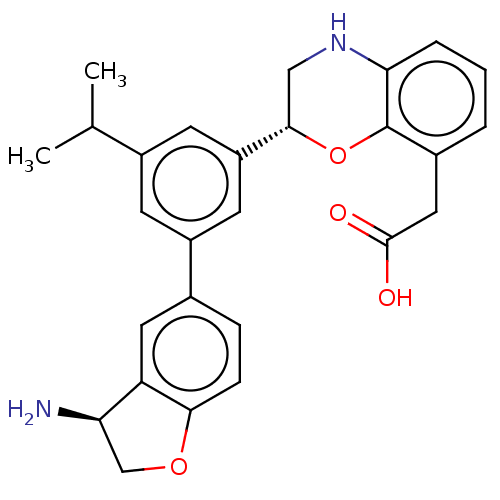
(CHEMBL4642845)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@@H]1CNc2cccc(CC(O)=O)c2O1 |r| Show InChI InChI=1S/C27H28N2O4/c1-15(2)18-8-19(16-6-7-24-21(11-16)22(28)14-32-24)10-20(9-18)25-13-29-23-5-3-4-17(12-26(30)31)27(23)33-25/h3-11,15,22,25,29H,12-14,28H2,1-2H3,(H,30,31)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542741
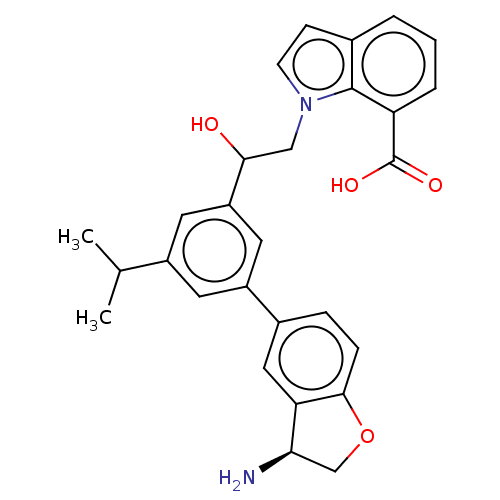
(CHEMBL4647950)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(O)Cn1ccc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H28N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-13,16,24-25,31H,14-15,29H2,1-2H3,(H,32,33)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542731

(CHEMBL4642845)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@@H]1CNc2cccc(CC(O)=O)c2O1 |r| Show InChI InChI=1S/C27H28N2O4/c1-15(2)18-8-19(16-6-7-24-21(11-16)22(28)14-32-24)10-20(9-18)25-13-29-23-5-3-4-17(12-26(30)31)27(23)33-25/h3-11,15,22,25,29H,12-14,28H2,1-2H3,(H,30,31)/t22-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542738

(CHEMBL4637027)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@H](O)Cn1ccc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H28N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-13,16,24-25,31H,14-15,29H2,1-2H3,(H,32,33)/t24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542740
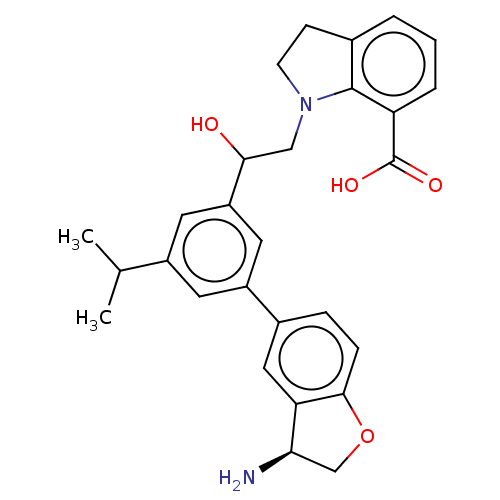
(CHEMBL4646398)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(O)CN1CCc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H30N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-7,10-13,16,24-25,31H,8-9,14-15,29H2,1-2H3,(H,32,33)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542724
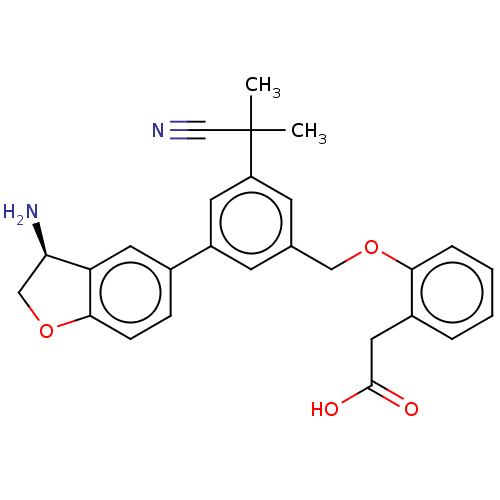
(CHEMBL4636415)Show SMILES CC(C)(C#N)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H26N2O4/c1-27(2,16-28)21-10-17(14-32-24-6-4-3-5-19(24)13-26(30)31)9-20(11-21)18-7-8-25-22(12-18)23(29)15-33-25/h3-12,23H,13-15,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542740

(CHEMBL4646398)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(O)CN1CCc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H30N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-7,10-13,16,24-25,31H,8-9,14-15,29H2,1-2H3,(H,32,33)/t24-,25?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542728
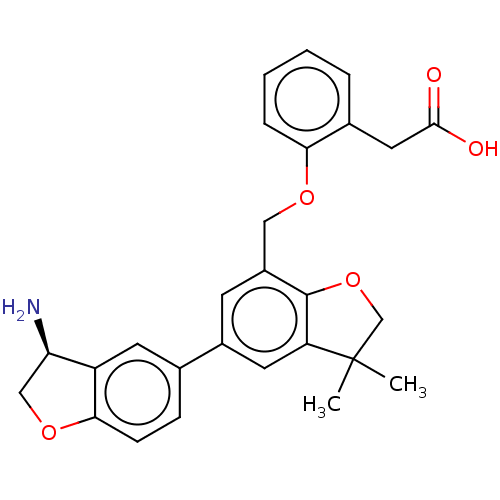
(CHEMBL4635912)Show SMILES CC1(C)COc2c1cc(cc2COc1ccccc1CC(O)=O)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H27NO5/c1-27(2)15-33-26-19(13-31-23-6-4-3-5-17(23)12-25(29)30)9-18(11-21(26)27)16-7-8-24-20(10-16)22(28)14-32-24/h3-11,22H,12-15,28H2,1-2H3,(H,29,30)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542733
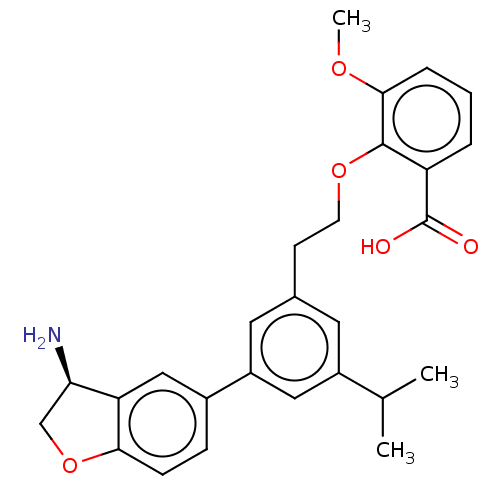
(CHEMBL4646141)Show SMILES COc1cccc(C(O)=O)c1OCCc1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(C)C |r| Show InChI InChI=1S/C27H29NO5/c1-16(2)19-11-17(9-10-32-26-21(27(29)30)5-4-6-25(26)31-3)12-20(13-19)18-7-8-24-22(14-18)23(28)15-33-24/h4-8,11-14,16,23H,9-10,15,28H2,1-3H3,(H,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542723
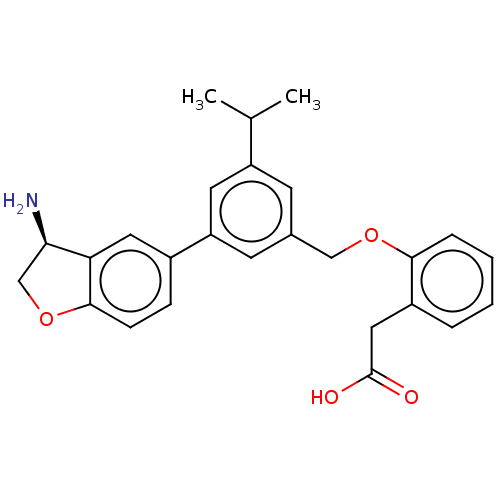
(CHEMBL4643449)Show SMILES CC(C)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO4/c1-16(2)20-9-17(14-30-24-6-4-3-5-19(24)13-26(28)29)10-21(11-20)18-7-8-25-22(12-18)23(27)15-31-25/h3-12,16,23H,13-15,27H2,1-2H3,(H,28,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542723

(CHEMBL4643449)Show SMILES CC(C)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO4/c1-16(2)20-9-17(14-30-24-6-4-3-5-19(24)13-26(28)29)10-21(11-20)18-7-8-25-22(12-18)23(27)15-31-25/h3-12,16,23H,13-15,27H2,1-2H3,(H,28,29)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542730
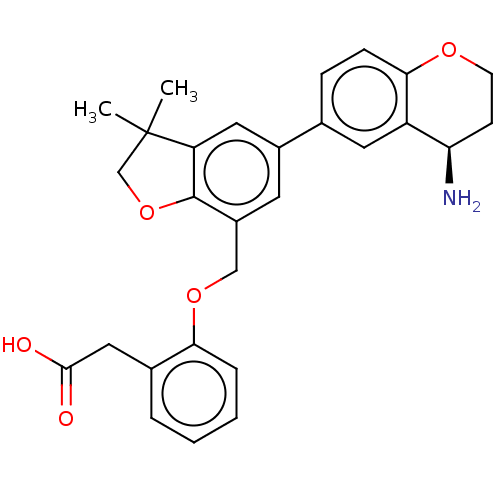
(CHEMBL4647909)Show SMILES CC1(C)COc2c1cc(cc2COc1ccccc1CC(O)=O)-c1ccc2OCC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C28H29NO5/c1-28(2)16-34-27-20(15-33-24-6-4-3-5-18(24)14-26(30)31)11-19(13-22(27)28)17-7-8-25-21(12-17)23(29)9-10-32-25/h3-8,11-13,23H,9-10,14-16,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542725
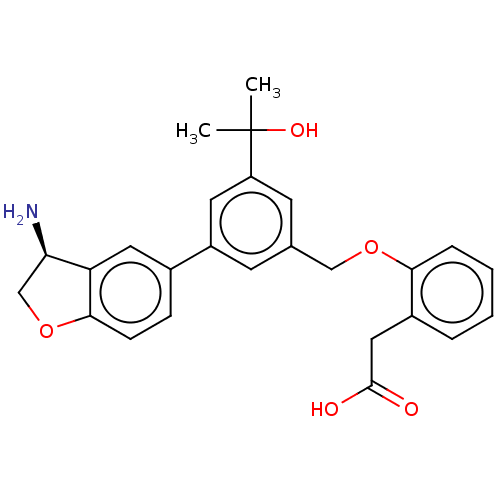
(CHEMBL4637683)Show SMILES CC(C)(O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO5/c1-26(2,30)20-10-16(14-31-23-6-4-3-5-18(23)13-25(28)29)9-19(11-20)17-7-8-24-21(12-17)22(27)15-32-24/h3-12,22,30H,13-15,27H2,1-2H3,(H,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542724

(CHEMBL4636415)Show SMILES CC(C)(C#N)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H26N2O4/c1-27(2,16-28)21-10-17(14-32-24-6-4-3-5-19(24)13-26(30)31)9-20(11-21)18-7-8-25-22(12-18)23(29)15-33-25/h3-12,23H,13-15,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361
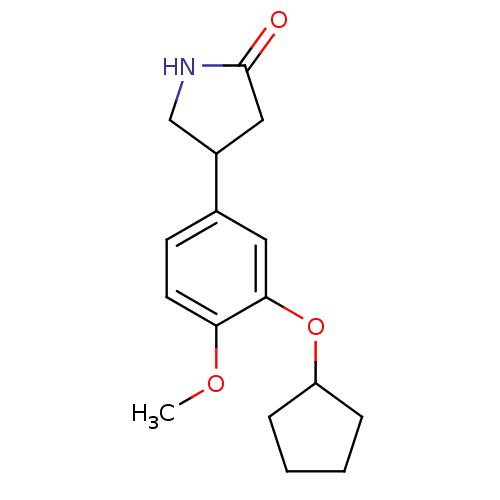
((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]rolipram binding to Wistar rat brain membranes |
J Med Chem 43: 4850-67 (2000)
Article DOI: 10.1021/jm000315p
BindingDB Entry DOI: 10.7270/Q25M68FS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542730

(CHEMBL4647909)Show SMILES CC1(C)COc2c1cc(cc2COc1ccccc1CC(O)=O)-c1ccc2OCC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C28H29NO5/c1-28(2)16-34-27-20(15-33-24-6-4-3-5-18(24)14-26(30)31)11-19(13-22(27)28)17-7-8-25-21(12-17)23(29)9-10-32-25/h3-8,11-13,23H,9-10,14-16,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542725

(CHEMBL4637683)Show SMILES CC(C)(O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO5/c1-26(2,30)20-10-16(14-31-23-6-4-3-5-18(23)13-25(28)29)9-19(11-20)17-7-8-24-21(12-17)22(27)15-32-24/h3-12,22,30H,13-15,27H2,1-2H3,(H,28,29)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data