 Found 3085 hits with Last Name = 'fan' and Initial = 'c'
Found 3085 hits with Last Name = 'fan' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50184069
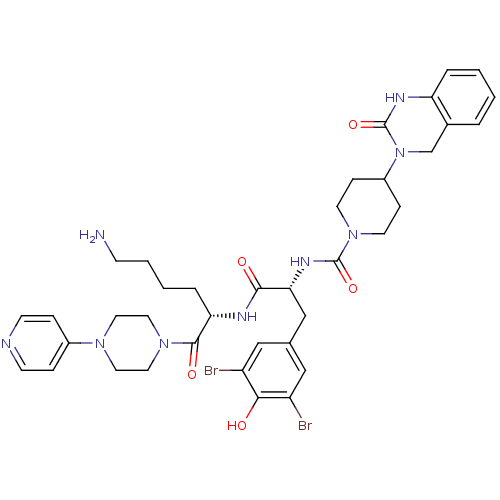
(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385312
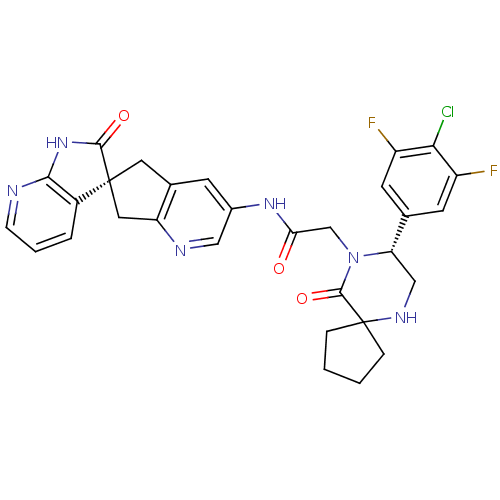
(CHEMBL2035984)Show SMILES Fc1cc(cc(F)c1Cl)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C30H27ClF2N6O3/c31-25-20(32)9-16(10-21(25)33)23-14-36-30(5-1-2-6-30)28(42)39(23)15-24(40)37-18-8-17-11-29(12-22(17)35-13-18)19-4-3-7-34-26(19)38-27(29)41/h3-4,7-10,13,23,36H,1-2,5-6,11-12,14-15H2,(H,37,40)(H,34,38,41)/t23-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385311
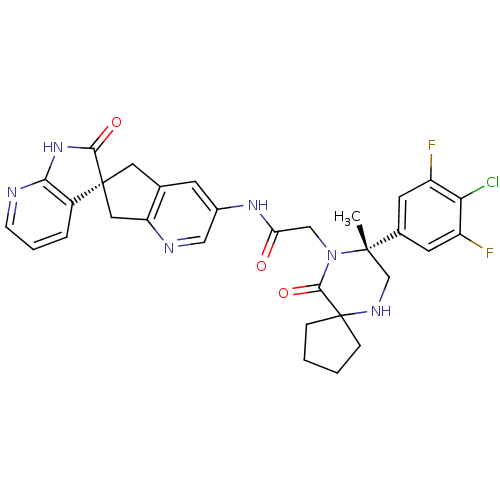
(CHEMBL2035985)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)c(Cl)c(F)c1 |r| Show InChI InChI=1S/C31H29ClF2N6O3/c1-29(18-10-21(33)25(32)22(34)11-18)16-37-31(6-2-3-7-31)28(43)40(29)15-24(41)38-19-9-17-12-30(13-23(17)36-14-19)20-5-4-8-35-26(20)39-27(30)42/h4-5,8-11,14,37H,2-3,6-7,12-13,15-16H2,1H3,(H,38,41)(H,35,39,42)/t29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50356282
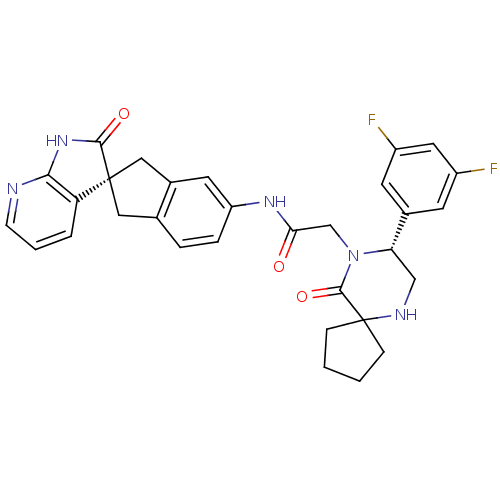
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385313

(CHEMBL2035982)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C30H28F2N6O3/c31-19-8-17(9-20(32)11-19)24-15-35-30(5-1-2-6-30)28(41)38(24)16-25(39)36-21-10-18-12-29(13-23(18)34-14-21)22-4-3-7-33-26(22)37-27(29)40/h3-4,7-11,14,24,35H,1-2,5-6,12-13,15-16H2,(H,36,39)(H,33,37,40)/t24-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50442585
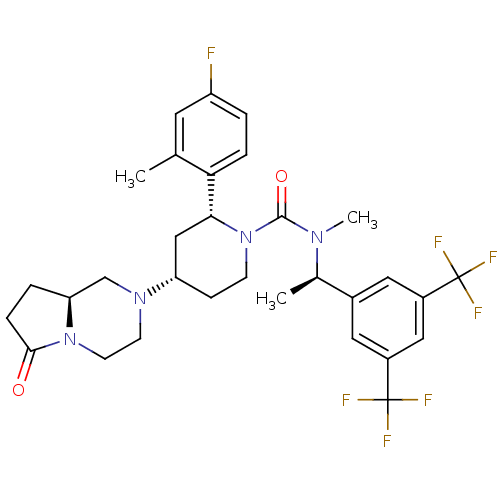
(GW823296X | ORVEPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR205171 from Mongolian gerbil brain NK1 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385314
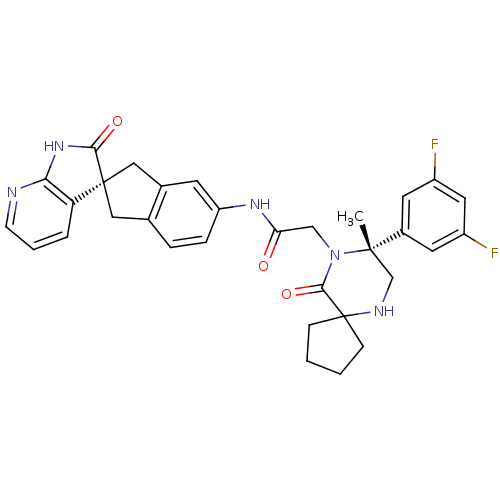
(CHEMBL2035981)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N5O3/c1-30(21-12-22(33)14-23(34)13-21)18-36-32(8-2-3-9-32)29(42)39(30)17-26(40)37-24-7-6-19-15-31(16-20(19)11-24)25-5-4-10-35-27(25)38-28(31)41/h4-7,10-14,36H,2-3,8-9,15-18H2,1H3,(H,37,40)(H,35,38,41)/t30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385309
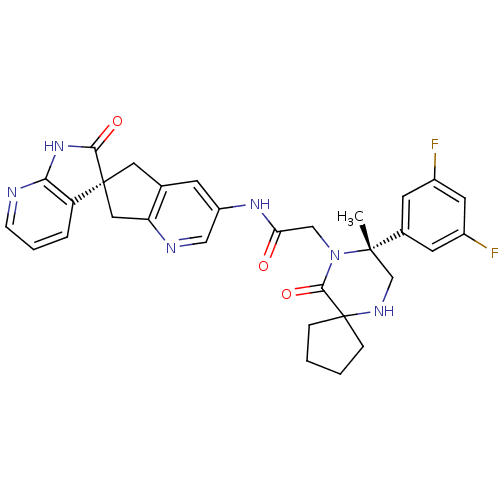
(CHEMBL2035983)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H30F2N6O3/c1-29(19-10-20(32)12-21(33)11-19)17-36-31(6-2-3-7-31)28(42)39(29)16-25(40)37-22-9-18-13-30(14-24(18)35-15-22)23-5-4-8-34-26(23)38-27(30)41/h4-5,8-12,15,36H,2-3,6-7,13-14,16-17H2,1H3,(H,37,40)(H,34,38,41)/t29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485494
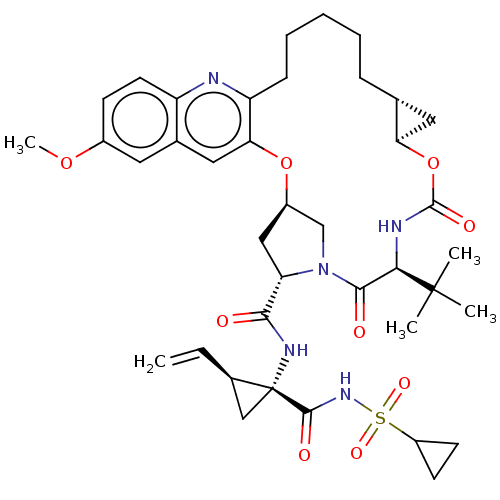
(CHEMBL2063089)Show SMILES [H][C@@]12C[C@@]1([H])OC(=O)N[C@H](C(=O)N1C[C@@]([H])(C[C@H]1C(=O)N[C@@]1(C[C@@]1([H])C=C)C(=O)NS(=O)(=O)C1CC1)Oc1cc3cc(OC)ccc3nc1CCCCC2)C(C)(C)C |r| Show InChI InChI=1S/C39H51N5O9S/c1-6-24-20-39(24,36(47)43-54(49,50)27-13-14-27)42-34(45)30-19-26-21-44(30)35(46)33(38(2,3)4)41-37(48)53-31-17-22(31)10-8-7-9-11-29-32(52-26)18-23-16-25(51-5)12-15-28(23)40-29/h6,12,15-16,18,22,24,26-27,30-31,33H,1,7-11,13-14,17,19-21H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t22-,24-,26-,30+,31-,33-,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50408664
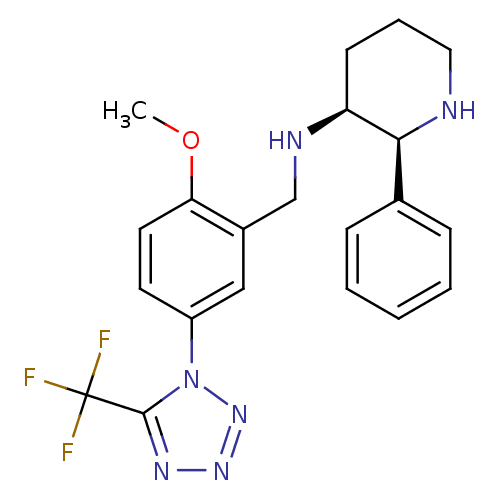
(GR-205171 | VOFOPITANT)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1nnnc1C(F)(F)F Show InChI InChI=1S/C21H23F3N6O/c1-31-18-10-9-16(30-20(21(22,23)24)27-28-29-30)12-15(18)13-26-17-8-5-11-25-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,25-26H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human recombinant NK1 receptor expressed in CHO cells |
J Med Chem 52: 3238-47 (2009)
Article DOI: 10.1021/jm900023b
BindingDB Entry DOI: 10.7270/Q2BP0425 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50385309

(CHEMBL2035983)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H30F2N6O3/c1-29(19-10-20(32)12-21(33)11-19)17-36-31(6-2-3-7-31)28(42)39(29)16-25(40)37-22-9-18-13-30(14-24(18)35-15-22)23-5-4-8-34-26(23)38-27(30)41/h4-5,8-12,15,36H,2-3,6-7,13-14,16-17H2,1H3,(H,37,40)(H,34,38,41)/t29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485491
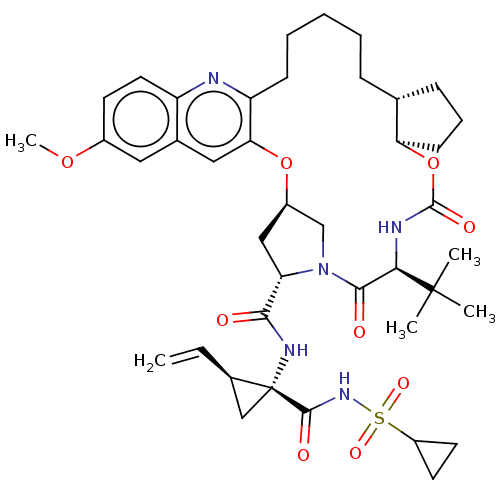
(CHEMBL2063088)Show SMILES [H][C@@]12C[C@H](N(C1)C(=O)[C@@H](NC(=O)O[C@]1([H])CCC[C@@]1([H])CCCCCc1nc3ccc(OC)cc3cc1O2)C(C)(C)C)C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C41H55N5O9S/c1-6-26-22-41(26,38(49)45-56(51,52)29-16-17-29)44-36(47)32-21-28-23-46(32)37(48)35(40(2,3)4)43-39(50)55-33-14-10-12-24(33)11-8-7-9-13-31-34(54-28)20-25-19-27(53-5)15-18-30(25)42-31/h6,15,18-20,24,26,28-29,32-33,35H,1,7-14,16-17,21-23H2,2-5H3,(H,43,50)(H,44,47)(H,45,49)/t24-,26-,28-,32+,33-,35-,41-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442585

(GW823296X | ORVEPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442588
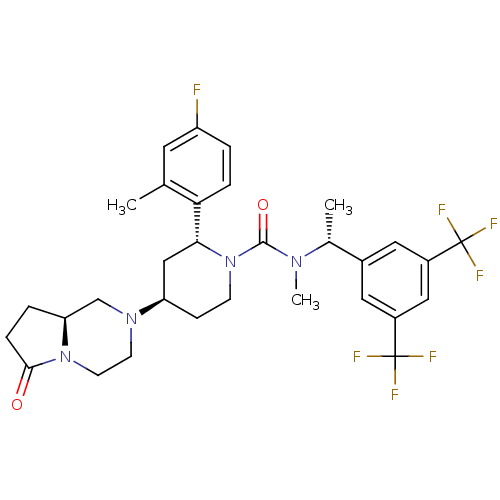
(CHEMBL2441373)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24-,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417977
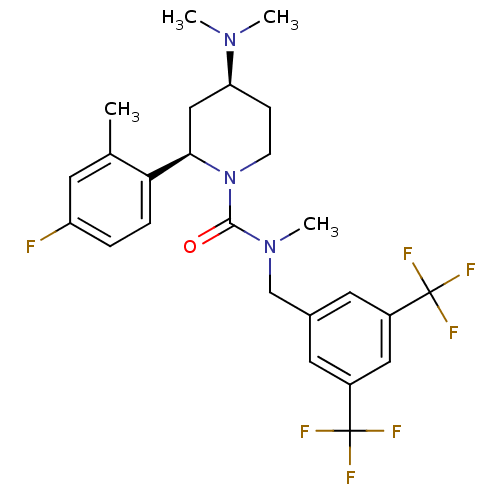
(CHEMBL1672044)Show SMILES CN(C)[C@H]1CCN([C@H](C1)c1ccc(F)cc1C)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H28F7N3O/c1-15-9-19(26)5-6-21(15)22-13-20(33(2)3)7-8-35(22)23(36)34(4)14-16-10-17(24(27,28)29)12-18(11-16)25(30,31)32/h5-6,9-12,20,22H,7-8,13-14H2,1-4H3/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417978
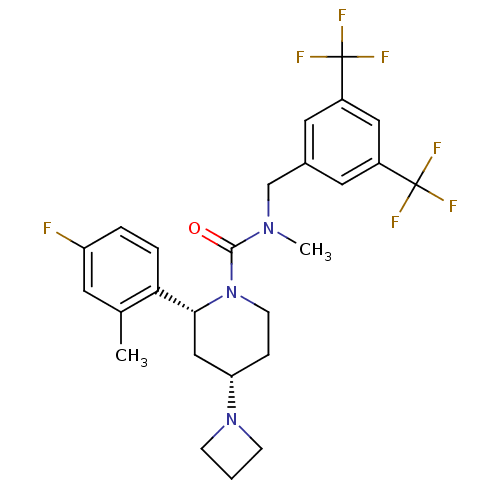
(CHEMBL1672047)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCC1 |r| Show InChI InChI=1S/C26H28F7N3O/c1-16-10-20(27)4-5-22(16)23-14-21(35-7-3-8-35)6-9-36(23)24(37)34(2)15-17-11-18(25(28,29)30)13-19(12-17)26(31,32)33/h4-5,10-13,21,23H,3,6-9,14-15H2,1-2H3/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967
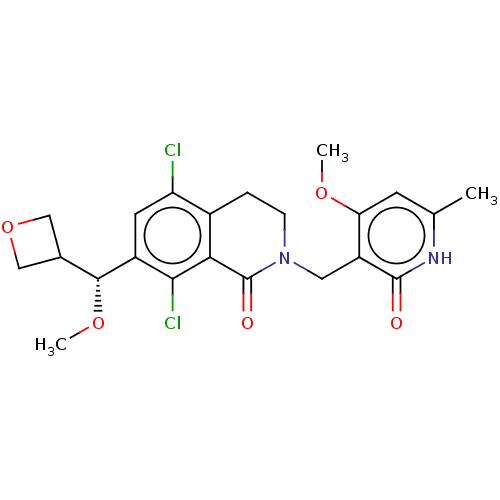
(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Binding affinity to EZH2 (unknown origin) |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417973
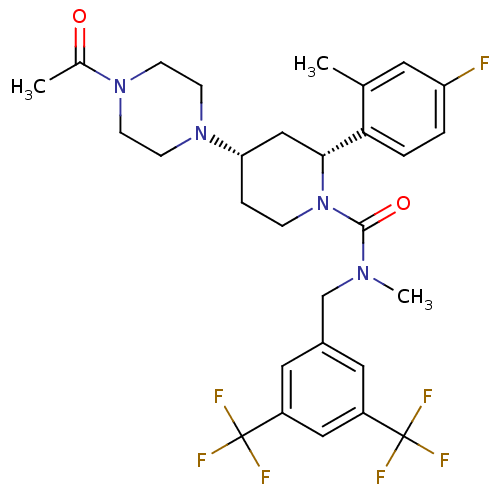
(CHEMBL1672053)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O |r| Show InChI InChI=1S/C29H33F7N4O2/c1-18-12-23(30)4-5-25(18)26-16-24(39-10-8-38(9-11-39)19(2)41)6-7-40(26)27(42)37(3)17-20-13-21(28(31,32)33)15-22(14-20)29(34,35)36/h4-5,12-15,24,26H,6-11,16-17H2,1-3H3/t24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50336575
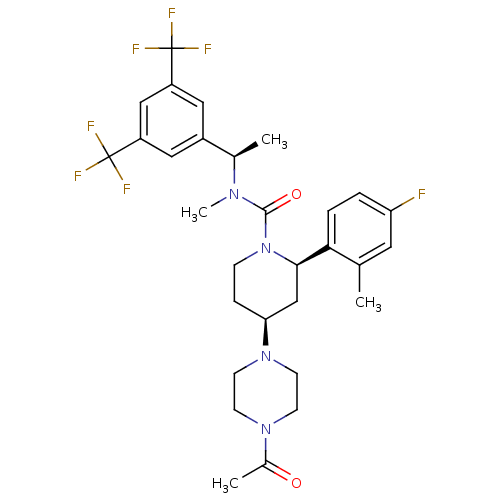
(CHEMBL1672054 | cis-(1'-Acetyl-N-{(1R)-1-[3,5-bis(...)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C30H35F7N4O2/c1-18-13-24(31)5-6-26(18)27-17-25(40-11-9-39(10-12-40)20(3)42)7-8-41(27)28(43)38(4)19(2)21-14-22(29(32,33)34)16-23(15-21)30(35,36)37/h5-6,13-16,19,25,27H,7-12,17H2,1-4H3/t19-,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR205171 from human NK1 receptor in cortex homogenate by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442589
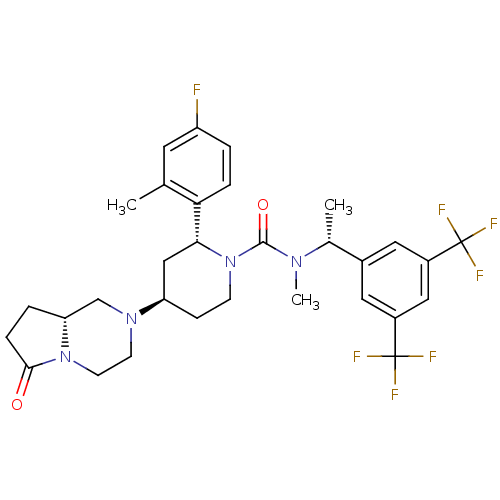
(CHEMBL2441371)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24-,25-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485492
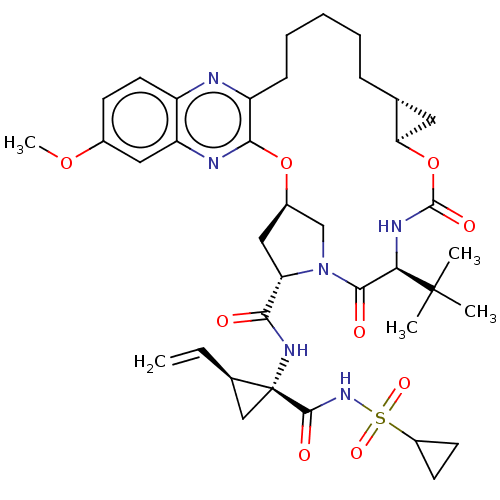
(Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...)Show SMILES [H][C@@]12C[C@@]1([H])OC(=O)N[C@H](C(=O)N1C[C@@]([H])(C[C@H]1C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1)Oc1nc3cc(OC)ccc3nc1CCCCC2)C(C)(C)C |r| Show InChI InChI=1S/C38H50N6O9S/c1-6-22-19-38(22,35(47)43-54(49,50)25-13-14-25)42-32(45)29-18-24-20-44(29)34(46)31(37(2,3)4)41-36(48)53-30-16-21(30)10-8-7-9-11-27-33(52-24)40-28-17-23(51-5)12-15-26(28)39-27/h6,12,15,17,21-22,24-25,29-31H,1,7-11,13-14,16,18-20H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t21-,22-,24-,29+,30-,31-,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417972
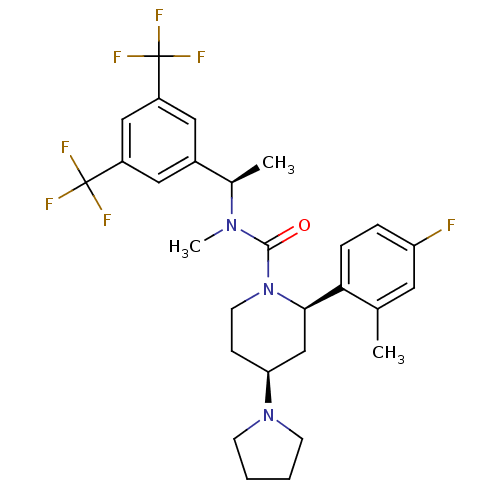
(CHEMBL1672051)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-17-12-22(29)6-7-24(17)25-16-23(37-9-4-5-10-37)8-11-38(25)26(39)36(3)18(2)19-13-20(27(30,31)32)15-21(14-19)28(33,34)35/h6-7,12-15,18,23,25H,4-5,8-11,16H2,1-3H3/t18-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417982
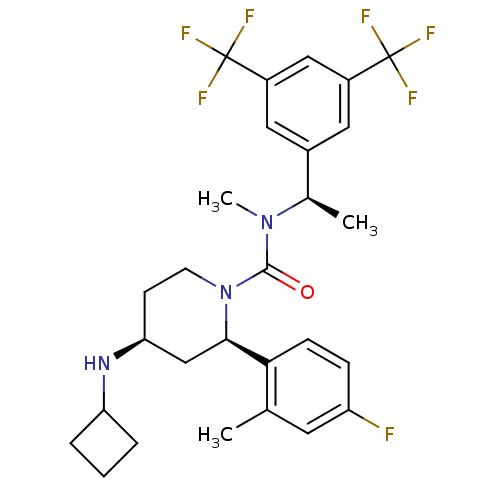
(CHEMBL1672058)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)NC1CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-16-11-21(29)7-8-24(16)25-15-23(36-22-5-4-6-22)9-10-38(25)26(39)37(3)17(2)18-12-19(27(30,31)32)14-20(13-18)28(33,34)35/h7-8,11-14,17,22-23,25,36H,4-6,9-10,15H2,1-3H3/t17-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417970
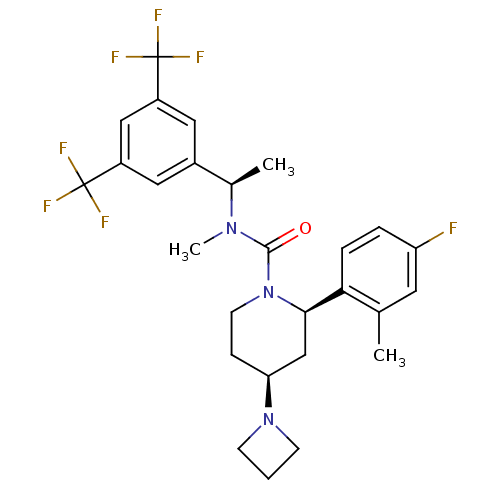
(CHEMBL1672048)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H30F7N3O/c1-16-11-21(28)5-6-23(16)24-15-22(36-8-4-9-36)7-10-37(24)25(38)35(3)17(2)18-12-19(26(29,30)31)14-20(13-18)27(32,33)34/h5-6,11-14,17,22,24H,4,7-10,15H2,1-3H3/t17-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417980
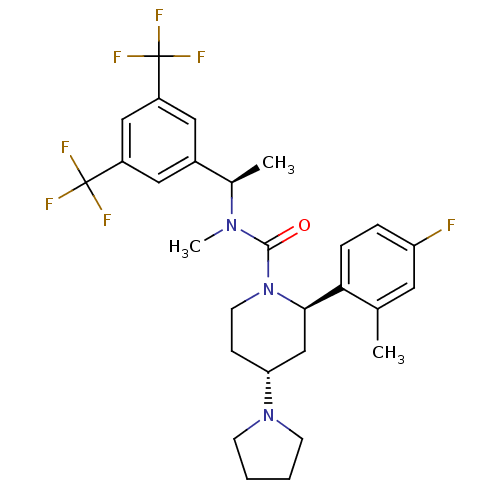
(CHEMBL1672052)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@H](C[C@@H]1c1ccc(F)cc1C)N1CCCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-17-12-22(29)6-7-24(17)25-16-23(37-9-4-5-10-37)8-11-38(25)26(39)36(3)18(2)19-13-20(27(30,31)32)15-21(14-19)28(33,34)35/h6-7,12-15,18,23,25H,4-5,8-11,16H2,1-3H3/t18-,23-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417974
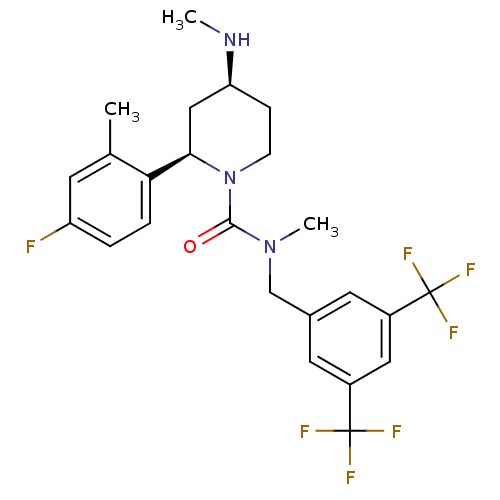
(CHEMBL1672056)Show SMILES CN[C@H]1CCN([C@H](C1)c1ccc(F)cc1C)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H26F7N3O/c1-14-8-18(25)4-5-20(14)21-12-19(32-2)6-7-34(21)22(35)33(3)13-15-9-16(23(26,27)28)11-17(10-15)24(29,30)31/h4-5,8-11,19,21,32H,6-7,12-13H2,1-3H3/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50486093
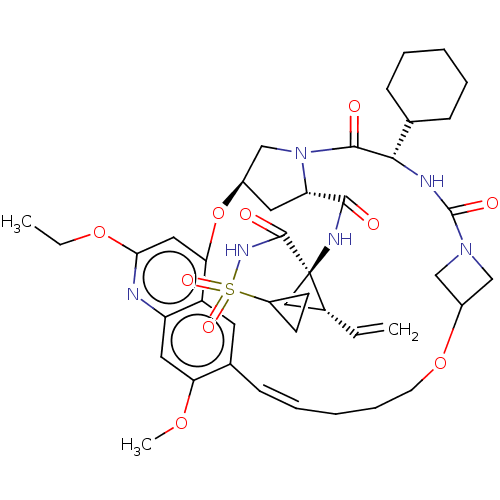
(CHEMBL2203889)Show SMILES CCOc1cc2O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)N3CC(C3)OCCC\C=C\c3cc2c(cc3OC)n1)C1CCCCC1)C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1 |r,t:28| Show InChI InChI=1S/C43H56N6O10S/c1-4-28-22-43(28,41(52)47-60(54,55)31-15-16-31)46-39(50)34-19-29-25-49(34)40(51)38(26-12-8-6-9-13-26)45-42(53)48-23-30(24-48)58-17-11-7-10-14-27-18-32-33(20-35(27)56-3)44-37(57-5-2)21-36(32)59-29/h4,10,14,18,20-21,26,28-31,34,38H,1,5-9,11-13,15-17,19,22-25H2,2-3H3,(H,45,53)(H,46,50)(H,47,52)/b14-10+/t28-,29-,34+,38+,43-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay |
Bioorg Med Chem Lett 22: 7207-13 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.061
BindingDB Entry DOI: 10.7270/Q28D0041 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50442590
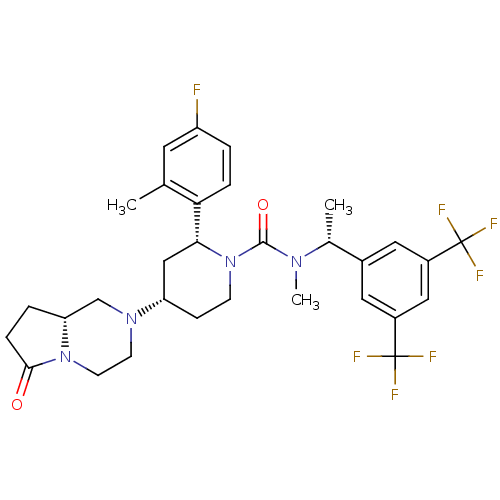
(CHEMBL2441372)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN2[C@H](CCC2=O)C1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 40 mins by scintillation counting analysis |
Bioorg Med Chem 21: 6264-73 (2013)
Article DOI: 10.1016/j.bmc.2013.09.001
BindingDB Entry DOI: 10.7270/Q2D79CWC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417976
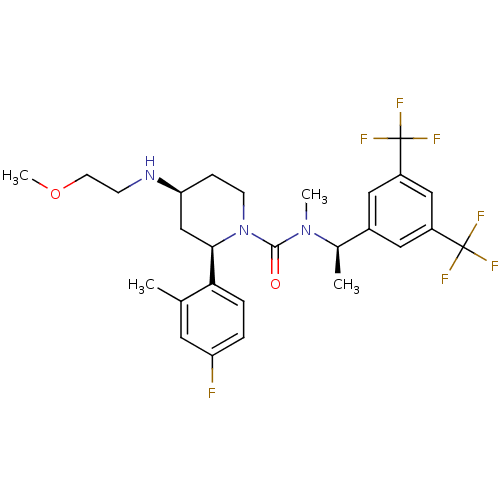
(CHEMBL1672059)Show SMILES COCCN[C@H]1CCN([C@H](C1)c1ccc(F)cc1C)C(=O)N(C)[C@H](C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H32F7N3O2/c1-16-11-21(28)5-6-23(16)24-15-22(35-8-10-39-4)7-9-37(24)25(38)36(3)17(2)18-12-19(26(29,30)31)14-20(13-18)27(32,33)34/h5-6,11-14,17,22,24,35H,7-10,15H2,1-4H3/t17-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413891

(VESTIPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CCNC[C@@H]1c1ccc(F)cc1C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F7N3O/c1-13-8-18(24)4-5-19(13)20-12-31-6-7-33(20)21(34)32(3)14(2)15-9-16(22(25,26)27)11-17(10-15)23(28,29)30/h4-5,8-11,14,20,31H,6-7,12H2,1-3H3/t14-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413891

(VESTIPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CCNC[C@@H]1c1ccc(F)cc1C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F7N3O/c1-13-8-18(24)4-5-19(13)20-12-31-6-7-33(20)21(34)32(3)14(2)15-9-16(22(25,26)27)11-17(10-15)23(28,29)30/h4-5,8-11,14,20,31H,6-7,12H2,1-3H3/t14-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human recombinant NK1 receptor expressed in CHO cells |
J Med Chem 52: 3238-47 (2009)
Article DOI: 10.1021/jm900023b
BindingDB Entry DOI: 10.7270/Q2BP0425 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417968
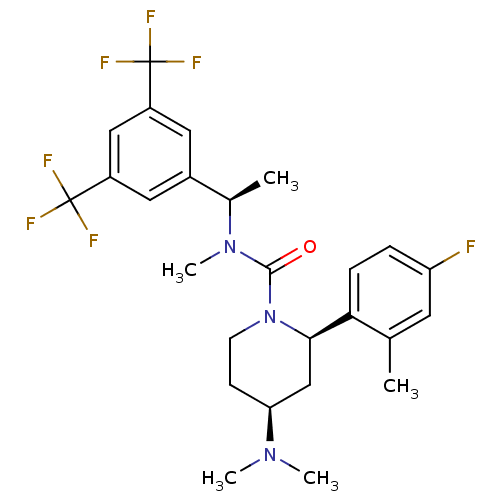
(CHEMBL1672045)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N(C)C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H30F7N3O/c1-15-10-20(27)6-7-22(15)23-14-21(34(3)4)8-9-36(23)24(37)35(5)16(2)17-11-18(25(28,29)30)13-19(12-17)26(31,32)33/h6-7,10-13,16,21,23H,8-9,14H2,1-5H3/t16-,21+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50385310

(CHEMBL2035986)Show SMILES CC[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N6O3/c1-2-32(20-11-21(33)13-22(34)12-20)18-37-31(7-3-4-8-31)29(43)40(32)17-26(41)38-23-10-19-14-30(15-25(19)36-16-23)24-6-5-9-35-27(24)39-28(30)42/h5-6,9-13,16,37H,2-4,7-8,14-15,17-18H2,1H3,(H,38,41)(H,35,39,42)/t30-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human recombinant CALCRL/RAMP1 receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem Lett 22: 3941-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.105
BindingDB Entry DOI: 10.7270/Q2FX7BG1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413891

(VESTIPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CCNC[C@@H]1c1ccc(F)cc1C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F7N3O/c1-13-8-18(24)4-5-19(13)20-12-31-6-7-33(20)21(34)32(3)14(2)15-9-16(22(25,26)27)11-17(10-15)23(28,29)30/h4-5,8-11,14,20,31H,6-7,12H2,1-3H3/t14-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 623-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.078
BindingDB Entry DOI: 10.7270/Q2H1338K |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417979
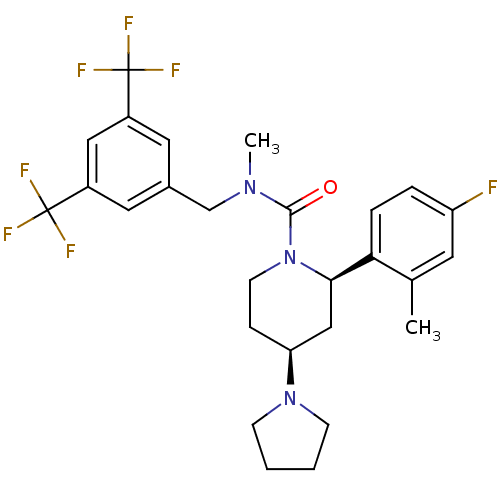
(CHEMBL1672050)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCCC1 |r| Show InChI InChI=1S/C27H30F7N3O/c1-17-11-21(28)5-6-23(17)24-15-22(36-8-3-4-9-36)7-10-37(24)25(38)35(2)16-18-12-19(26(29,30)31)14-20(13-18)27(32,33)34/h5-6,11-14,22,24H,3-4,7-10,15-16H2,1-2H3/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50020310
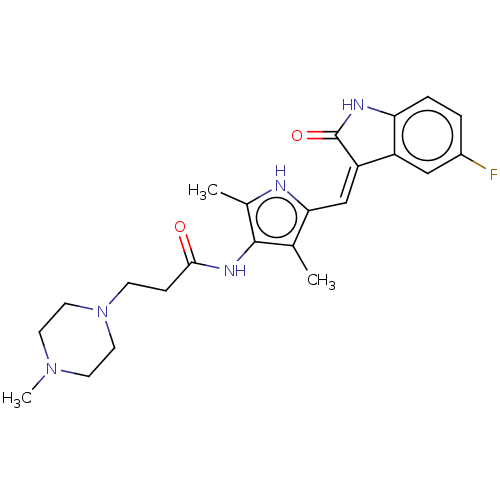
(CHEMBL3288854)Show SMILES CN1CCN(CCC(=O)Nc2c(C)[nH]c(\C=C3/C(=O)Nc4ccc(F)cc34)c2C)CC1 Show InChI InChI=1S/C23H28FN5O2/c1-14-20(13-18-17-12-16(24)4-5-19(17)26-23(18)31)25-15(2)22(14)27-21(30)6-7-29-10-8-28(3)9-11-29/h4-5,12-13,25H,6-11H2,1-3H3,(H,26,31)(H,27,30)/b18-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to VEGFR3 (unknown origin) |
Eur J Med Chem 82: 139-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.051
BindingDB Entry DOI: 10.7270/Q2F1918D |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50020310

(CHEMBL3288854)Show SMILES CN1CCN(CCC(=O)Nc2c(C)[nH]c(\C=C3/C(=O)Nc4ccc(F)cc34)c2C)CC1 Show InChI InChI=1S/C23H28FN5O2/c1-14-20(13-18-17-12-16(24)4-5-19(17)26-23(18)31)25-15(2)22(14)27-21(30)6-7-29-10-8-28(3)9-11-29/h4-5,12-13,25H,6-11H2,1-3H3,(H,26,31)(H,27,30)/b18-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to FLT3 (unknown origin) |
Eur J Med Chem 82: 139-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.051
BindingDB Entry DOI: 10.7270/Q2F1918D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50004767
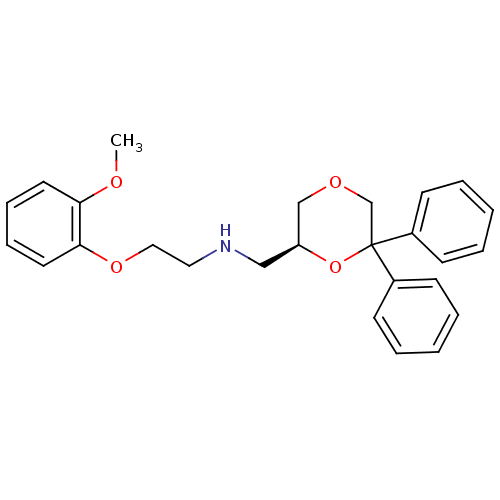
(CHEMBL2312536)Show SMILES COc1ccccc1OCCNC[C@H]1COCC(O1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H29NO4/c1-28-24-14-8-9-15-25(24)30-17-16-27-18-23-19-29-20-26(31-23,21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,27H,16-20H2,1H3/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... |
Eur J Med Chem 125: 233-244 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.026
BindingDB Entry DOI: 10.7270/Q21C20BZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417975
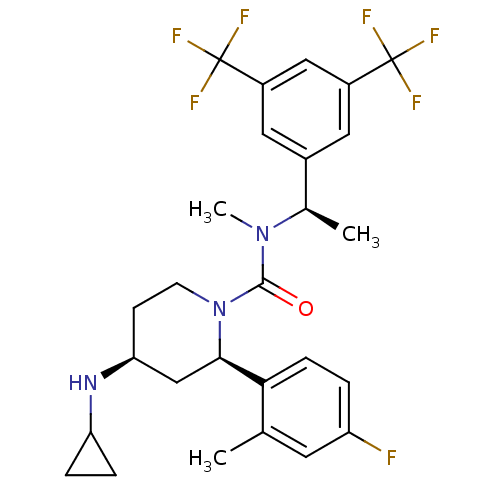
(CHEMBL1672057)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)NC1CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H30F7N3O/c1-15-10-20(28)4-7-23(15)24-14-22(35-21-5-6-21)8-9-37(24)25(38)36(3)16(2)17-11-18(26(29,30)31)13-19(12-17)27(32,33)34/h4,7,10-13,16,21-22,24,35H,5-6,8-9,14H2,1-3H3/t16-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50257034
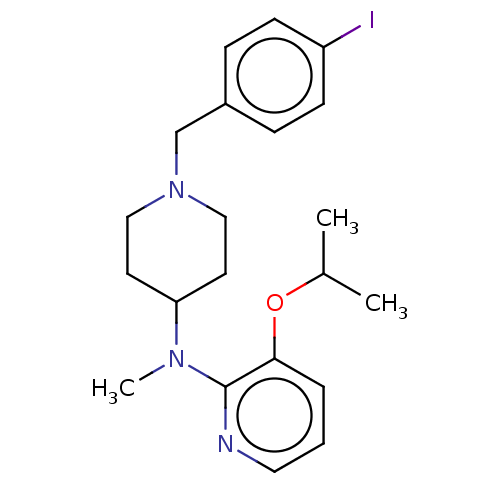
(CHEMBL4096543)Show InChI InChI=1S/C21H28IN3O/c1-16(2)26-20-5-4-12-23-21(20)24(3)19-10-13-25(14-11-19)15-17-6-8-18(22)9-7-17/h4-9,12,16,19H,10-11,13-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708
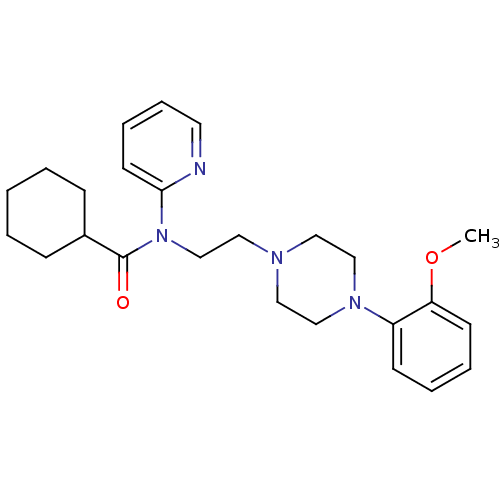
(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrove; di Firenze
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 109-17 (2005)
Article DOI: 10.1124/jpet.105.087809
BindingDB Entry DOI: 10.7270/Q2VD6X2V |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50257034

(CHEMBL4096543)Show InChI InChI=1S/C21H28IN3O/c1-16(2)26-20-5-4-12-23-21(20)24(3)19-10-13-25(14-11-19)15-17-6-8-18(22)9-7-17/h4-9,12,16,19H,10-11,13-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scuola di Scienze del Farmaco e dei Prodotti della Salute , UniversitÓ di Camerino , Via S. Agostino 1 , 62032 Camerino , Italy.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human dopamine D4 receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method |
J Med Chem 61: 3712-3725 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00265
BindingDB Entry DOI: 10.7270/Q20K2C2P |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50336575

(CHEMBL1672054 | cis-(1'-Acetyl-N-{(1R)-1-[3,5-bis(...)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C30H35F7N4O2/c1-18-13-24(31)5-6-26(18)27-17-25(40-11-9-39(10-12-40)20(3)42)7-8-41(27)28(43)38(4)19(2)21-14-22(29(32,33)34)16-23(15-21)30(35,36)37/h5-6,13-16,19,25,27H,7-12,17H2,1-4H3/t19-,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50026917
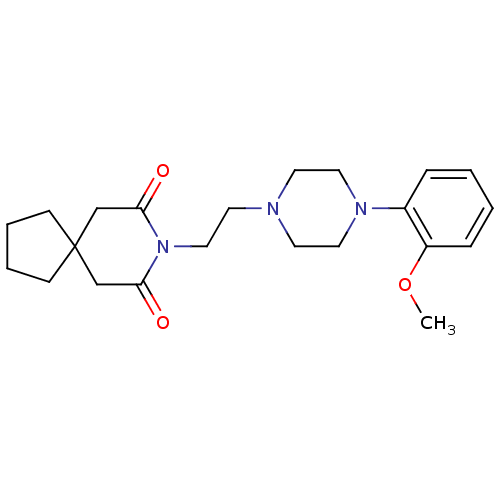
(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... |
Eur J Med Chem 125: 233-244 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.026
BindingDB Entry DOI: 10.7270/Q21C20BZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50004768
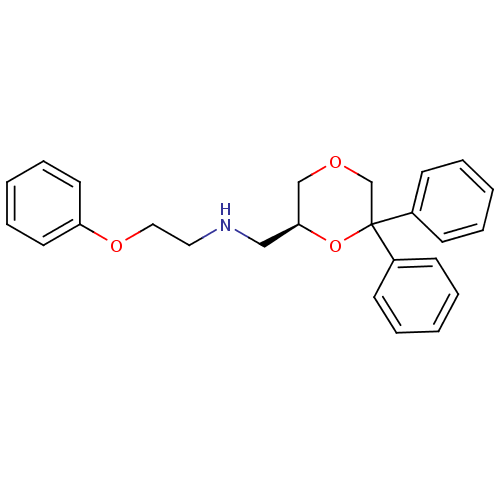
(CHEMBL2312538)Show SMILES C(COc1ccccc1)NC[C@H]1COCC(O1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H27NO3/c1-4-10-21(11-5-1)25(22-12-6-2-7-13-22)20-27-19-24(29-25)18-26-16-17-28-23-14-8-3-9-15-23/h1-15,24,26H,16-20H2/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... |
Eur J Med Chem 125: 233-244 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.026
BindingDB Entry DOI: 10.7270/Q21C20BZ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50486108

(CHEMBL2203884)Show SMILES [H][C@]12CCN(C1)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@@]([H])(C[C@H]1C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1)Oc1cc(OCC)nc3cc(OC)c(cc13)\C=C\CO2 |r,t:64| Show InChI InChI=1S/C42H54N6O10S/c1-4-27-22-42(27,40(51)46-59(53,54)30-13-14-30)45-38(49)33-19-29-24-48(33)39(50)37(25-10-7-6-8-11-25)44-41(52)47-16-15-28(23-47)57-17-9-12-26-18-31-32(20-34(26)55-3)43-36(56-5-2)21-35(31)58-29/h4,9,12,18,20-21,25,27-30,33,37H,1,5-8,10-11,13-17,19,22-24H2,2-3H3,(H,44,52)(H,45,49)(H,46,51)/b12-9+/t27-,28+,29-,33+,37+,42-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay |
Bioorg Med Chem Lett 22: 7207-13 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.061
BindingDB Entry DOI: 10.7270/Q28D0041 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50401953

(CHEMBL2207643)Show InChI InChI=1S/C22H24N2O/c1-25-22-10-6-5-9-21(22)24-15-13-23(14-16-24)17-19-12-11-18-7-3-2-4-8-20(18)19/h2-12H,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413892
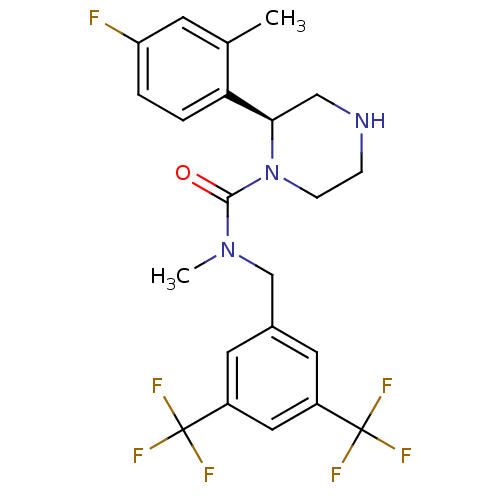
(CHEMBL490926)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CCNC[C@@H]1c1ccc(F)cc1C |r| Show InChI InChI=1S/C22H22F7N3O/c1-13-7-17(23)3-4-18(13)19-11-30-5-6-32(19)20(33)31(2)12-14-8-15(21(24,25)26)10-16(9-14)22(27,28)29/h3-4,7-10,19,30H,5-6,11-12H2,1-2H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human recombinant NK1 receptor expressed in CHO cells |
J Med Chem 52: 3238-47 (2009)
Article DOI: 10.1021/jm900023b
BindingDB Entry DOI: 10.7270/Q2BP0425 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM85093
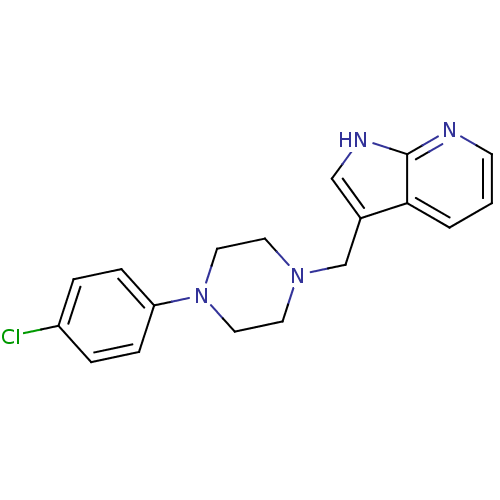
(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417981
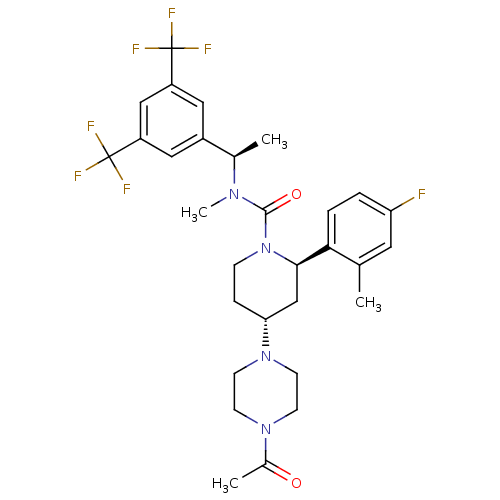
(CHEMBL1672055)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C30H35F7N4O2/c1-18-13-24(31)5-6-26(18)27-17-25(40-11-9-39(10-12-40)20(3)42)7-8-41(27)28(43)38(4)19(2)21-14-22(29(32,33)34)16-23(15-21)30(35,36)37/h5-6,13-16,19,25,27H,7-12,17H2,1-4H3/t19-,25-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data