

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha2-beta2 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha3-beta2 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50095611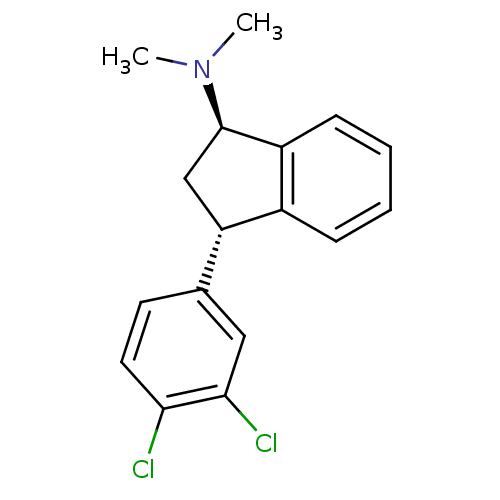 (CHEMBL356750 | [3-(3,4-Dichloro-phenyl)-indan-1-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI | J Med Chem 43: 4981-92 (2001) BindingDB Entry DOI: 10.7270/Q2SX6DXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50095611 (CHEMBL356750 | [3-(3,4-Dichloro-phenyl)-indan-1-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI | J Med Chem 43: 4981-92 (2001) BindingDB Entry DOI: 10.7270/Q2SX6DXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha2-beta4 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121640 (2-[2-Dimethylamino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Binding affinity of compound towards the Opioid receptor delta 1 in rat synaptosomal membrane was determined using [3H]-DPDPE as radioligand | J Med Chem 45: 5506-13 (2002) BindingDB Entry DOI: 10.7270/Q2DN44DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha4-beta4 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121639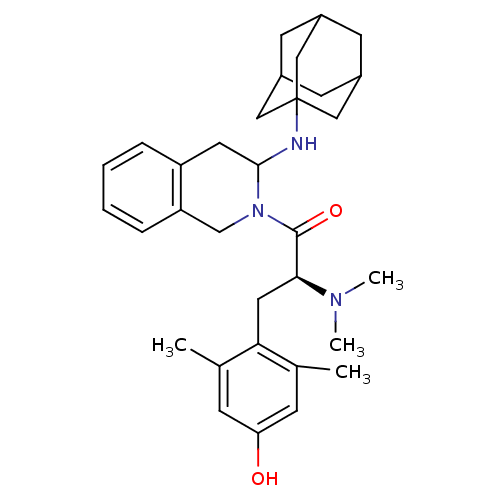 (1-[3-(Adamantan-1-ylamino)-3,4-dihydro-1H-isoquino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Binding affinity of compound towards the Opioid receptor delta 1 in rat synaptosomal membrane was determined using [3H]-DPDPE as radioligand | J Med Chem 45: 5506-13 (2002) BindingDB Entry DOI: 10.7270/Q2DN44DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121638 (1-[3-(Adamantan-1-ylamino)-3,4-dihydro-1H-isoquino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Binding affinity of compound towards the Opioid receptor delta 1 in rat synaptosomal membrane was determined using [3H]-DPDPE as radioligand | J Med Chem 45: 5506-13 (2002) BindingDB Entry DOI: 10.7270/Q2DN44DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001054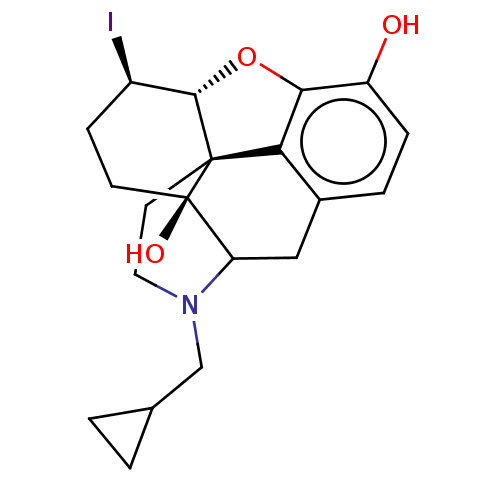 (4-cyclopropylmethyl-14-iodo-(14R,17S)-12-oxa-4-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001053 (14-bromo-4-cyclopropylmethyl-(13R,14R,17S)-12-oxa-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50369770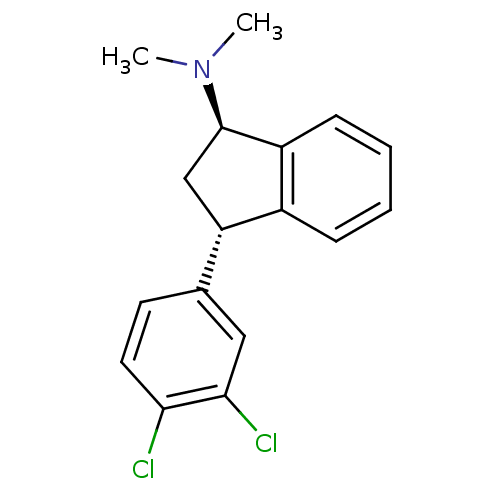 (CHEMBL1788140) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharm-Eco Laboratories Curated by ChEMBL | Assay Description Binding affinity against cloned serotonin human transporter using 40-80 PM [125I]RTI | J Med Chem 43: 4981-92 (2001) BindingDB Entry DOI: 10.7270/Q2SX6DXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50067447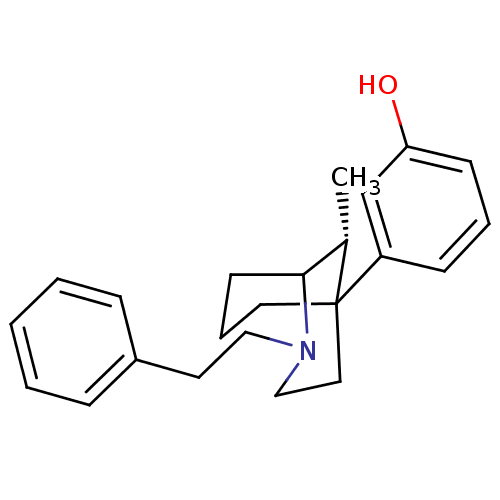 (3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by DAMGO (Opioid receptor mu 1) | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236918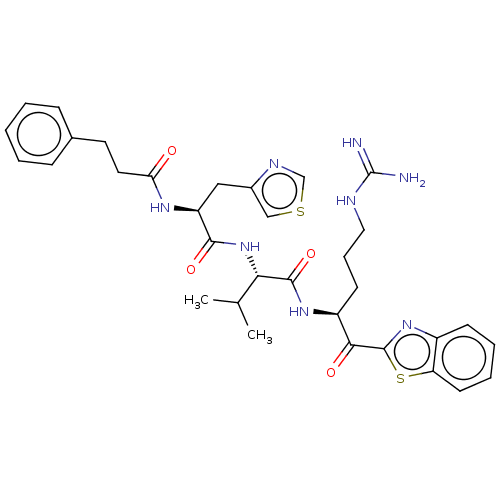 (CHEMBL4081543) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal V5-His-tagged matriptase-2 expressed in Drosophila S2 cells using Boc-Gln-Ala-ArgAMC as substrate measured... | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001054 (4-cyclopropylmethyl-14-iodo-(14R,17S)-12-oxa-4-aza...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50088336 (9-(3,4-Dichloro-benzylidene)-7-aza-tricyclo[4.3.1....) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity using [125I]RTI-55 as a radioligand in HEK-hSERT cells expressing human serotonin transporter | J Med Chem 43: 2064-71 (2000) BindingDB Entry DOI: 10.7270/Q2GX4C8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity was determined as ability to displace [3H]-U-69, radioligand from Opioid receptor kappa 1 | Bioorg Med Chem Lett 8: 3149-52 (1999) BindingDB Entry DOI: 10.7270/Q2S75FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 using [3H]naloxone as radioligand. | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha3-beta4 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121638 (1-[3-(Adamantan-1-ylamino)-3,4-dihydro-1H-isoquino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Binding affinity of compound towards the Opioid receptor mu 1 in rat synaptosomal membrane was determined using [3H]-DAGO as radioligand | J Med Chem 45: 5506-13 (2002) BindingDB Entry DOI: 10.7270/Q2DN44DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001054 (4-cyclopropylmethyl-14-iodo-(14R,17S)-12-oxa-4-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace [3H]cyclofoxy (Kd = 0.8 nM and concentration is 1.3 nM) from mu and kappa2 receptor in rat brain membranes... | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50228072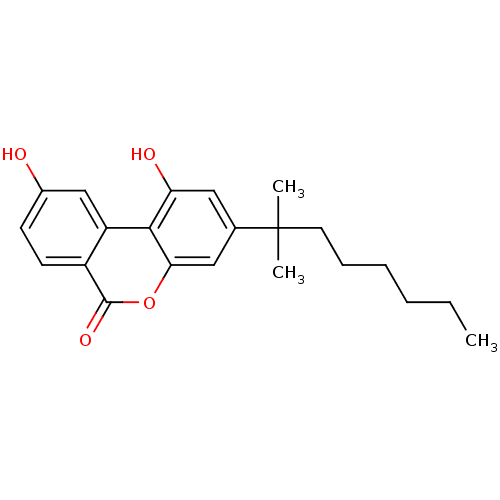 (1,9-dihydroxy-3-(1',1'-dimethylheptyl)-6H-benzo[c]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from CB2 receptor in mouse spleen membranes | J Med Chem 50: 6493-500 (2007) Article DOI: 10.1021/jm070441u BindingDB Entry DOI: 10.7270/Q2736QN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000780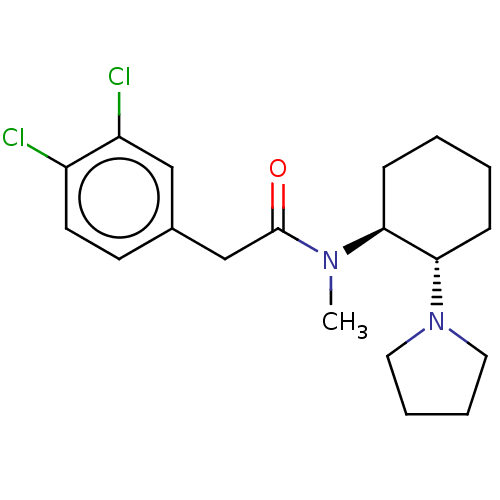 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Digestive Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards kappa opioid receptor using [3H](-)-U-69,593 in guinea pig ileum | J Med Chem 32: 1996-2002 (1989) BindingDB Entry DOI: 10.7270/Q2ZG6STP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039026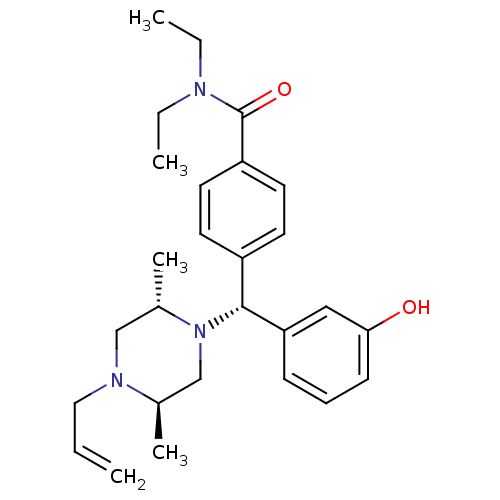 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Affinity of [H]DADLE to the delta opioid receptor from rat brain | J Med Chem 44: 972-87 (2001) BindingDB Entry DOI: 10.7270/Q2V69K8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by DAMGO (Opioid receptor mu 1) | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Compound was tested for the inhibition of [35S]-GTP-gammaS, binding in Guinea pig Caudate stimulated by the opioid receptor agonist Mu-DAMGO | Bioorg Med Chem Lett 8: 3149-52 (1999) BindingDB Entry DOI: 10.7270/Q2S75FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50366775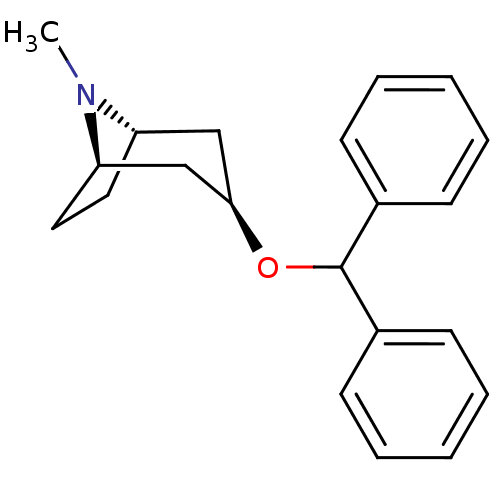 (BENZTROPINE | Benzatropine) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50061113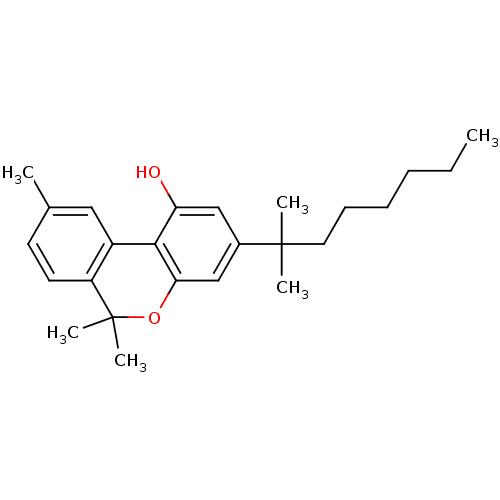 (3-(1',1'-dimethylheptyl)-6,6,9-trimethyl-6H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from CB1 receptor in rat brain synaptosomes | J Med Chem 50: 6493-500 (2007) Article DOI: 10.1021/jm070441u BindingDB Entry DOI: 10.7270/Q2736QN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50080448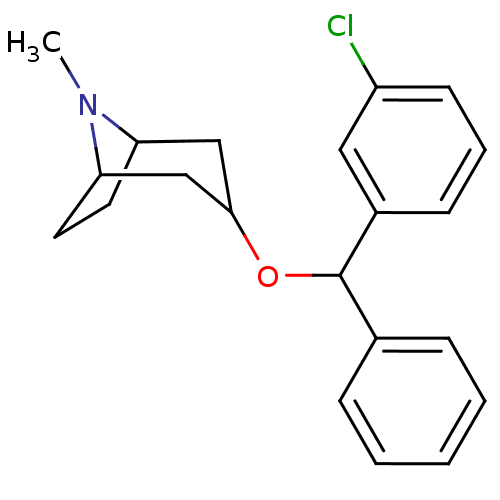 (3-[(3-Chloro-phenyl)-phenyl-methoxy]-8-methyl-8-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for muscarinic m1 receptor was determined in vitro in rat brain using [3H]pirenzepine as radioligand. | J Med Chem 44: 633-40 (2001) BindingDB Entry DOI: 10.7270/Q2MP540N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236924 (CHEMBL4093487) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-L-Fucosidase of human placenta | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50061113 (3-(1',1'-dimethylheptyl)-6,6,9-trimethyl-6H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55,940 from CB2 receptor in mouse spleen membranes | J Med Chem 50: 6493-500 (2007) Article DOI: 10.1021/jm070441u BindingDB Entry DOI: 10.7270/Q2736QN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50094798 (CHEMBL543688 | [3-(3,4-Dichloro-phenyl)-6-methoxy-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for serotonin transporter labeled with [125 I]RTI-55 | J Med Chem 43: 4868-76 (2000) BindingDB Entry DOI: 10.7270/Q2639QD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50453903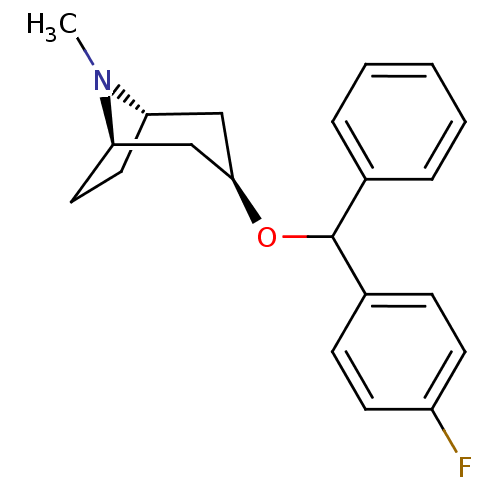 (CHEMBL3084883) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Affinity for rat M1 acetylcholine receptor using [3H]pirenzepine displacement. | J Med Chem 38: 3933-40 (1995) BindingDB Entry DOI: 10.7270/Q20V8DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121639 (1-[3-(Adamantan-1-ylamino)-3,4-dihydro-1H-isoquino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Binding affinity of compound towards the Opioid receptor mu 1 in rat synaptosomal membrane was determined using [3H]-DAGO as radioligand | J Med Chem 45: 5506-13 (2002) BindingDB Entry DOI: 10.7270/Q2DN44DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236911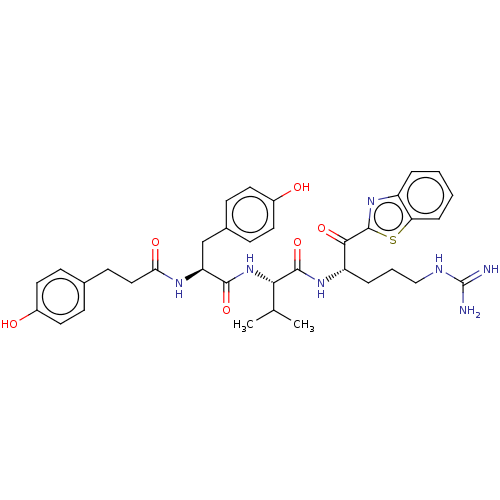 (CHEMBL4103740) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibitory activity against alpha-L-Fucosidase of bovine epididymis expressed as Ki | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001053 (14-bromo-4-cyclopropylmethyl-(13R,14R,17S)-12-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001053 (14-bromo-4-cyclopropylmethyl-(13R,14R,17S)-12-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50067447 (3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by U69,593 in Opioid receptor kappa 1 | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding activity against Opioid receptor mu 1 using [3H]-DAMGO as radioligand in rat brain membranes. | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity was determined as ability to displace [3H]-DAMGO radioligand from Mu opioid receptor | Bioorg Med Chem Lett 8: 3149-52 (1999) BindingDB Entry DOI: 10.7270/Q2S75FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50045767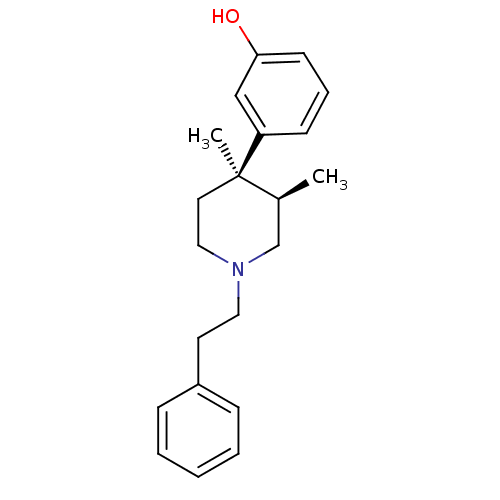 ((+)-N-phenethyl trans-3(R),4(R)-dimethyl-4-(3-hydr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity was determined against Mu opioid receptor using [3H]-Naltrexone as radioligand | Bioorg Med Chem Lett 8: 3149-52 (1999) BindingDB Entry DOI: 10.7270/Q2S75FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236933 (CHEMBL4101897) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal V5-His-tagged matriptase-2 expressed in Drosophila S2 cells using Boc-Gln-Ala-ArgAMC as substrate measured... | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001054 (4-cyclopropylmethyl-14-iodo-(14R,17S)-12-oxa-4-aza...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace [3H]BRM (Kd = 1.0 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045767 ((+)-N-phenethyl trans-3(R),4(R)-dimethyl-4-(3-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 using [3H]-Naloxone as radioligand. | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Affinity of [H]DADLE to the delta opioid receptor from rat brain | J Med Chem 44: 972-87 (2001) BindingDB Entry DOI: 10.7270/Q2V69K8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001054 (4-cyclopropylmethyl-14-iodo-(14R,17S)-12-oxa-4-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes. | J Med Chem 35: 2826-35 (1992) BindingDB Entry DOI: 10.7270/Q2639QB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236914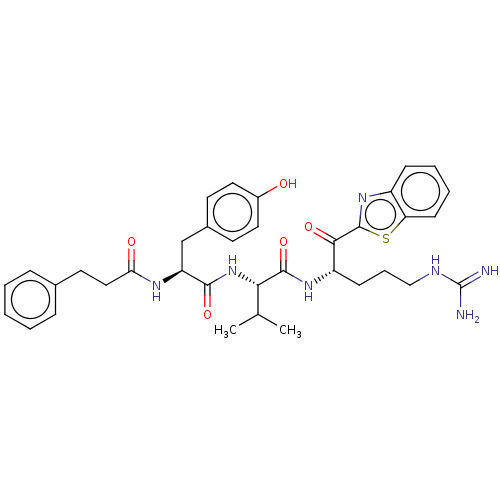 (CHEMBL4091809) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal V5-His-tagged matriptase-2 expressed in Drosophila S2 cells using Boc-Gln-Ala-ArgAMC as substrate measured... | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50094791 (CHEMBL542500 | [3-(3,4-Dichloro-phenyl)-4-methoxy-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for dopamine transporter (DAT) labeled with [125 I]RTI-55 | J Med Chem 43: 4868-76 (2000) BindingDB Entry DOI: 10.7270/Q2639QD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1466 total ) | Next | Last >> |