

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acid phosphatase (Francisella tularensis) | BDBM92477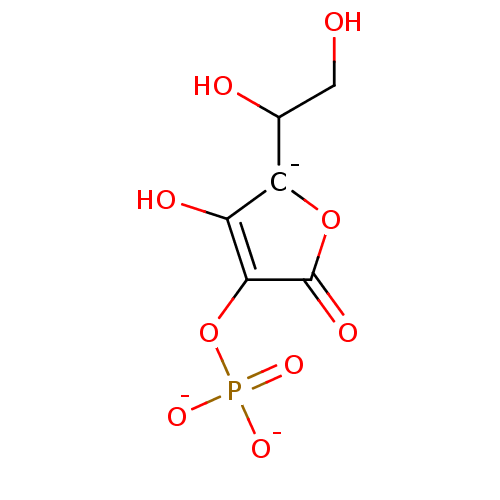 (2-phospho-L-ascorbic acid) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid phosphatase (Francisella tularensis) | BDBM59083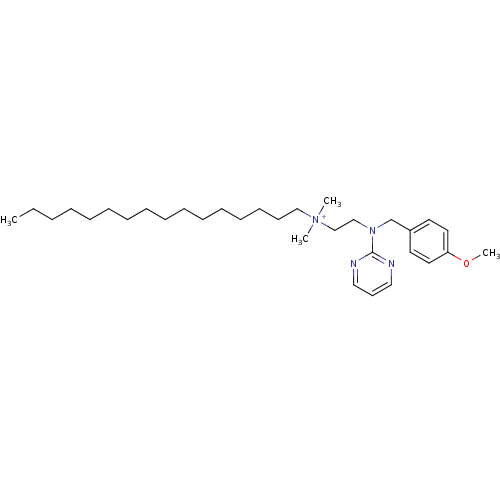 (MLS002154065 | SMR001233380 | THONZONIUM BROMIDE |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid phosphatase (Francisella tularensis) | BDBM92476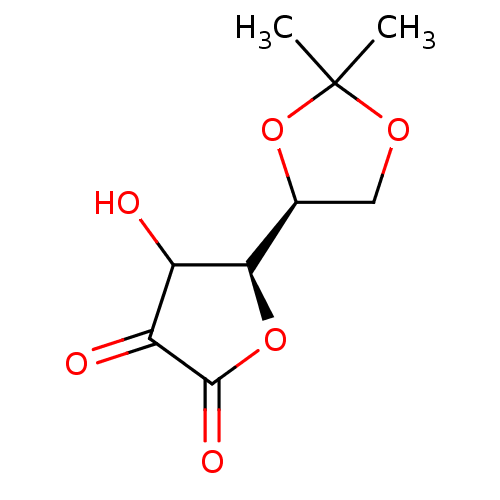 ((+)-5,6-O-Isopropylidene-L-ascorbic acid) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid phosphatase (Francisella tularensis) | BDBM39344 (4-amino-N-[2-(diethylamino)ethyl]benzamide;hydroch...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid phosphatase (Francisella tularensis) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid phosphatase (Francisella tularensis) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid phosphatase (Francisella tularensis) | BDBM92478 (Atracurium besylate | CHEMBL1360) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid phosphatase (Francisella tularensis) | BDBM50090256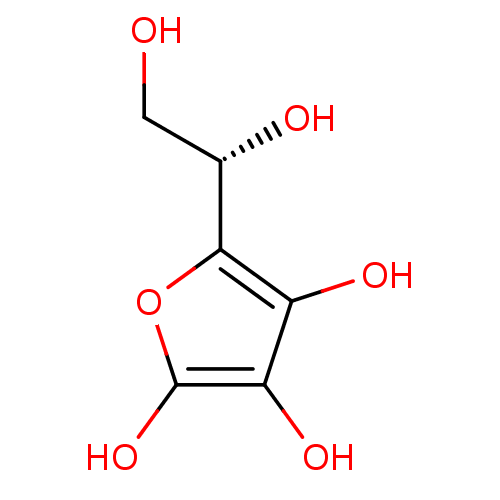 ((R)-2-((S)-1,2-dihydroxyethyl)-4,5-dihydroxyfuran-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical... | J Biol Chem 285: 5171-7 (2010) Article DOI: 10.1074/jbc.M109.039511 BindingDB Entry DOI: 10.7270/Q2348HZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of src kinase | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199773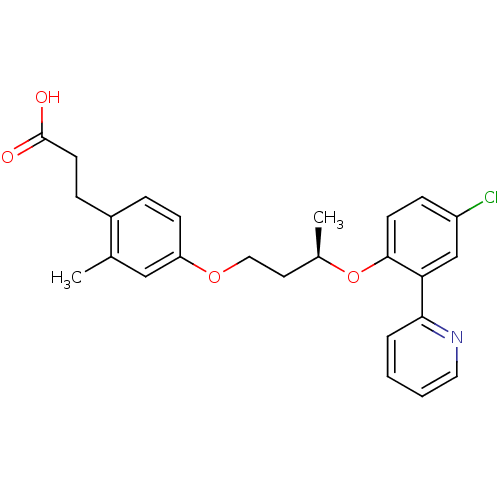 (3-(4-((R)-3-(4-chloro-2-(pyridin-2-yl)phenoxy)buto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-23 receptor (Homo sapiens) | BDBM50459752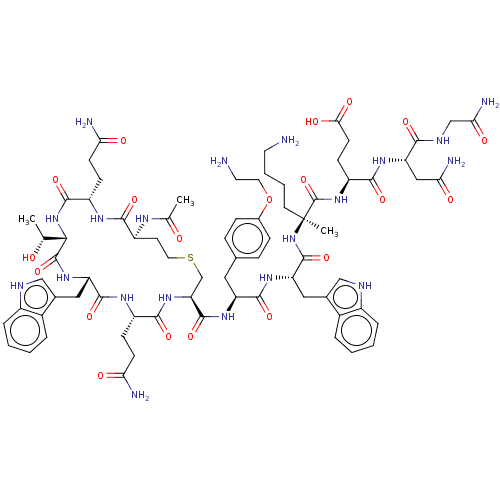 (CHEMBL4206167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci£n Lilly S.A. Curated by ChEMBL | Assay Description Binding affinity to recombinant human flag/His-tagged IL-23R (1 to 353 residues) expressed in HEK293F cells assessed as disruption of biotinylated IL... | ACS Med Chem Lett 9: 912-916 (2018) Article DOI: 10.1021/acsmedchemlett.8b00255 BindingDB Entry DOI: 10.7270/Q25B054G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50194627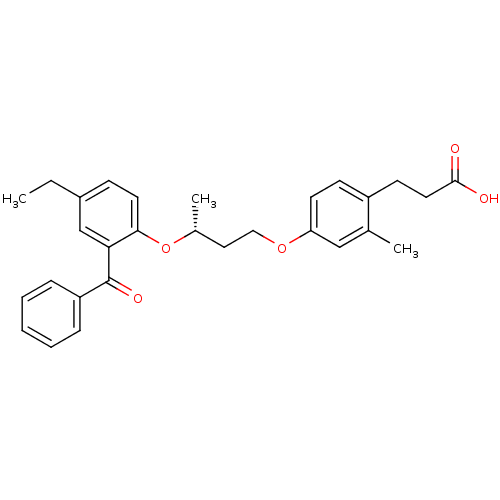 ((R)-3-(4-(3-(2-benzoyl-4-ethylphenoxy)butoxy)-2-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199778 (3-(4-((R)-3-(4-ethyl-2-(thiazol-4-yl)phenoxy)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199781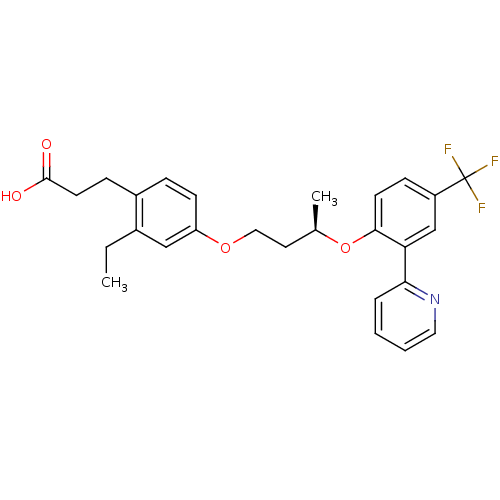 (3-(2-ethyl-4-((R)-3-(2-(pyridin-2-yl)-4-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199776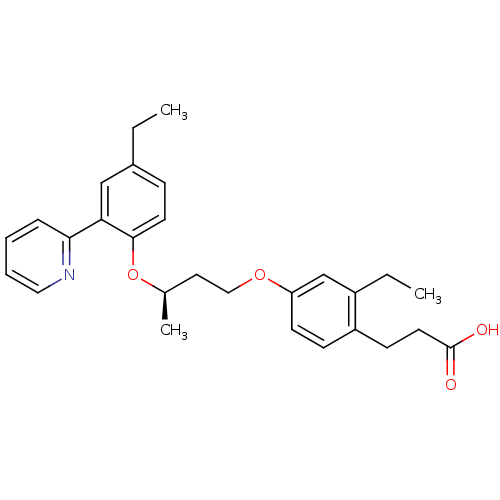 (3-(2-ethyl-4-((R)-3-(4-ethyl-2-(pyridin-2-yl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199779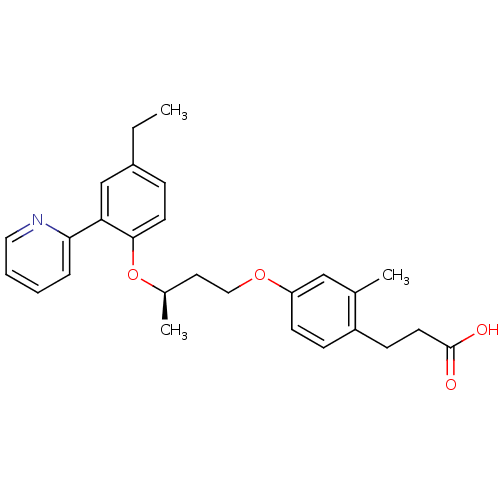 (3-(4-((R)-3-(4-ethyl-2-(pyridin-2-yl)phenoxy)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199777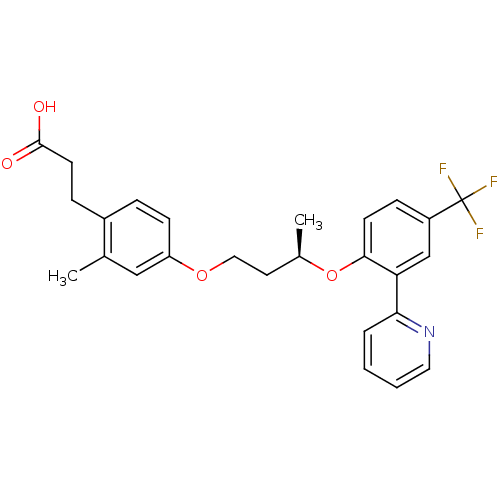 (3-(2-methyl-4-((R)-3-(2-(pyridin-2-yl)-4-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM17055 ((18S)-18-[(dimethylamino)methyl]-17-oxa-4,14,21-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50052034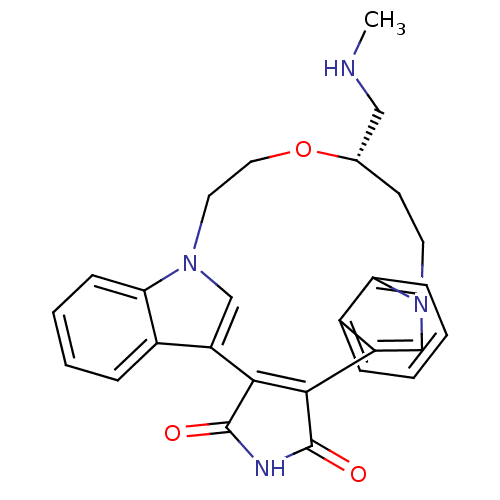 (18-methylaminomethyl-(18S)-17-oxa-4,14,21-triazahe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199774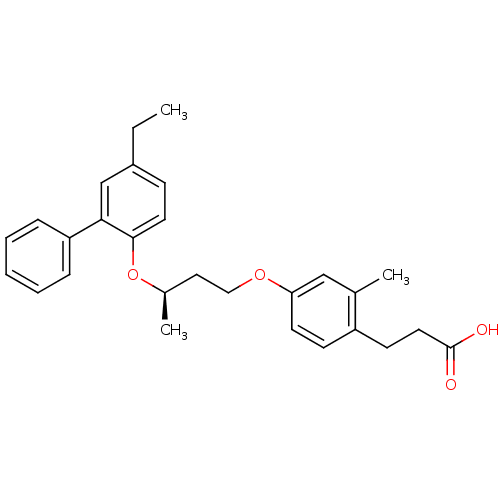 (3-{4-[(R)-3-(5-ethyl-biphenyl-2-yloxy)-butoxy]-2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50194627 ((R)-3-(4-(3-(2-benzoyl-4-ethylphenoxy)butoxy)-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-[porpyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-propyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199783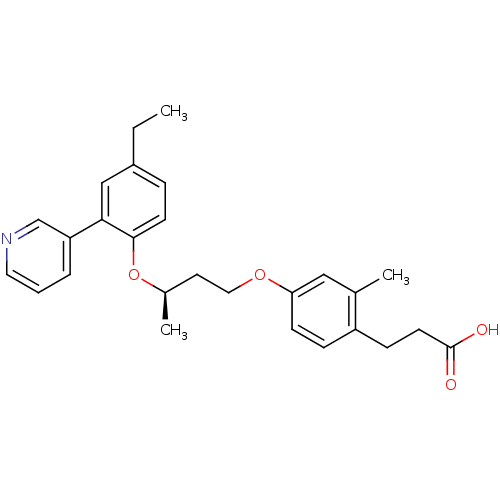 (3-(4-((R)-3-(4-ethyl-2-(pyridin-3-yl)phenoxy)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199785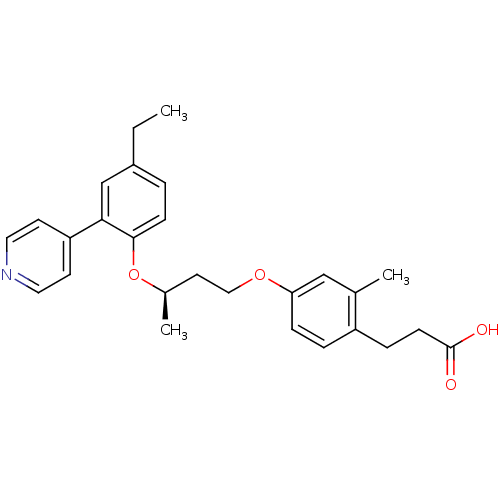 ((R)-3-(4-(3-(4-ethyl-2-(pyridin-4-yl)phenoxy)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50052034 (18-methylaminomethyl-(18S)-17-oxa-4,14,21-triazahe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 2 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199782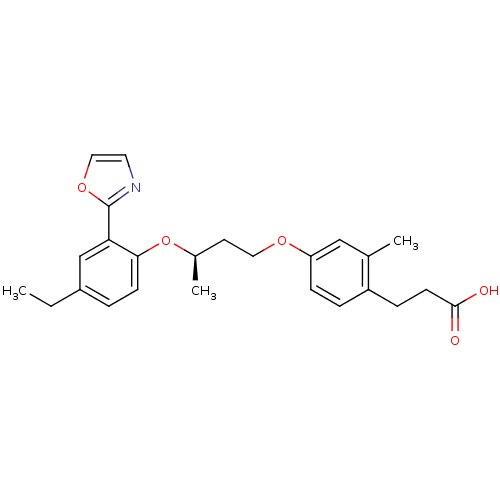 (3-(4-((R)-3-(4-ethyl-2-(oxazol-2-yl)phenoxy)butoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-23 receptor (Homo sapiens) | BDBM50459752 (CHEMBL4206167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci£n Lilly S.A. Curated by ChEMBL | Assay Description Binding affinity to recombinant human flag/His-tagged IL-23R (1 to 353 residues) expressed in HEK293F cells assessed as disruption of biotinylated IL... | ACS Med Chem Lett 9: 912-916 (2018) Article DOI: 10.1021/acsmedchemlett.8b00255 BindingDB Entry DOI: 10.7270/Q25B054G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine palmitoyltransferase 1 (Homo sapiens (Human)) | BDBM50535848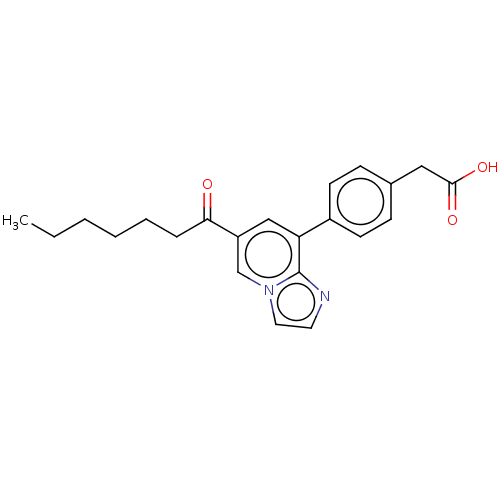 (CHEMBL4584997) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human SPT1 expressed in microsomes of HEK293 cells incubated for 1hr by LC/MS analysis | J Med Chem 59: 5904-10 (2016) Article DOI: 10.1021/acs.jmedchem.5b01851 BindingDB Entry DOI: 10.7270/Q29K4FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM17055 ((18S)-18-[(dimethylamino)methyl]-17-oxa-4,14,21-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 2 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199772 (3-(4-((R)-3-(4-ethyl-2-(thiophen-3-yl)phenoxy)buto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054042 ((E)-6-(4-Hydroxy-7-methyl-3-oxo-6-vinyl-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant inosine monophosphate dehydrogenase type II . | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199775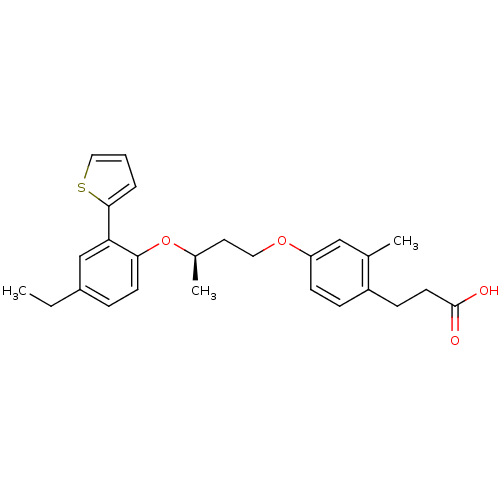 (3-(4-((R)-3-(4-ethyl-2-(thiophen-2-yl)phenoxy)buto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199771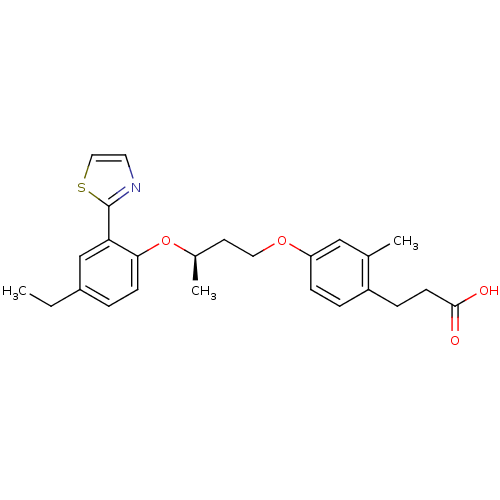 (3-(4-((R)-3-(4-ethyl-2-(thiazol-2-yl)phenoxy)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054021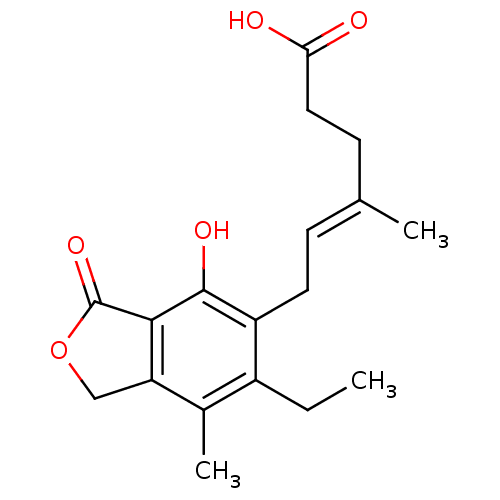 ((E)-6-(6-Ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant inosine monophosphate dehydrogenase type II . | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199784 (3-(4-((R)-3-(4-ethyl-2-(oxazol-4-yl)phenoxy)butoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50199780 (3-(4-((R)-3-(4-ethyl-2-(furan-2-yl)phenoxy)butoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdelta | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50199781 (3-(2-ethyl-4-((R)-3-(2-(pyridin-2-yl)-4-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-[porpyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-propyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C epsilon | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054019 ((E)-6-(4-Hydroxy-6,7-dimethyl-3-oxo-1,3-dihydro-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant type II Inosine Monophosphate Dehydrogenase. at pH 8.0 | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 2 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50199777 (3-(2-methyl-4-((R)-3-(2-(pyridin-2-yl)-4-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-[porpyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-propyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 17: 1052-5 (2007) Article DOI: 10.1016/j.bmcl.2006.11.029 BindingDB Entry DOI: 10.7270/Q2BP02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50052041 (18-(4-methylhexahydro-1-pyrazinylmethyl)-(18S)-17-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 2 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Syntex Research Curated by ChEMBL | Assay Description Compound was tested for inhibition of human recombinant type II Inosine Monophosphate Dehydrogenase at pH 8.0 | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Syntex Research Curated by ChEMBL | Assay Description Compound was tested for inhibition of human recombinant type II Inosine Monophosphate Dehydrogenase at pH 8.0 | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C delta | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50052039 (18-hydroxymethyl-(18S)-17-oxa-4,14,21-triazahexacy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 2 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50052041 (18-(4-methylhexahydro-1-pyrazinylmethyl)-(18S)-17-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50052034 (18-methylaminomethyl-(18S)-17-oxa-4,14,21-triazahe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50052037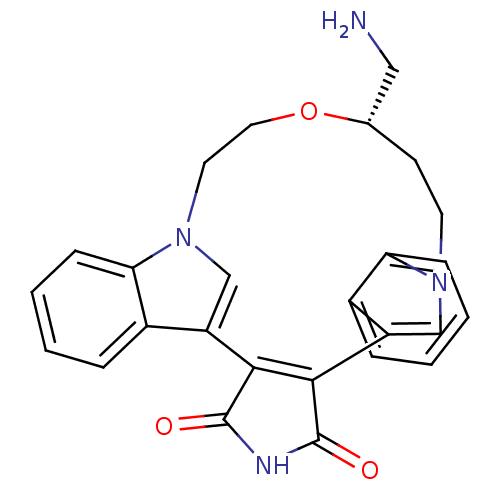 (18-aminomethyl-(18S)-17-oxa-4,14,21-triazahexacycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 2 | J Med Chem 39: 2664-71 (1996) Article DOI: 10.1021/jm950588y BindingDB Entry DOI: 10.7270/Q25H7FBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 259 total ) | Next | Last >> |