

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50051232 (8-(4-Fluoro-benzyl)-2-furan-2-yl-8H-pyrazolo[4,3-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Binding affinity to rat A2A adenosine receptor | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Displacement of MRS5346 from C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pich... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50051226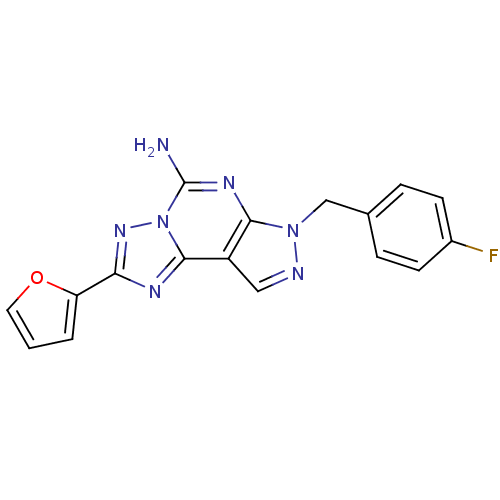 (7-(4-Fluoro-benzyl)-2-furan-2-yl-7H-pyrazolo[4,3-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Binding affinity to rat A2A adenosine receptor | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254384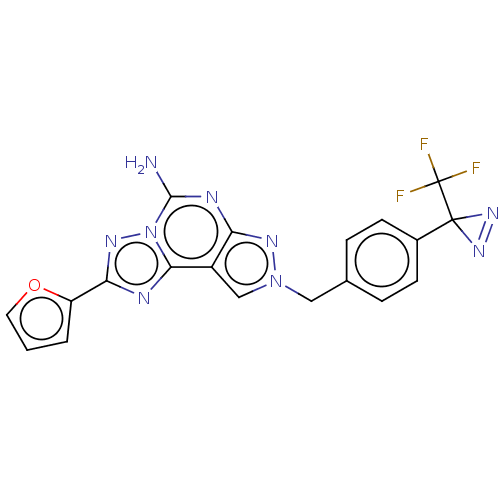 (CHEMBL4079067) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Displacement of MRS5346 from C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pich... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50207816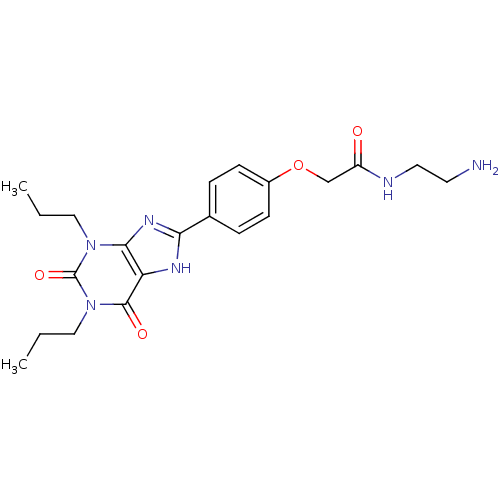 (CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Displacement of MRS5346 from C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pich... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 481 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Displacement of MRS5346 from C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pich... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254363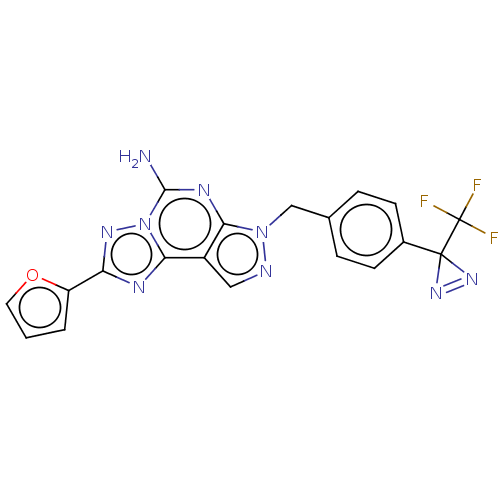 (CHEMBL4103324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Displacement of MRS5346 from C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pich... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of wild type human N-terminal GST-fusion tagged TRKB kinase domain (456 to 822 residues) expressed in baculovirus expression system by HTR... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of wild type human His-tagged TRKA kinase domain (441 to 796 residues) expressed in baculovirus expression system by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50232542 (CHEMBL4090531 | US10323022, Example 135) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkA expressed in DHFR deficient CHO cells assessed as inhibition of human beta-nerve growth factor-induced calcium i... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of TRKA E735A mutant (unknown origin) expressed in baculovirus infected sf9 cells assessed as kinase domain dimerization by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50232542 (CHEMBL4090531 | US10323022, Example 135) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkB expressed in DHFR deficient CHO cells assessed as inhibition of human brain-derived neurotrophic factor-induced ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkC expressed in DHFR deficient CHO cells assessed as inhibition of human neurotrophin-3-induced calcium influx by F... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region deficient recombinant human N-terminal His6-tagged/GST-tagged TrkA (498 to 796 residues) expressed in baculovirus ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkA expressed in DHFR deficient CHO cells assessed as inhibition of human beta-nerve growth factor-induced calcium i... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50198370 (CHEMBL223873 | N-(2,6-dimethyl-phenyl)-5-phenylimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of LCK (unknown origin) | Bioorg Med Chem 16: 10311-8 (2008) Article DOI: 10.1016/j.bmc.2008.10.041 BindingDB Entry DOI: 10.7270/Q2W37W5C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022672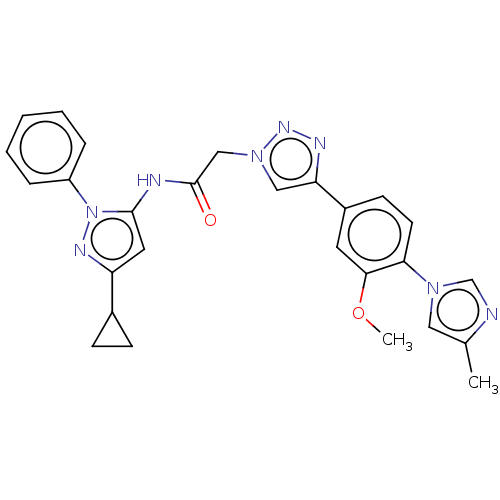 (CHEMBL3298266) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022672 (CHEMBL3298266) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50022672 (CHEMBL3298266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkB expressed in DHFR deficient CHO cells assessed as inhibition of human brain-derived neurotrophic factor-induced ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50022672 (CHEMBL3298266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkC expressed in DHFR deficient CHO cells assessed as inhibition of human neurotrophin-3-induced calcium influx by F... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50510995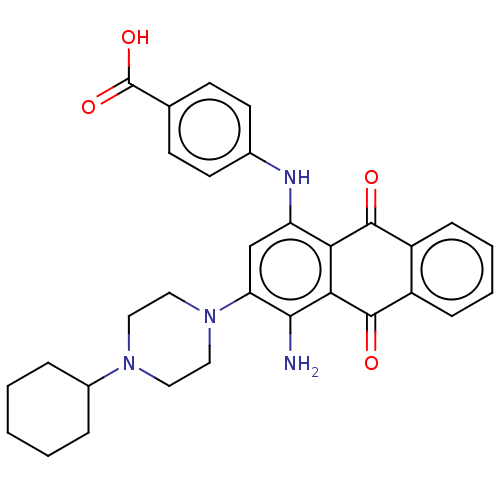 (CHEMBL4556229) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of wild type human His-tagged TRKA kinase domain (441 to 796 residues) expressed in baculovirus expression system by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50246553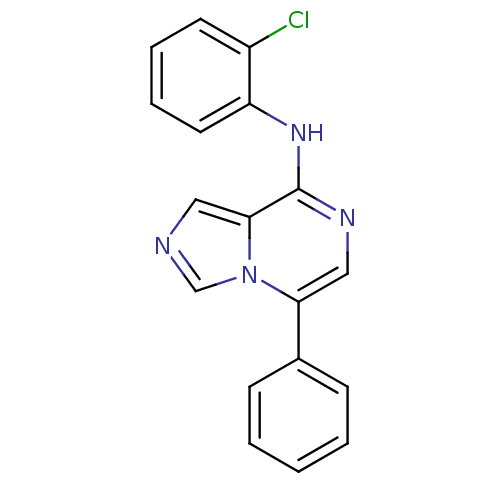 (CHEMBL462228 | N-(2-chlorophenyl)-5-phenylimidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of LCK (unknown origin) | Bioorg Med Chem 16: 10311-8 (2008) Article DOI: 10.1016/j.bmc.2008.10.041 BindingDB Entry DOI: 10.7270/Q2W37W5C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022672 (CHEMBL3298266) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region deficient recombinant human N-terminal His6-tagged/GST-tagged TrkA (498 to 796 residues) expressed in baculovirus ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50232542 (CHEMBL4090531 | US10323022, Example 135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkB expressed in DHFR deficient CHO cells assessed as inhibition of human brain-derived neurotrophic factor-induced ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50232542 (CHEMBL4090531 | US10323022, Example 135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of recombinant human TrkC expressed in DHFR deficient CHO cells assessed as inhibition of human neurotrophin-3-induced calcium influx by F... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435857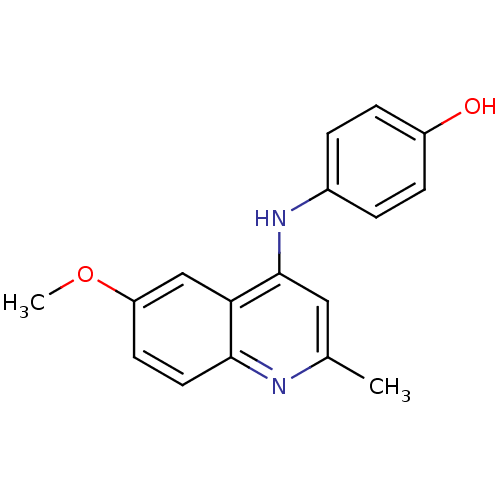 (CHEMBL2393579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435854 (CHEMBL2393583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435849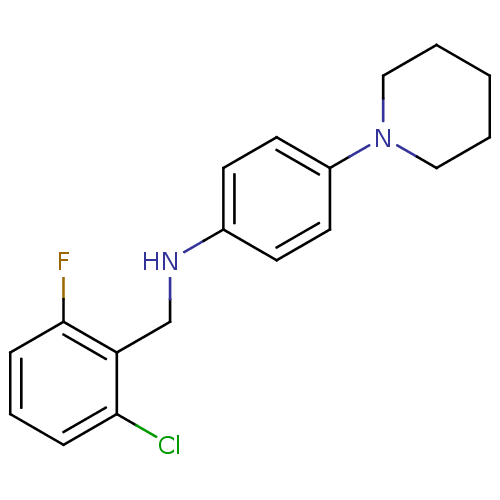 (CHEMBL2391046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435850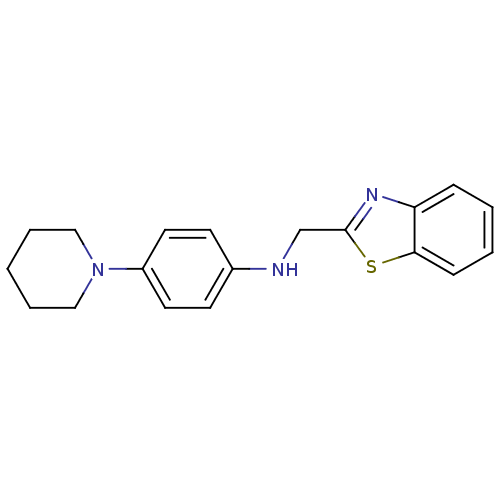 (CHEMBL2391045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435855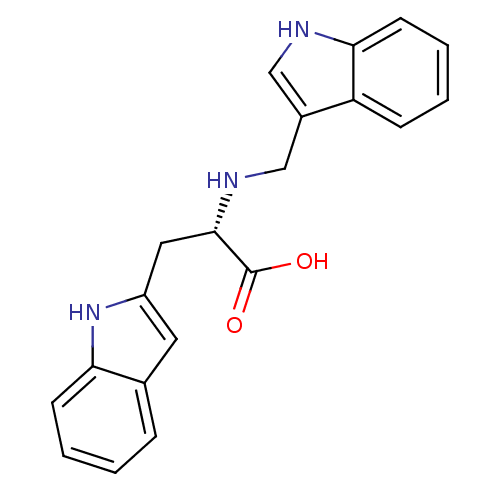 (CHEMBL2393581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435851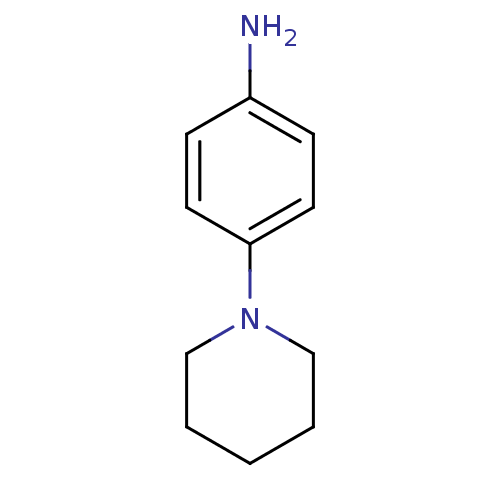 (CHEMBL2391044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50232542 (CHEMBL4090531 | US10323022, Example 135) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of juxtamembrane region deficient recombinant human N-terminal His6-tagged/GST-tagged TrkA (498 to 796 residues) expressed in baculovirus ... | Bioorg Med Chem Lett 27: 1233-1236 (2017) Article DOI: 10.1016/j.bmcl.2017.01.056 BindingDB Entry DOI: 10.7270/Q2TT4T51 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50510995 (CHEMBL4556229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University Curated by ChEMBL | Assay Description Inhibition of wild type human N-terminal GST-fusion tagged TRKB kinase domain (456 to 822 residues) expressed in baculovirus expression system by HTR... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126775 BindingDB Entry DOI: 10.7270/Q2Q243K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435853 (CHEMBL2393584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435856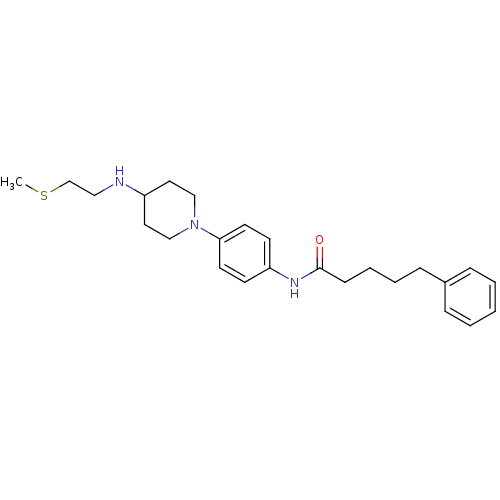 (CHEMBL2393580) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Mycobacterium tuberculosis) | BDBM50435852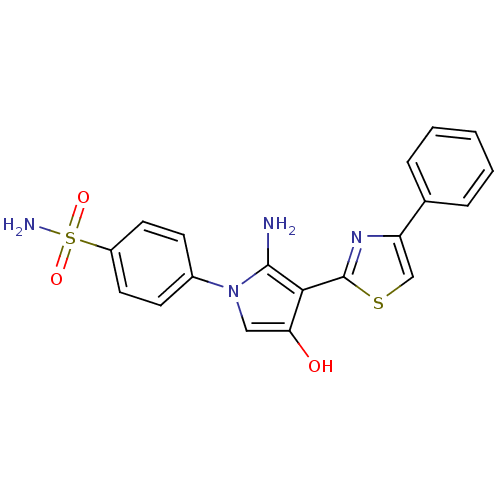 (CHEMBL2393585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis TrxR after 10 mins by microplate reader analysis | J Med Chem 56: 4849-59 (2013) Article DOI: 10.1021/jm3015734 BindingDB Entry DOI: 10.7270/Q21C1Z88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50342519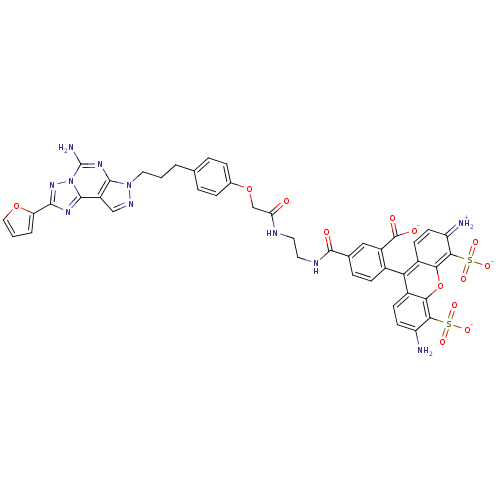 (CHEMBL1771809 | triethylammonium 5-(2-(2-(4-(3-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Binding affinity to C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pichia pastor... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50342519 (CHEMBL1771809 | triethylammonium 5-(2-(2-(4-(3-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Binding affinity to human A2A adenosine receptor expressed in HEK293 cells after 1 hr by fluorescence polarization assay | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50342519 (CHEMBL1771809 | triethylammonium 5-(2-(2-(4-(3-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Binding affinity to C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pichia pastor... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50342519 (CHEMBL1771809 | triethylammonium 5-(2-(2-(4-(3-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Binding affinity to C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pichia pastor... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50342519 (CHEMBL1771809 | triethylammonium 5-(2-(2-(4-(3-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a |
Central Research Laboratories, Kissei Pharmaceutical Co., Ltd., 4365-1 Kashiwabara, Hotaka, Azumino, Nagano 399-8304, Japan. Curated by ChEMBL | Assay Description Binding affinity to C-terminal 10xHis and 1D4-tagged C-terminal-truncated human A2A adenosine receptor (1 to 316 residues) expressed in Pichia pastor... | ACS Med Chem Lett 8: 660-665 (2017) Article DOI: 10.1021/acsmedchemlett.7b00138 BindingDB Entry DOI: 10.7270/Q2GT5QMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558569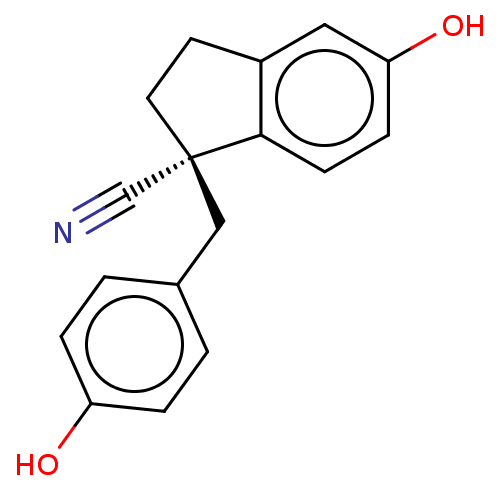 (CHEMBL4795911) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558570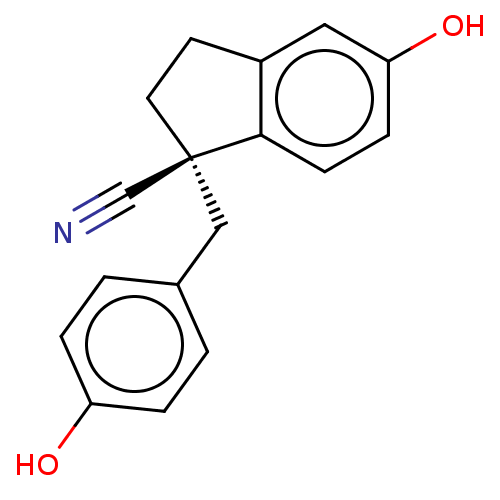 (CHEMBL4789805) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558571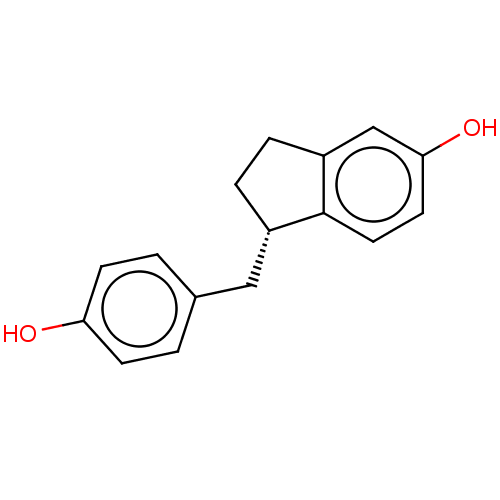 (CHEMBL4744050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558572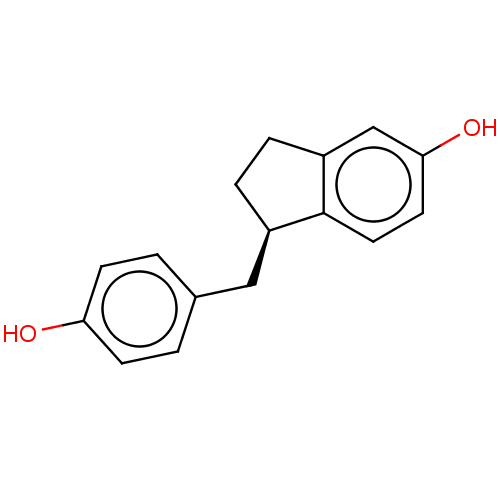 (CHEMBL4784379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558573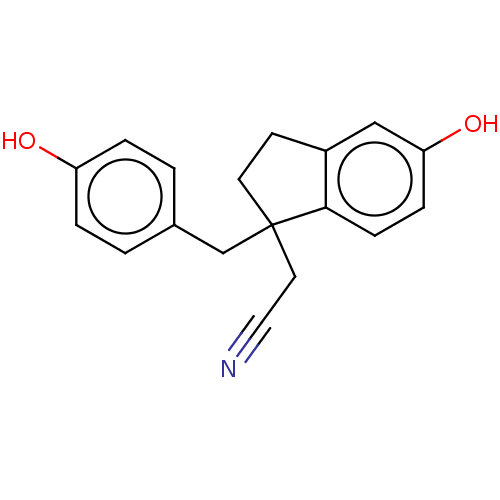 (CHEMBL4758549) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558574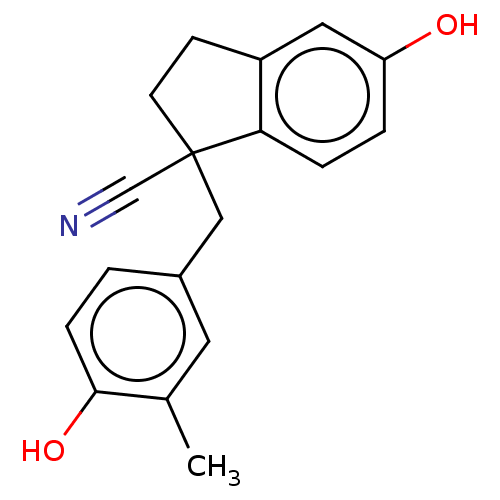 (CHEMBL4795356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558575 (CHEMBL4796518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human ERbeta receptor expressed in HEK293T cells transfected with pGL4.27-(ERE)3-Luc assessed as transcriptional activation measu... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.047 BindingDB Entry DOI: 10.7270/Q2RX9GRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |