 Found 230 hits with Last Name = 'jagusch' and Initial = 'c'
Found 230 hits with Last Name = 'jagusch' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322794

(4'-(2-methyl-1-(pyridin-4-yl)prop-1-enyl)biphenyl-...)Show SMILES [#6]\[#6](-[#6])=[#6](/c1ccncc1)-c1ccc(cc1)-c1ccc(-[#8])c(-[#8])c1 Show InChI InChI=1S/C21H19NO2/c1-14(2)21(17-9-11-22-12-10-17)16-5-3-15(4-6-16)18-7-8-19(23)20(24)13-18/h3-13,23-24H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322784

(4'-(2-methyl-1-(pyridin-4-yl)prop-1-enyl)biphenyl-...)Show SMILES [#6]\[#6](-[#6])=[#6](/c1ccncc1)-c1ccc(cc1)-c1cccc(-[#7])c1 Show InChI InChI=1S/C21H20N2/c1-15(2)21(18-10-12-23-13-11-18)17-8-6-16(7-9-17)19-4-3-5-20(22)14-19/h3-14H,22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322793
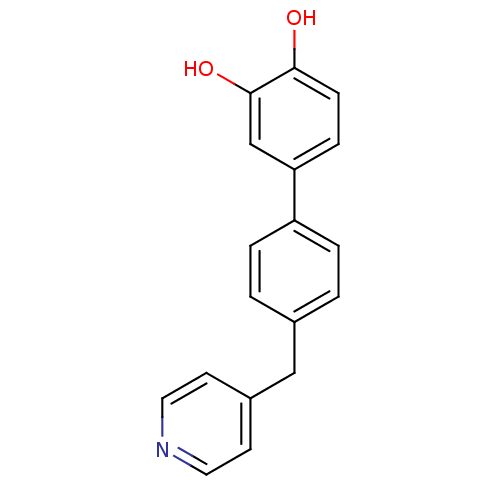
(4'-(Pyridin-4-ylmethyl)biphenyl-3,4-diol | CHEMBL1...)Show InChI InChI=1S/C18H15NO2/c20-17-6-5-16(12-18(17)21)15-3-1-13(2-4-15)11-14-7-9-19-10-8-14/h1-10,12,20-21H,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322793

(4'-(Pyridin-4-ylmethyl)biphenyl-3,4-diol | CHEMBL1...)Show InChI InChI=1S/C18H15NO2/c20-17-6-5-16(12-18(17)21)15-3-1-13(2-4-15)11-14-7-9-19-10-8-14/h1-10,12,20-21H,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322790

(4'-(2-methyl-1-(pyridin-4-yl)prop-1-enyl)biphenyl-...)Show SMILES [#6]\[#6](-[#6])=[#6](/c1ccncc1)-c1ccc(cc1)-c1cccc(-[#8])c1 Show InChI InChI=1S/C21H19NO/c1-15(2)21(18-10-12-22-13-11-18)17-8-6-16(7-9-17)19-4-3-5-20(23)14-19/h3-14,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322787
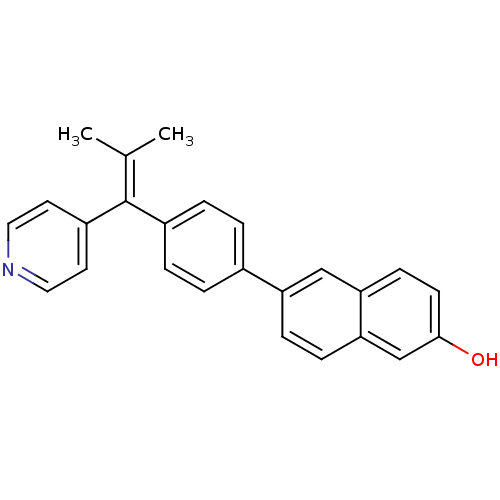
(6-(4-(2-methyl-1-(pyridin-4-yl)prop-1-enyl)phenyl)...)Show SMILES [#6]\[#6](-[#6])=[#6](/c1ccncc1)-c1ccc(cc1)-c1ccc2cc(-[#8])ccc2c1 Show InChI InChI=1S/C25H21NO/c1-17(2)25(20-11-13-26-14-12-20)19-5-3-18(4-6-19)21-7-8-23-16-24(27)10-9-22(23)15-21/h3-16,27H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25457
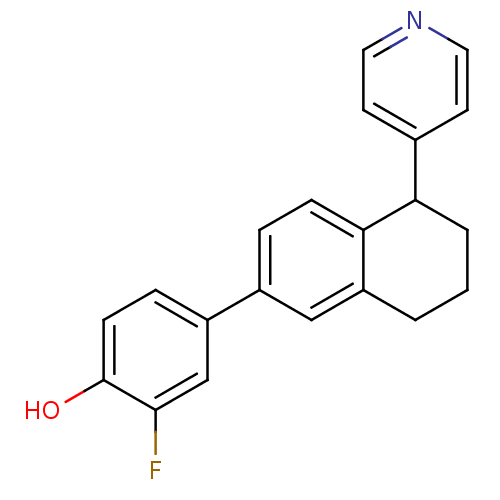
(2-fluoro-4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronap...)Show InChI InChI=1S/C21H18FNO/c22-20-13-16(5-7-21(20)24)15-4-6-19-17(12-15)2-1-3-18(19)14-8-10-23-11-9-14/h4-13,18,24H,1-3H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University
| Assay Description
The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster V79MZh cells |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
| Assay Description
The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
Bioorg Med Chem Lett 18: 267-73 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.079
BindingDB Entry DOI: 10.7270/Q2833RSP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase |
Bioorg Med Chem 16: 1992-2010 (2008)
Article DOI: 10.1016/j.bmc.2007.10.094
BindingDB Entry DOI: 10.7270/Q29W0GBN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
| Assay Description
To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University
| Assay Description
The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322792

(3-fluoro-4'-(2-methyl-1-(pyridin-4-yl)prop-1-enyl)...)Show SMILES [#6]\[#6](-[#6])=[#6](/c1ccncc1)-c1ccc(cc1)-c1ccc(-[#8])c(F)c1 Show InChI InChI=1S/C21H18FNO/c1-14(2)21(17-9-11-23-12-10-17)16-5-3-15(4-6-16)18-7-8-20(24)19(22)13-18/h3-13,24H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322786
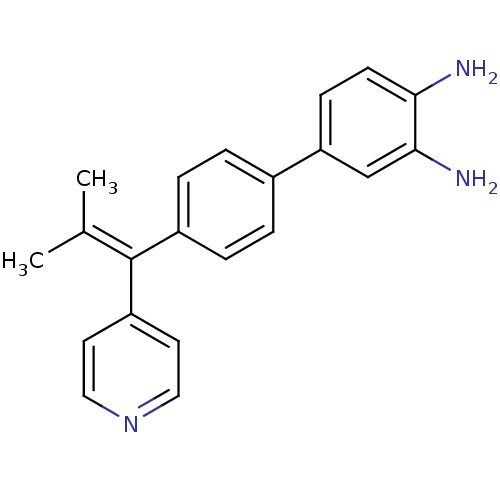
(4'-(2-methyl-1-(pyridin-4-yl)prop-1-enyl)biphenyl-...)Show SMILES [#6]\[#6](-[#6])=[#6](/c1ccncc1)-c1ccc(cc1)-c1ccc(-[#7])c(-[#7])c1 Show InChI InChI=1S/C21H21N3/c1-14(2)21(17-9-11-24-12-10-17)16-5-3-15(4-6-16)18-7-8-19(22)20(23)13-18/h3-13H,22-23H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322789

(4'-(pyridin-4-ylmethyl)biphenyl-3-ol | 4-[(3'-Hydr...)Show InChI InChI=1S/C18H15NO/c20-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-19-11-9-15/h1-11,13,20H,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322789

(4'-(pyridin-4-ylmethyl)biphenyl-3-ol | 4-[(3'-Hydr...)Show InChI InChI=1S/C18H15NO/c20-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-19-11-9-15/h1-11,13,20H,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50322791

(3-Fluoro-4'-(pyridin-4-ylmethyl)biphenyl-4-ol | CH...)Show InChI InChI=1S/C18H14FNO/c19-17-12-16(5-6-18(17)21)15-3-1-13(2-4-15)11-14-7-9-20-10-8-14/h1-10,12,21H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster V79MZh cells |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM25448

(4-[6-(4-fluorophenyl)-3,4-dihydronaphthalen-1-yl]p...)Show SMILES Fc1ccc(cc1)-c1ccc2C(=CCCc2c1)c1ccncc1 |c:12| Show InChI InChI=1S/C21H16FN/c22-19-7-4-15(5-8-19)17-6-9-21-18(14-17)2-1-3-20(21)16-10-12-23-13-11-16/h3-14H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
| Assay Description
The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
| Assay Description
The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79MZh cells |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25456
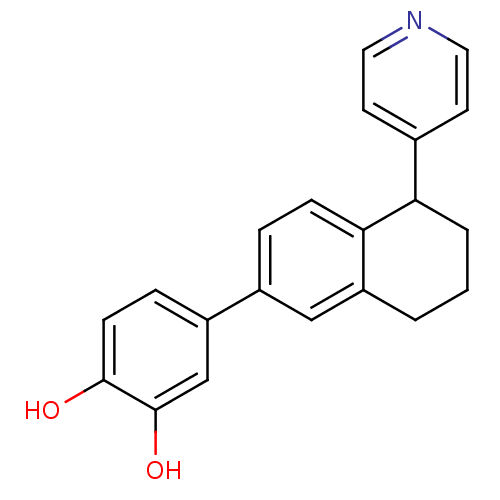
(4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronaphthalen-2...)Show InChI InChI=1S/C21H19NO2/c23-20-7-5-16(13-21(20)24)15-4-6-19-17(12-15)2-1-3-18(19)14-8-10-22-11-9-14/h4-13,18,23-24H,1-3H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University
| Assay Description
The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25446

(4-[6-(4-fluorophenyl)-1H-inden-3-yl]pyridine | Abi...)Show SMILES Fc1ccc(cc1)-c1ccc2C(=CCc2c1)c1ccncc1 |c:12| Show InChI InChI=1S/C20H14FN/c21-18-5-1-14(2-6-18)16-3-7-20-17(13-16)4-8-19(20)15-9-11-22-12-10-15/h1-3,5-13H,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
| Assay Description
To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25454

(4-[6-(4-fluorophenyl)-1,2,3,4-tetrahydronaphthalen...)Show InChI InChI=1S/C21H18FN/c22-19-7-4-15(5-8-19)17-6-9-21-18(14-17)2-1-3-20(21)16-10-12-23-13-11-16/h4-14,20H,1-3H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University
| Assay Description
The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322791

(3-Fluoro-4'-(pyridin-4-ylmethyl)biphenyl-4-ol | CH...)Show InChI InChI=1S/C18H14FNO/c19-17-12-16(5-6-18(17)21)15-3-1-13(2-4-15)11-14-7-9-20-10-8-14/h1-10,12,21H,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322791

(3-Fluoro-4'-(pyridin-4-ylmethyl)biphenyl-4-ol | CH...)Show InChI InChI=1S/C18H14FNO/c19-17-12-16(5-6-18(17)21)15-3-1-13(2-4-15)11-14-7-9-20-10-8-14/h1-10,12,21H,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25451

(2-fluoro-4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen...)Show SMILES Oc1ccc(cc1F)-c1ccc2C(=CCCc2c1)c1ccncc1 |c:13| Show InChI InChI=1S/C21H16FNO/c22-20-13-16(5-7-21(20)24)15-4-6-19-17(12-15)2-1-3-18(19)14-8-10-23-11-9-14/h3-13,24H,1-2H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University
| Assay Description
The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322795

(4'-(1-(pyridin-4-yl)propyl)biphenyl-3-ol | CHEMBL1...)Show InChI InChI=1S/C20H19NO/c1-2-20(17-10-12-21-13-11-17)16-8-6-15(7-9-16)18-4-3-5-19(22)14-18/h3-14,20,22H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50322791

(3-Fluoro-4'-(pyridin-4-ylmethyl)biphenyl-4-ol | CH...)Show InChI InChI=1S/C18H14FNO/c19-17-12-16(5-6-18(17)21)15-3-1-13(2-4-15)11-14-7-9-20-10-8-14/h1-10,12,21H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat testicular CYP17 |
Bioorg Med Chem 16: 1992-2010 (2008)
Article DOI: 10.1016/j.bmc.2007.10.094
BindingDB Entry DOI: 10.7270/Q29W0GBN |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322783

(4'-(pyridin-4-ylmethyl)biphenyl-3-amine | CHEMBL11...)Show InChI InChI=1S/C18H16N2/c19-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-20-11-9-15/h1-11,13H,12,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli coexpressing NADPH-P450 reductase |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50322783

(4'-(pyridin-4-ylmethyl)biphenyl-3-amine | CHEMBL11...)Show InChI InChI=1S/C18H16N2/c19-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-20-11-9-15/h1-11,13H,12,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50324625

(5-[4-(Pyridin-4-ylmethyl)phenyl]-1H-indole | CHEMB...)Show InChI InChI=1S/C20H16N2/c1-3-17(18-5-6-20-19(14-18)9-12-22-20)4-2-15(1)13-16-7-10-21-11-8-16/h1-12,14,22H,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25453
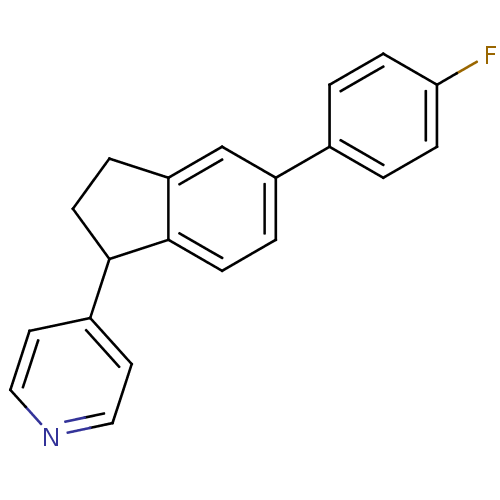
(4-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...)Show InChI InChI=1S/C20H16FN/c21-18-5-1-14(2-6-18)16-3-7-20-17(13-16)4-8-19(20)15-9-11-22-12-10-15/h1-3,5-7,9-13,19H,4,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University
| Assay Description
The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50371480

(CHEMBL403808)Show InChI InChI=1S/C17H17FN2S/c1-3-17(20-7-6-19-11-20)14-5-4-13(8-16(14)18)15-10-21-9-12(15)2/h4-11,17H,3H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase |
Bioorg Med Chem 16: 1992-2010 (2008)
Article DOI: 10.1016/j.bmc.2007.10.094
BindingDB Entry DOI: 10.7270/Q29W0GBN |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50324611

(4-[(4'-Hydroxybiphenyl-4-yl)methyl]pyridine | CHEM...)Show InChI InChI=1S/C18H15NO2/c20-17-7-5-14(6-8-17)13-1-3-15(4-2-13)18(21)16-9-11-19-12-10-16/h1-12,18,20-21H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50324611

(4-[(4'-Hydroxybiphenyl-4-yl)methyl]pyridine | CHEM...)Show InChI InChI=1S/C18H15NO2/c20-17-7-5-14(6-8-17)13-1-3-15(4-2-13)18(21)16-9-11-19-12-10-16/h1-12,18,20-21H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50322789

(4'-(pyridin-4-ylmethyl)biphenyl-3-ol | 4-[(3'-Hydr...)Show InChI InChI=1S/C18H15NO/c20-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-19-11-9-15/h1-11,13,20H,12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50322789

(4'-(pyridin-4-ylmethyl)biphenyl-3-ol | 4-[(3'-Hydr...)Show InChI InChI=1S/C18H15NO/c20-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-19-11-9-15/h1-11,13,20H,12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster V79MZh cells |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50371479

(CHEMBL403852)Show InChI InChI=1S/C16H15ClN2S/c1-2-15(19-9-8-18-11-19)13-5-3-12(4-6-13)14-7-10-20-16(14)17/h3-11,15H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase |
Bioorg Med Chem 16: 1992-2010 (2008)
Article DOI: 10.1016/j.bmc.2007.10.094
BindingDB Entry DOI: 10.7270/Q29W0GBN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50324617

(4-((3',4'-Difluorobiphenyl-4-yl)methyl)pyridine | ...)Show InChI InChI=1S/C18H13F2N/c19-17-6-5-16(12-18(17)20)15-3-1-13(2-4-15)11-14-7-9-21-10-8-14/h1-10,12H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50322783

(4'-(pyridin-4-ylmethyl)biphenyl-3-amine | CHEMBL11...)Show InChI InChI=1S/C18H16N2/c19-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-20-11-9-15/h1-11,13H,12,19H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster V79MZh cells |
J Med Chem 53: 5049-53 (2010)
Article DOI: 10.1021/jm100400a
BindingDB Entry DOI: 10.7270/Q2W37X9K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50322783

(4'-(pyridin-4-ylmethyl)biphenyl-3-amine | CHEMBL11...)Show InChI InChI=1S/C18H16N2/c19-18-3-1-2-17(13-18)16-6-4-14(5-7-16)12-15-8-10-20-11-9-15/h1-11,13H,12,19H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM25441
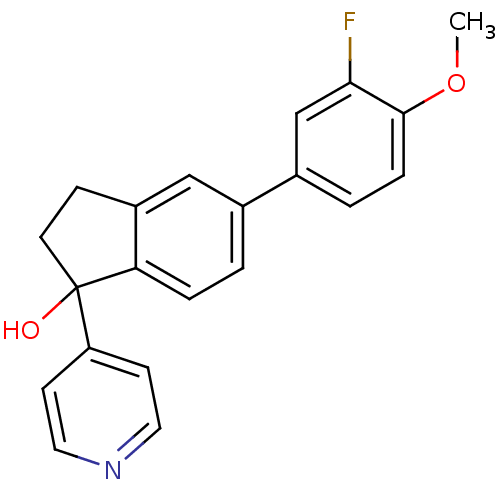
(5-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-2,3-...)Show InChI InChI=1S/C21H18FNO2/c1-25-20-5-3-15(13-19(20)22)14-2-4-18-16(12-14)6-9-21(18,24)17-7-10-23-11-8-17/h2-5,7-8,10-13,24H,6,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
| Assay Description
The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50324614

(CHEMBL1215730 | N-(4'-Isonicotinoylbiphenyl-3-yl)a...)Show InChI InChI=1S/C20H18N2O/c1-15(23)22-20-4-2-3-19(14-20)18-7-5-16(6-8-18)13-17-9-11-21-12-10-17/h2-12,14H,13H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 307 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25450

(4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen-2-yl]ben...)Show SMILES Oc1ccc(cc1O)-c1ccc2C(=CCCc2c1)c1ccncc1 |c:13| Show InChI InChI=1S/C21H17NO2/c23-20-7-5-16(13-21(20)24)15-4-6-19-17(12-15)2-1-3-18(19)14-8-10-22-11-9-14/h3-13,23-24H,1-2H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 307 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University
| Assay Description
The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM25449
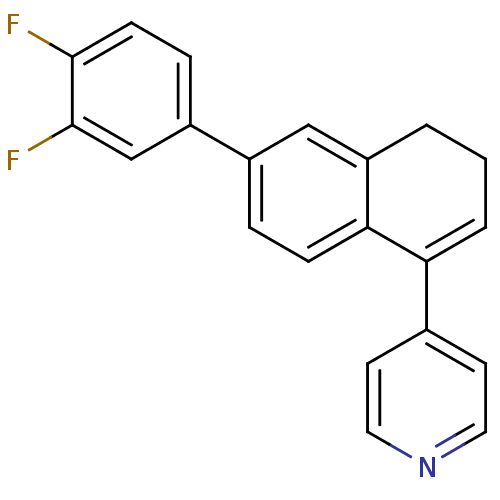
(4-[6-(3,4-difluorophenyl)-3,4-dihydronaphthalen-1-...)Show SMILES Fc1ccc(cc1F)-c1ccc2C(=CCCc2c1)c1ccncc1 |c:13| Show InChI InChI=1S/C21H15F2N/c22-20-7-5-16(13-21(20)23)15-4-6-19-17(12-15)2-1-3-18(19)14-8-10-24-11-9-14/h3-13H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
| Assay Description
The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... |
J Med Chem 51: 5009-18 (2008)
Article DOI: 10.1021/jm800355c
BindingDB Entry DOI: 10.7270/Q2TB156N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50324623
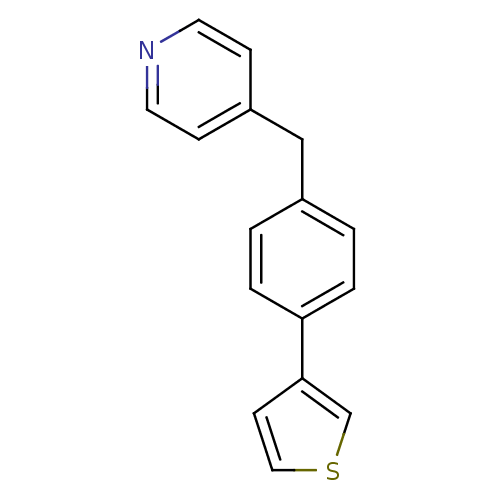
(4-(4-(thiophen-3-yl)benzyl)pyridine | CHEMBL121473...)Show InChI InChI=1S/C16H13NS/c1-3-15(16-7-10-18-12-16)4-2-13(1)11-14-5-8-17-9-6-14/h1-10,12H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in hamster fibroblast |
J Med Chem 53: 5749-58 (2010)
Article DOI: 10.1021/jm100317b
BindingDB Entry DOI: 10.7270/Q24B31J1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data