

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | -50.4 | 13 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178095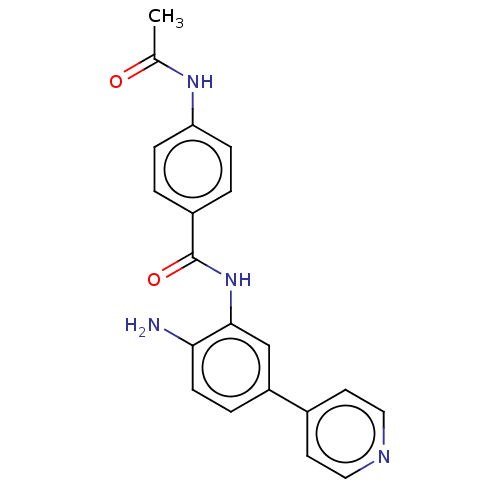 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50037187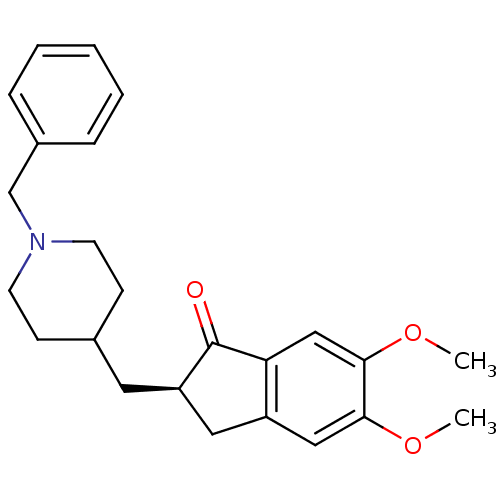 ((2R)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Binding affinity tested against acetylcholinesterase in Torpedo californica | J Med Chem 47: 2839-52 (2004) Article DOI: 10.1021/jm031032a BindingDB Entry DOI: 10.7270/Q2P84CN5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM178095 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.8 | 19 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50037176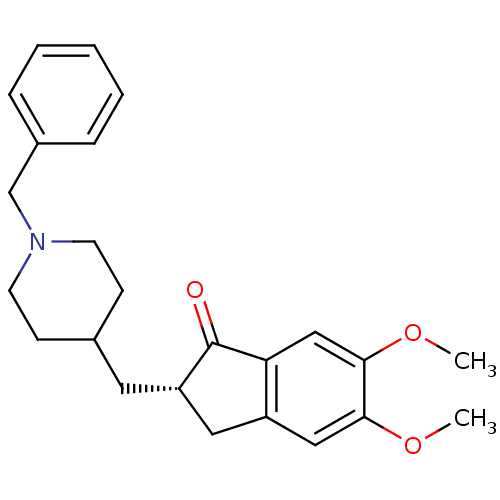 ((2S)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg Curated by ChEMBL | Assay Description Binding affinity tested against acetylcholinesterase in Torpedo californica | J Med Chem 47: 2839-52 (2004) Article DOI: 10.1021/jm031032a BindingDB Entry DOI: 10.7270/Q2P84CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.4 | 46 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM178100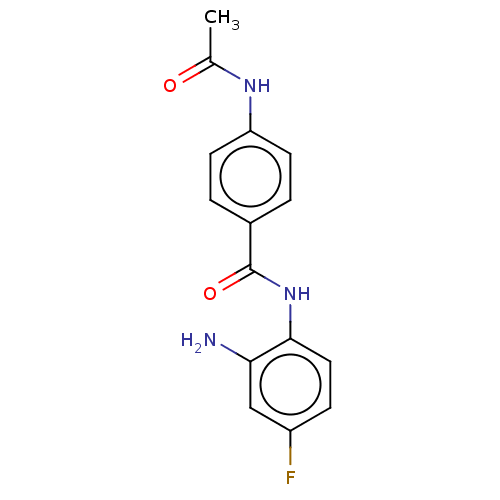 (BRD3308 | US11377423, Cmpd 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 29 | -43.0 | 64 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | -42.4 | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50064015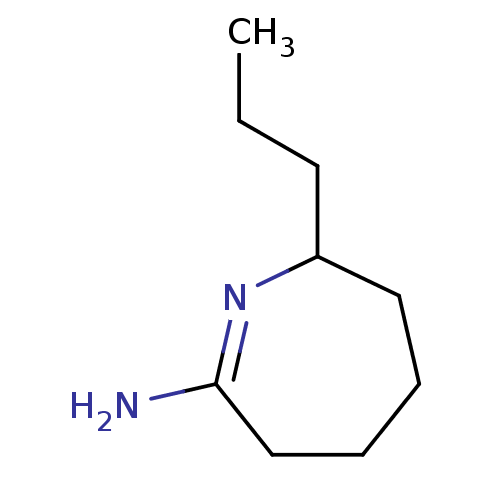 (7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-arginine binding to Inducible nitric oxide synthase | J Med Chem 41: 1361-6 (1998) Article DOI: 10.1021/jm9704715 BindingDB Entry DOI: 10.7270/Q2348M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 223 | -38.0 | 147 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | -36.0 | 398 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-10 (Rattus norvegicus) | BDBM50366779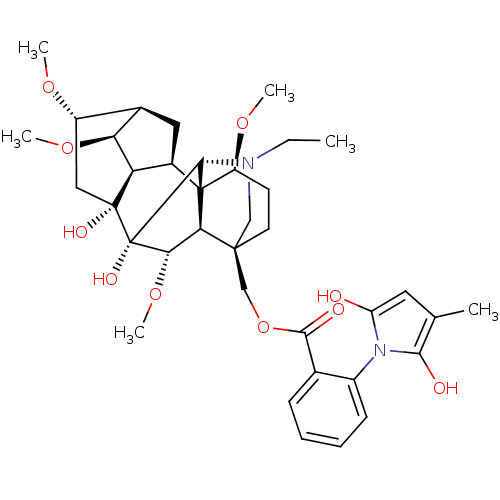 (METHYLLYCACONITINE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Binding affinity towards alpha3-beta4 neuronal nicotinic acetylcholine receptor | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178100 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | -30.2 | 1.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM178100 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | -29.7 | 1.15E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM178095 (BRD2492) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | -27.7 | 2.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50035297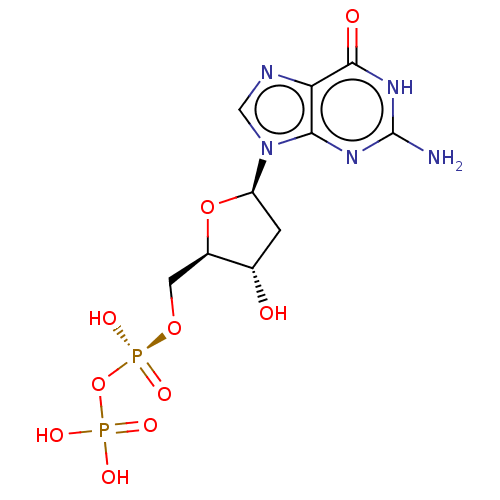 (CHEBI:28862 | CHEMBL1232205) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 5.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM92459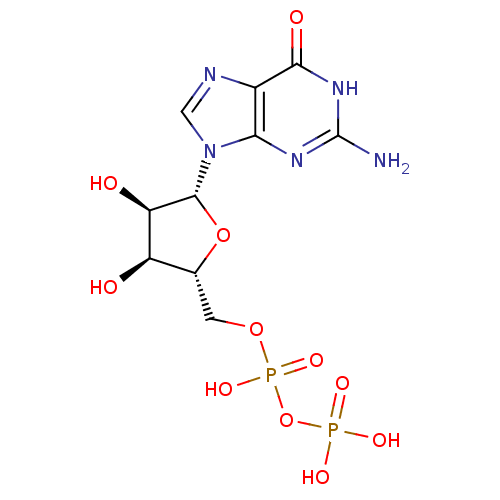 (CHEMBL384759 | GDP | Guanosine Diphosphate) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50370754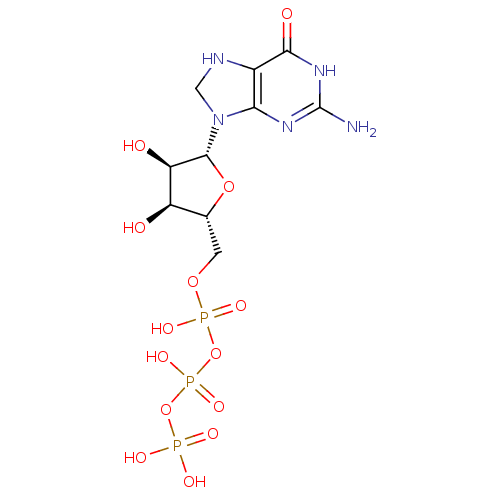 (CHEMBL252929) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50035296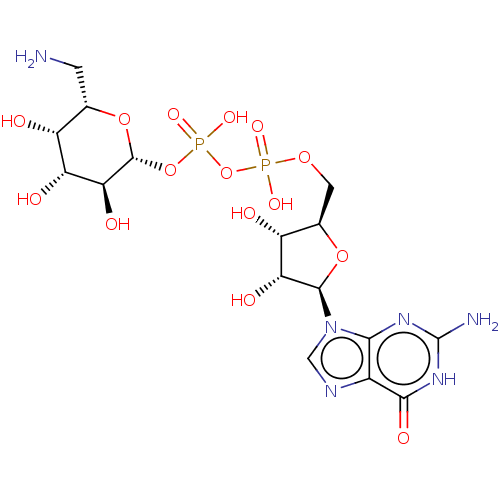 (CHEMBL3343356) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50035295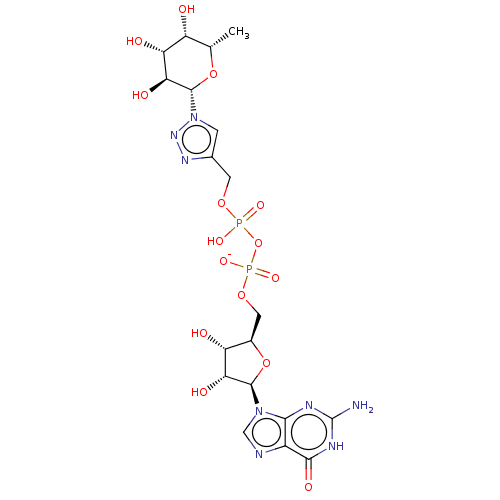 (CHEMBL3343357) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50035293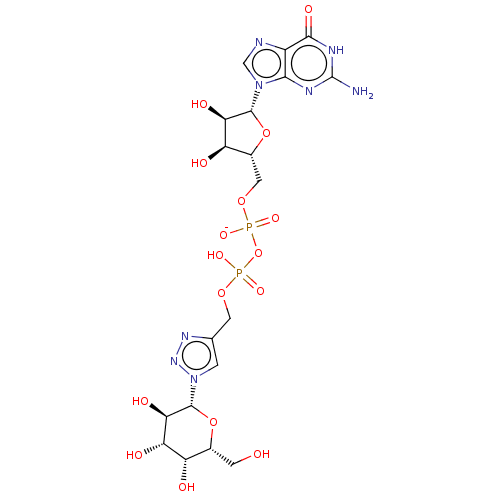 (CHEMBL3343359) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase 9 (Homo sapiens (Human)) | BDBM50035294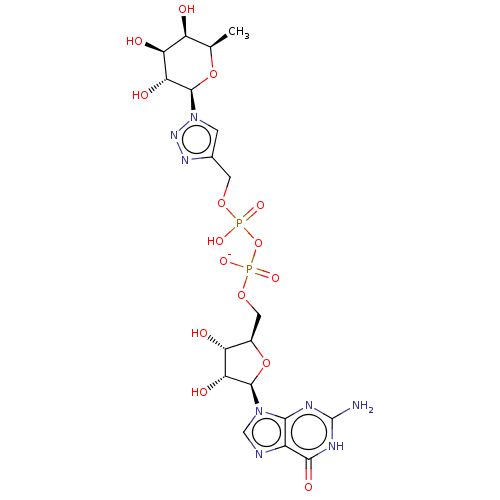 (CHEMBL3343358) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hamburg University Curated by ChEMBL | Assay Description Inhibition of human recombinant alpha1,3-fucosyltransferase 9 using GDP-[14C]-fucose preincubated for 30 mins by liquid scintillation counting | Bioorg Med Chem 22: 6430-7 (2014) Article DOI: 10.1016/j.bmc.2014.09.038 BindingDB Entry DOI: 10.7270/Q2G73GC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50385150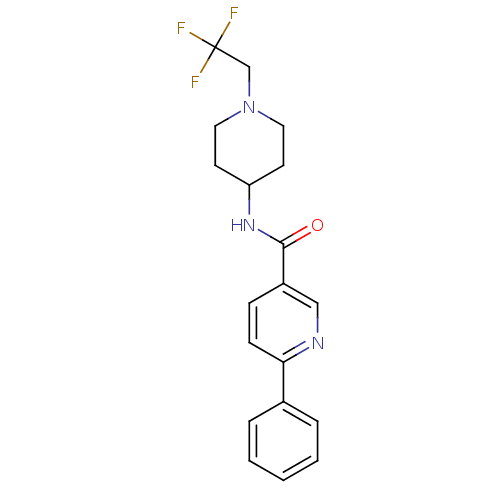 (CHEMBL2035650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HPGDS | Bioorg Med Chem Lett 22: 3795-9 (2012) Article DOI: 10.1016/j.bmcl.2012.04.004 BindingDB Entry DOI: 10.7270/Q28C9X82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509092 (S-(1-{2-[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.271 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509099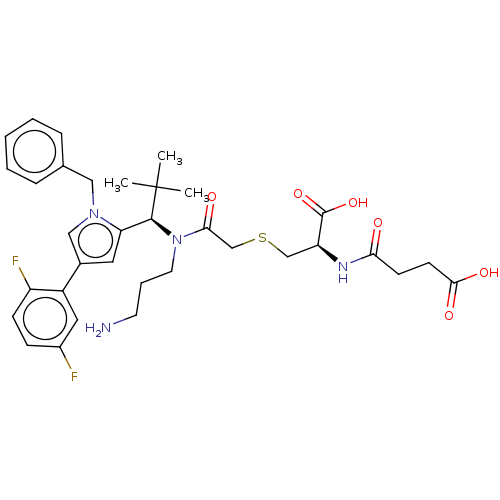 (4-{[(1R)-2-({2-[(3-Aminopropyl) {(1R)-1-[1-benzyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.446 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509093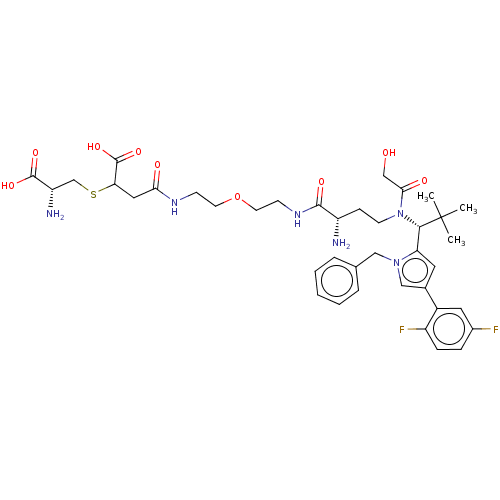 ((3R,7S)-7-Amino-17-{[(2R)-2-amino-2-carboxyethyl]s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.457 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509097 ((2R,28R)-28-Amino-2-[({2-[(3-aminopropyl){(1R)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509095 (S-(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.621 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509107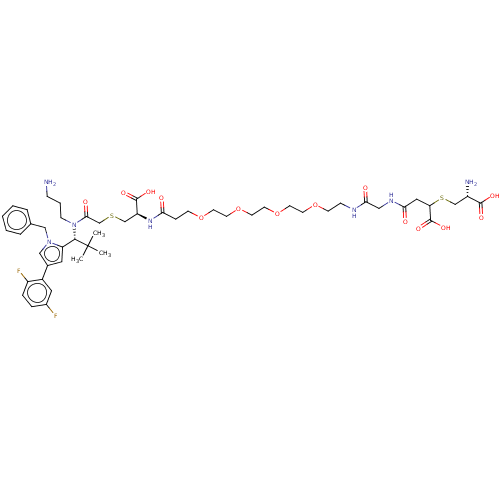 ((1R,4R,27R,33R)-1-Amino-32-(3-aminopropyl)-33-[1-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.827 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509113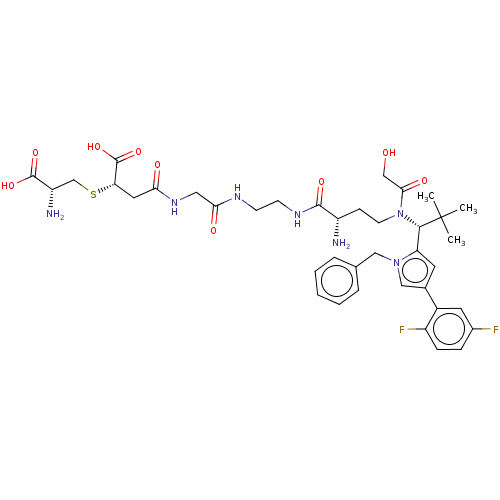 (4-[(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.944 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509102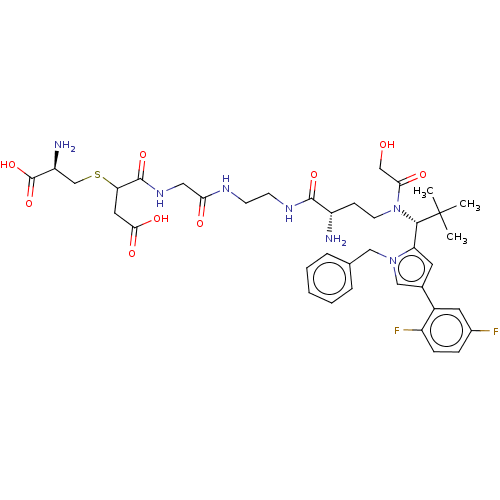 (4-[(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.964 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509096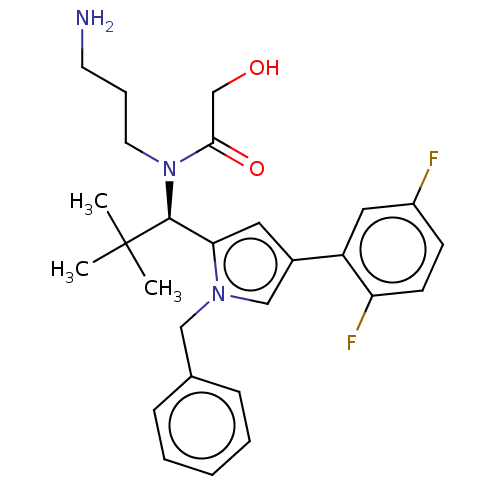 (N-(3-Aminopropyl)-N-{(1R)-1-[1-benzyl-4-(2,5-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509098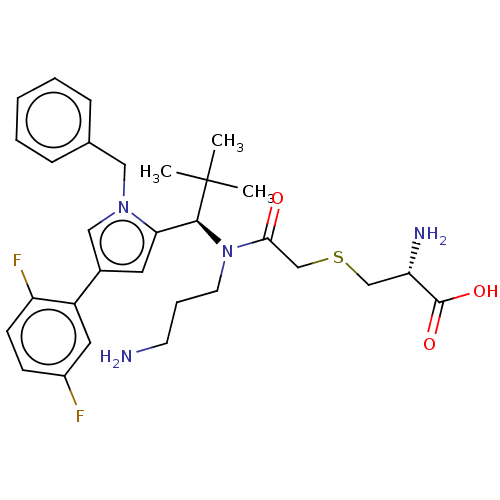 (S-{2-[(3-Aminopropyl){(1R)-1-[1-benzyl-4-(2,5-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509110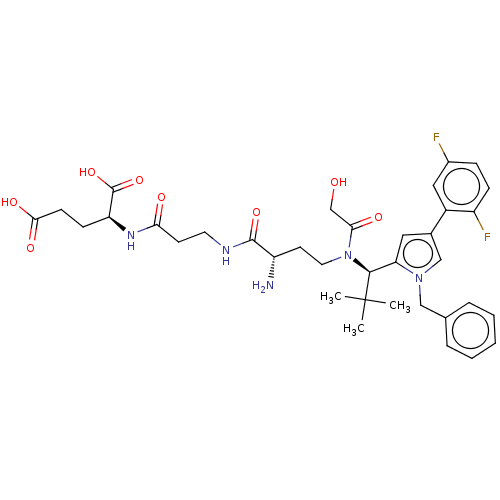 (N-{(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2,5-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509102 (4-[(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509107 ((1R,4R,27R,33R)-1-Amino-32-(3-aminopropyl)-33-[1-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509090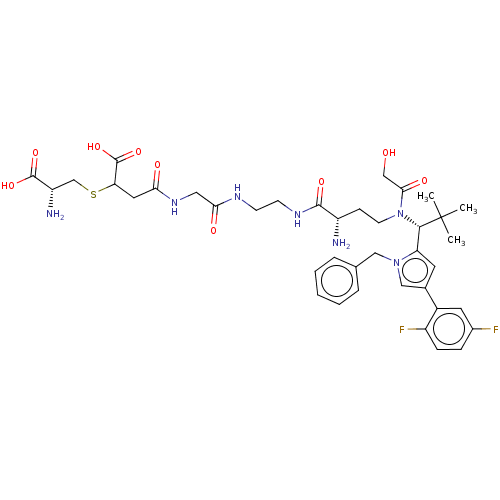 (4-[(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509091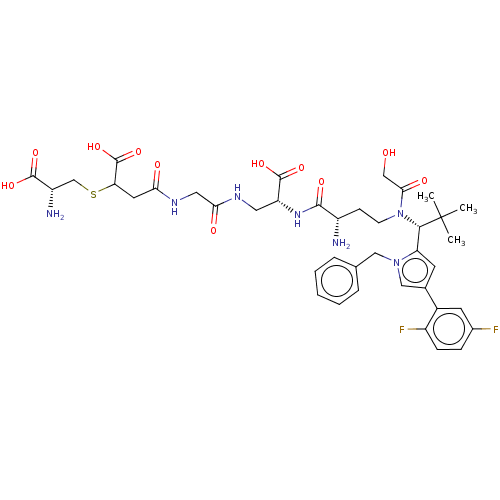 (4-[(2-{[(2R)-2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509094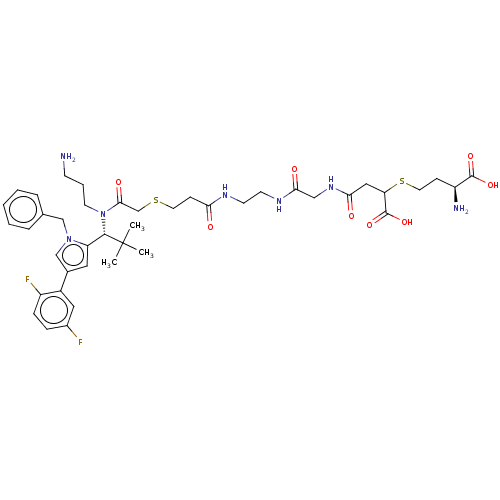 (2-{[(2R)-2-Amino-2-carboxyethyl]sulphanyl}-4-({(14...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM111911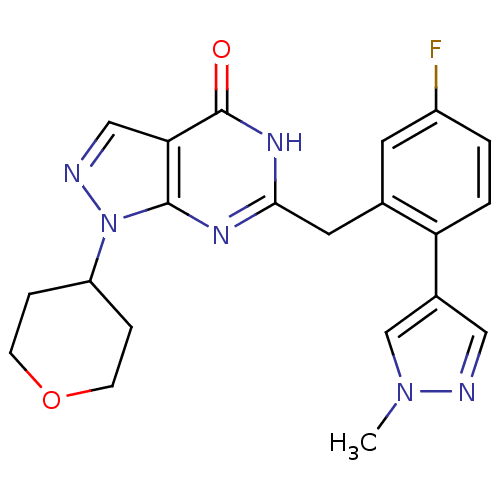 (US8623901, 239) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 0 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The PDE9A2 enzymatic activity assay was run as scintillation proximity assay (SPA), in general according to the protocol of the manufacturer (GE Heal... | US Patent US8623901 (2014) BindingDB Entry DOI: 10.7270/Q2Z036TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509089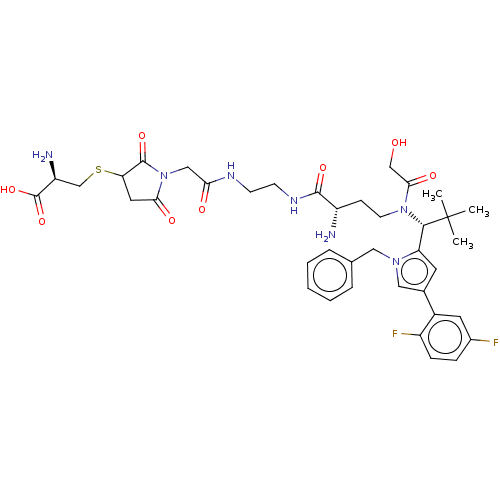 (S-[1-(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.01 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509102 (4-[(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM509090 (4-[(2-{[2-({(2S)-2-Amino-4-[{(1R)-1-[1-benzyl-4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The motor domain of the human kinesin spindle protein KSP/Eg5 (tebu-bio/Cytoskeleton Inc, No. 027EG01-XL) was incubated in a concentration of 10 nM w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N30139 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1062 total ) | Next | Last >> |