 Found 9478 hits with Last Name = 'tan' and Initial = 'c'
Found 9478 hits with Last Name = 'tan' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156455
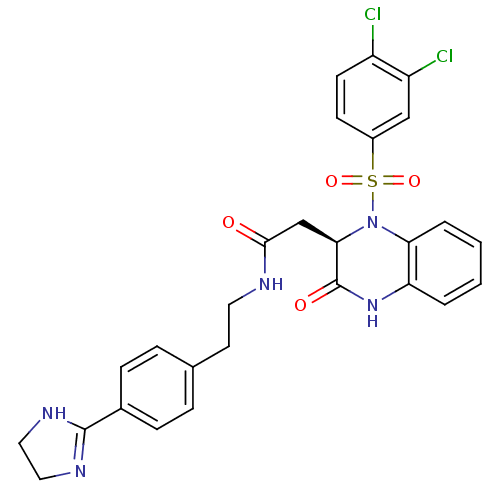
((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)C2=NCCN2)C(=O)Nc2ccccc12 |r,t:27| Show InChI InChI=1S/C27H25Cl2N5O4S/c28-20-10-9-19(15-21(20)29)39(37,38)34-23-4-2-1-3-22(23)33-27(36)24(34)16-25(35)30-12-11-17-5-7-18(8-6-17)26-31-13-14-32-26/h1-10,15,24H,11-14,16H2,(H,30,35)(H,31,32)(H,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human BK1 receptor E273 mutant |
Bioorg Med Chem Lett 16: 2791-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.112
BindingDB Entry DOI: 10.7270/Q28915GX |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50273370
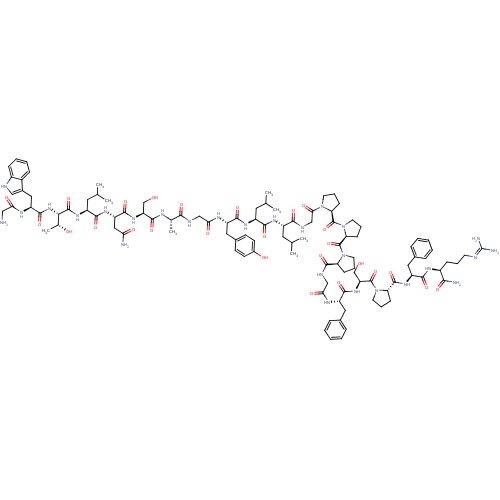
(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
FEBS Lett 405: 285-90 (1997)
Article DOI: 10.1016/s0014-5793(97)00196-8
BindingDB Entry DOI: 10.7270/Q2VH5MBC |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156455

((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)C2=NCCN2)C(=O)Nc2ccccc12 |r,t:27| Show InChI InChI=1S/C27H25Cl2N5O4S/c28-20-10-9-19(15-21(20)29)39(37,38)34-23-4-2-1-3-22(23)33-27(36)24(34)16-25(35)30-12-11-17-5-7-18(8-6-17)26-31-13-14-32-26/h1-10,15,24H,11-14,16H2,(H,30,35)(H,31,32)(H,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human BK1 receptor |
Bioorg Med Chem Lett 16: 2791-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.112
BindingDB Entry DOI: 10.7270/Q28915GX |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156455

((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)C2=NCCN2)C(=O)Nc2ccccc12 |r,t:27| Show InChI InChI=1S/C27H25Cl2N5O4S/c28-20-10-9-19(15-21(20)29)39(37,38)34-23-4-2-1-3-22(23)33-27(36)24(34)16-25(35)30-12-11-17-5-7-18(8-6-17)26-31-13-14-32-26/h1-10,15,24H,11-14,16H2,(H,30,35)(H,31,32)(H,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human BK1 receptor N298 mutant |
Bioorg Med Chem Lett 16: 2791-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.112
BindingDB Entry DOI: 10.7270/Q28915GX |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50184183
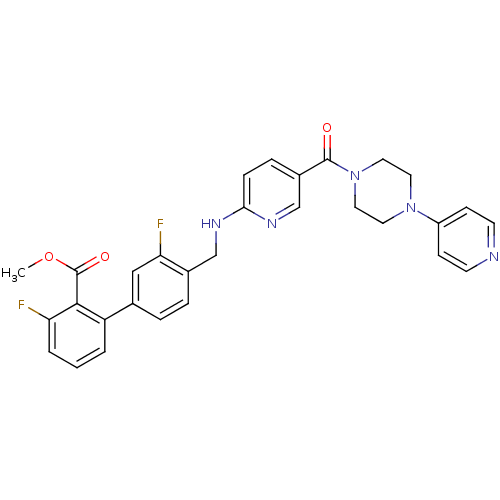
(3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(CNc2ccc(cn2)C(=O)N2CCN(CC2)c2ccncc2)c(F)c1 Show InChI InChI=1S/C30H27F2N5O3/c1-40-30(39)28-24(3-2-4-25(28)31)20-5-6-21(26(32)17-20)18-34-27-8-7-22(19-35-27)29(38)37-15-13-36(14-16-37)23-9-11-33-12-10-23/h2-12,17,19H,13-16,18H2,1H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human BK1 receptor |
Bioorg Med Chem Lett 16: 2791-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.112
BindingDB Entry DOI: 10.7270/Q28915GX |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156455

((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)C2=NCCN2)C(=O)Nc2ccccc12 |r,t:27| Show InChI InChI=1S/C27H25Cl2N5O4S/c28-20-10-9-19(15-21(20)29)39(37,38)34-23-4-2-1-3-22(23)33-27(36)24(34)16-25(35)30-12-11-17-5-7-18(8-6-17)26-31-13-14-32-26/h1-10,15,24H,11-14,16H2,(H,30,35)(H,31,32)(H,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human BK1 receptor D291 mutant |
Bioorg Med Chem Lett 16: 2791-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.112
BindingDB Entry DOI: 10.7270/Q28915GX |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50184183

(3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(CNc2ccc(cn2)C(=O)N2CCN(CC2)c2ccncc2)c(F)c1 Show InChI InChI=1S/C30H27F2N5O3/c1-40-30(39)28-24(3-2-4-25(28)31)20-5-6-21(26(32)17-20)18-34-27-8-7-22(19-35-27)29(38)37-15-13-36(14-16-37)23-9-11-33-12-10-23/h2-12,17,19H,13-16,18H2,1H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human BK1 receptor E273 mutant |
Bioorg Med Chem Lett 16: 2791-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.112
BindingDB Entry DOI: 10.7270/Q28915GX |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM85070
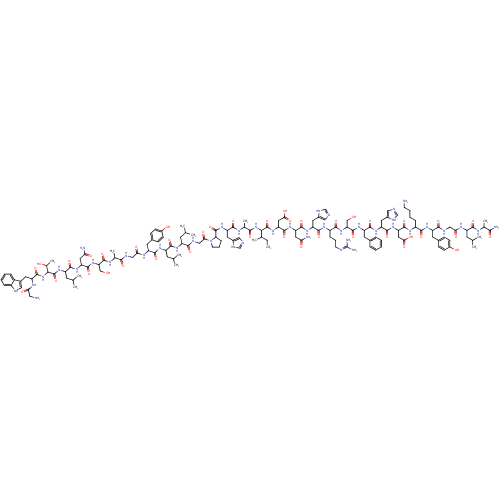
(Galanin, Porcine)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(C)C(N)=O |(22.15,12.84,;20.71,12.32,;20.44,10.8,;21.61,9.81,;18.99,10.28,;18.72,8.76,;19.9,7.77,;21.34,8.3,;19.63,6.25,;18.18,5.73,;20.8,5.26,;20.53,3.75,;19.09,3.22,;21.71,2.75,;21.44,1.24,;19.99,.71,;18.72,1.58,;17.5,.63,;18.03,-.82,;19.57,-.77,;23.16,3.28,;24.34,2.29,;24.07,.77,;25.79,2.81,;26.21,4.29,;27.75,4.34,;28.28,2.89,;27.06,1.95,;27.11,.41,;25.81,-.41,;28.47,-.32,;28.52,-1.86,;29.88,-2.58,;31.19,-1.77,;29.93,-4.12,;31.29,-4.85,;32.6,-4.03,;33.96,-4.76,;32.55,-2.49,;28.62,-4.93,;28.68,-6.47,;30.2,-6.69,;27.73,-7.69,;26.22,-8,;25.19,-6.86,;23.68,-7.17,;25.67,-5.39,;28.3,-9.11,;27.35,-10.33,;25.82,-10.11,;27.92,-11.76,;26.97,-12.97,;25.45,-12.75,;24.87,-11.32,;23.35,-11.1,;22.4,-12.32,;20.88,-12.1,;22.97,-13.75,;24.5,-13.96,;29.45,-11.97,;30.02,-13.4,;29.07,-14.61,;31.55,-13.62,;32.12,-15.05,;33.65,-15.26,;34.6,-14.05,;34.22,-16.69,;33.27,-17.9,;35.75,-16.91,;36.32,-18.34,;35.37,-19.55,;37.85,-18.56,;38.8,-17.34,;38.22,-15.91,;38.42,-19.98,;39.94,-20.2,;40.89,-18.99,;40.52,-21.63,;42.04,-21.85,;42.99,-20.64,;44.52,-20.85,;42.42,-19.21,;39.57,-22.84,;40.14,-24.27,;41.65,-23.95,;39.66,-25.74,;38.36,-26.55,;37,-25.82,;35.69,-26.64,;36.95,-24.28,;40.69,-26.88,;40.21,-28.35,;38.71,-28.66,;41.24,-29.49,;40.76,-30.96,;41.79,-32.1,;43.3,-31.78,;41.31,-33.57,;39.81,-33.88,;39.33,-35.35,;40.23,-36.59,;39.33,-37.83,;37.87,-37.36,;36.54,-38.13,;35.2,-37.36,;35.2,-35.82,;36.54,-35.05,;37.87,-35.82,;42.34,-34.71,;43.85,-34.39,;44.33,-32.93,;44.88,-35.54,;46.39,-35.22,;42.75,-29.17,;43.78,-30.32,;43.23,-27.71,;17.81,11.27,;16.36,10.75,;18.08,12.79,;16.9,13.78,;15.45,13.25,;14.28,14.25,;12.83,13.72,;14.55,15.76,;17.17,15.3,;15.99,16.29,;18.62,15.82,;18.89,17.34,;17.37,17.61,;16.85,19.05,;15.33,19.32,;17.84,20.23,;20.34,17.86,;21.52,16.87,;21.33,19.04,;22.85,18.77,;23.37,17.32,;24.89,17.05,;26,18.12,;27.36,17.39,;27.09,15.88,;25.56,15.66,;23.84,19.95,;25.35,19.68,;23.31,21.39,;24.31,22.57,;25.82,22.3,;26.81,23.48,;28.33,23.21,;29.32,24.39,;30.84,24.12,;31.83,25.3,;31.36,22.67,;23.78,24.02,;22.27,24.29,;24.77,25.2,;24.25,26.65,;22.73,26.92,;22.21,28.36,;25.24,27.82,;26.76,27.55,;24.72,29.27,;25.71,30.45,;27.23,30.18,;28.22,31.36,;29.73,31.09,;30.73,32.27,;30.2,33.71,;28.69,33.98,;27.69,32.81,;25.19,31.9,;26.18,33.08,;23.67,32.17,;23.14,33.62,;24.14,34.79,;23.61,36.24,;22.13,36.67,;22.08,38.21,;23.53,38.73,;24.48,37.52,;21.63,33.89,;21.1,35.33,;20.64,32.71,;19.12,32.98,;18.6,34.43,;17.42,35.42,;17.69,36.93,;15.97,34.89,;18.13,31.8,;18.65,30.35,;16.61,32.07,;15.62,30.89,;16.14,29.44,;15.15,28.27,;15.68,26.82,;14.69,25.64,;15.21,24.19,;14.1,31.16,;13.58,32.61,;13.11,29.98,;11.6,30.25,;11.07,31.7,;9.56,31.97,;9.03,33.42,;7.52,33.69,;6.52,32.51,;5.01,32.78,;7.05,31.06,;8.56,30.79,;10.6,29.08,;9.09,29.35,;11.13,27.63,;10.14,26.45,;8.62,26.72,;8.1,28.17,;7.63,25.54,;6.11,25.81,;5.59,27.26,;4.07,27.53,;3.55,28.98,;3.08,26.35,;5.12,24.63,;3.6,24.9,;5.64,23.19,;4.65,22.01,;3.14,22.28,;5.18,20.56,;4.19,19.38,;6.69,20.29,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
FEBS Lett 405: 285-90 (1997)
Article DOI: 10.1016/s0014-5793(97)00196-8
BindingDB Entry DOI: 10.7270/Q2VH5MBC |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50444605
(CHEMBL3099899)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2cccnc2)-c2cc(Cl)cc(Cl)c2)cc1OC Show InChI InChI=1S/C26H21Cl2N3O3/c1-33-23-6-5-16(8-24(23)34-2)13-31-26(32)22-11-19(18-9-20(27)12-21(28)10-18)15-30-25(22)17-4-3-7-29-14-17/h3-12,14-15H,13H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 23: 6620-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.045
BindingDB Entry DOI: 10.7270/Q2WH2RFJ |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM85072
(Galanin, Human)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O |wU:212.220,131.139,139.152,71.79,166.175,182.187,54.57,211.217,20.25,37.39,112.114,97.99,155.160,wD:202.210,102.111,123.131,93.95,81.87,174.183,160.167,60.68,186.203,4.4,12.16,43.51,29.33,218.224,(13.66,18.44,;13.66,16.9,;12.32,16.13,;14.99,16.13,;14.99,14.59,;16.32,13.82,;17.86,14.59,;17.86,16.13,;19.2,13.82,;20.53,14.59,;22.07,13.82,;22.07,12.28,;23.4,14.59,;23.4,16.13,;22.07,16.9,;22.07,18.44,;20.74,16.13,;24.74,13.82,;26.28,14.59,;26.28,16.13,;27.61,13.82,;27.61,12.28,;28.95,11.51,;28.95,9.97,;30.28,9.2,;31.61,9.97,;28.95,14.59,;30.49,13.82,;30.49,12.28,;31.82,14.59,;31.82,16.13,;33.15,16.9,;33.15,18.44,;34.49,16.13,;33.15,13.82,;32.43,3.03,;32.43,1.49,;31.1,3.8,;31.1,5.34,;32.43,6.11,;29.76,3.03,;28.22,3.8,;28.22,5.34,;26.89,3.03,;26.89,1.49,;28.22,.72,;28.22,-.82,;29.56,-1.59,;30.89,-.82,;30.89,.72,;29.56,1.49,;25.56,3.8,;24.02,3.03,;24.02,1.49,;22.68,3.8,;22.68,5.34,;24.02,6.11,;21.35,3.03,;19.81,3.8,;19.81,5.34,;18.47,3.03,;18.47,1.49,;19.81,.72,;19.81,-.82,;21.14,-1.59,;22.48,-.82,;23.81,-1.59,;22.48,.72,;17.14,3.8,;15.6,3.03,;15.6,1.49,;14.27,3.8,;14.27,5.34,;15.6,6.11,;17.01,5.49,;18.04,6.63,;17.27,7.97,;15.76,7.65,;12.93,3.03,;11.39,3.8,;11.39,5.34,;10.06,3.03,;10.06,1.49,;11.39,.72,;11.39,-.82,;12.73,1.49,;8.73,3.8,;7.19,3.03,;7.19,1.49,;5.85,3.8,;4.52,3.03,;5.24,-7.75,;5.24,-9.29,;6.57,-6.98,;7.91,-7.75,;9.45,-6.98,;9.45,-5.44,;10.78,-7.75,;10.78,-9.29,;12.12,-6.98,;13.66,-7.75,;13.66,-9.29,;14.99,-6.98,;14.99,-5.44,;13.66,-4.67,;12.25,-5.29,;11.22,-4.15,;11.99,-2.81,;13.49,-3.13,;16.32,-7.75,;17.66,-6.98,;17.66,-5.44,;18.99,-7.75,;19.15,-9.28,;20.66,-9.6,;21.43,-8.26,;20.4,-7.12,;20.72,-5.61,;19.57,-4.58,;22.18,-5.14,;23.33,-6.17,;24.79,-5.69,;25.11,-4.19,;25.94,-6.72,;27.4,-6.25,;27.72,-4.74,;29.19,-4.26,;26.58,-3.71,;25.62,-8.23,;26.76,-9.26,;28.22,-8.78,;26.44,-10.77,;24.98,-11.24,;24.65,-12.75,;23.19,-13.22,;25.8,-13.78,;27.58,-11.8,;29.05,-11.32,;29.37,-9.81,;30.19,-12.35,;29.87,-13.86,;31.02,-14.89,;30.7,-16.39,;31.84,-17.42,;33.31,-16.95,;34.45,-17.98,;33.63,-15.44,;32.48,-14.41,;31.66,-11.87,;31.98,-10.37,;30.83,-9.34,;33.44,-9.89,;33.76,-8.39,;35.23,-7.91,;35.55,-6.4,;36.37,-8.94,;36.05,-10.45,;37.84,-8.46,;38.98,-9.49,;38.66,-11,;40.45,-9.02,;40.77,-7.51,;42.23,-7.04,;41.59,-10.05,;43.06,-9.57,;43.38,-8.07,;44.2,-10.6,;43.88,-12.11,;45.02,-13.14,;44.7,-14.65,;46.49,-12.66,;45.66,-10.13,;45.98,-8.62,;44.84,-7.59,;47.45,-8.15,;47.77,-6.64,;46.62,-5.61,;46.94,-4.1,;45.16,-6.08,;48.59,-9.18,;50.06,-8.7,;50.38,-7.19,;51.2,-9.73,;52.67,-9.25,;53.81,-10.29,;53.49,-11.79,;55.28,-9.81,;55.6,-8.3,;57.06,-7.83,;58.3,-8.73,;59.54,-7.83,;59.07,-6.37,;59.85,-5.04,;59.08,-3.71,;57.54,-3.7,;56.77,-5.04,;57.53,-6.37,;56.42,-10.84,;56.1,-12.35,;54.64,-12.82,;57.25,-13.38,;56.93,-14.88,;50.88,-11.24,;49.42,-11.71,;52.03,-12.27,;6.57,-5.44,;5.24,-4.67,;7.91,-4.67,;13.66,13.82,;13.66,12.28,;12.12,14.59,;10.78,13.82,;10.78,12.28,;12.12,11.51,;9.45,11.51,;9.45,14.59,;9.45,16.13,;7.91,13.82,;6.57,14.59,;6.57,16.13,;5.24,16.9,;5.24,13.82,;3.91,14.59,;5.24,12.28,)| Show InChI InChI=1S/C139H210N42O43/c1-65(2)38-84(165-121(206)86(40-67(5)6)166-123(208)88(43-75-31-33-79(188)34-32-75)161-106(193)55-151-114(199)70(11)157-131(216)97(59-182)175-127(212)95(49-104(144)191)170-122(207)87(41-68(7)8)173-136(221)112(72(13)186)180-130(215)90(159-105(192)51-141)44-76-52-150-81-27-19-18-26-80(76)81)116(201)154-58-109(196)181-37-23-30-101(181)134(219)172-91(45-77-53-147-63-155-77)120(205)158-71(12)115(200)178-111(69(9)10)135(220)153-57-108(195)162-94(48-103(143)190)126(211)168-92(46-78-54-148-64-156-78)125(210)164-83(29-22-36-149-139(145)146)119(204)174-98(60-183)132(217)167-89(42-74-24-16-15-17-25-74)124(209)176-99(61-184)133(218)171-96(50-110(197)198)128(213)163-82(28-20-21-35-140)118(203)169-93(47-102(142)189)117(202)152-56-107(194)160-85(39-66(3)4)129(214)179-113(73(14)187)137(222)177-100(62-185)138(223)224/h15-19,24-27,31-34,52-54,63-73,82-101,111-113,150,182-188H,20-23,28-30,35-51,55-62,140-141H2,1-14H3,(H2,142,189)(H2,143,190)(H2,144,191)(H,147,155)(H,148,156)(H,151,199)(H,152,202)(H,153,220)(H,154,201)(H,157,216)(H,158,205)(H,159,192)(H,160,194)(H,161,193)(H,162,195)(H,163,213)(H,164,210)(H,165,206)(H,166,208)(H,167,217)(H,168,211)(H,169,203)(H,170,207)(H,171,218)(H,172,219)(H,173,221)(H,174,204)(H,175,212)(H,176,209)(H,177,222)(H,178,200)(H,179,214)(H,180,215)(H,197,198)(H,223,224)(H4,145,146,149)/t70-,71-,72+,73+,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,111-,112-,113-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
FEBS Lett 405: 285-90 (1997)
Article DOI: 10.1016/s0014-5793(97)00196-8
BindingDB Entry DOI: 10.7270/Q2VH5MBC |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50184183

(3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(CNc2ccc(cn2)C(=O)N2CCN(CC2)c2ccncc2)c(F)c1 Show InChI InChI=1S/C30H27F2N5O3/c1-40-30(39)28-24(3-2-4-25(28)31)20-5-6-21(26(32)17-20)18-34-27-8-7-22(19-35-27)29(38)37-15-13-36(14-16-37)23-9-11-33-12-10-23/h2-12,17,19H,13-16,18H2,1H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human BK1 receptor D291 mutant |
Bioorg Med Chem Lett 16: 2791-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.112
BindingDB Entry DOI: 10.7270/Q28915GX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347380

(CHEMBL1801350)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(Br)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H20BrClFN5O2/c1-14(30-24(35)25(8-9-25)31-23(34)15-10-16(26)13-29-12-15)18-7-6-17(11-20(18)28)33-21-5-3-2-4-19(21)22(27)32-33/h2-7,10-14H,8-9H2,1H3,(H,30,35)(H,31,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50451631

(CHEMBL4212454)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(nn1C)C(C)(C)C |r| Show InChI InChI=1S/C21H28N6O2/c1-21(2,3)18-13-17(26(5)24-18)19(28)23-16(12-15-10-8-7-9-11-15)20(29)27(6)25(4)14-22/h7-11,13,16H,12H2,1-6H3,(H,23,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
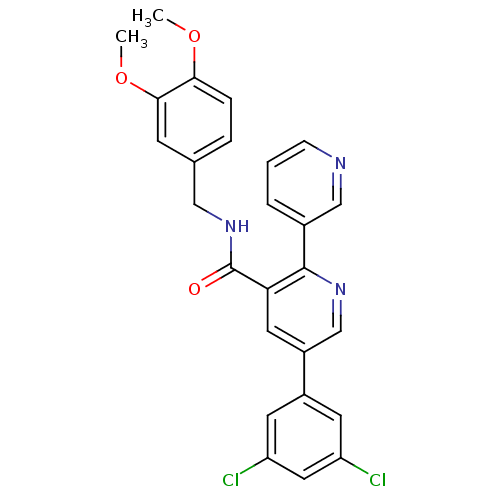
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50202421
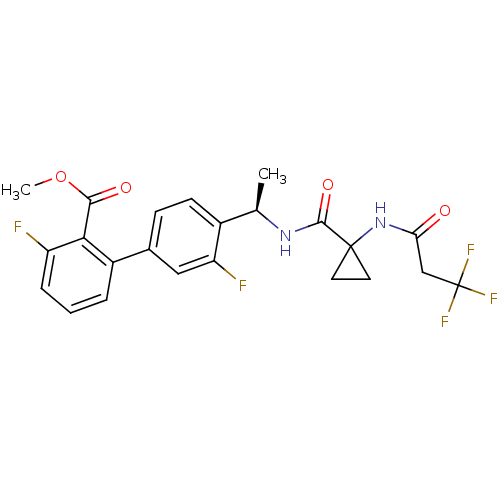
(3,3'-difluoro-4'-((R)-1-{[1-(3,3,3-trifluoro-propi...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)CC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C23H21F5N2O4/c1-12(29-21(33)22(8-9-22)30-18(31)11-23(26,27)28)14-7-6-13(10-17(14)25)15-4-3-5-16(24)19(15)20(32)34-2/h3-7,10,12H,8-9,11H2,1-2H3,(H,29,33)(H,30,31)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-Arg10 Leu9 kallidin from human bradykinin B1 receptor expressed in CHO cells |
J Med Chem 50: 272-82 (2007)
Article DOI: 10.1021/jm061094b
BindingDB Entry DOI: 10.7270/Q2X63MMQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85951
(hAc-MCH(6-16)-NH2)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6]-1-[#7]-[#6](=O)-[#6](-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6]-1=O)-[#6](-[#6])-[#6])-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O Show InChI InChI=1S/C58H97N21O13S3/c1-30(2)25-39-47(84)69-27-44(82)71-36(12-8-21-67-57(62)63)50(87)78-45(31(3)4)54(91)75-40(26-33-15-17-34(81)18-16-33)51(88)73-38(13-9-22-68-58(64)65)55(92)79-23-10-14-43(79)53(90)76-41(46(59)83)28-94-95-29-42(52(89)72-37(19-24-93-6)49(86)74-39)77-48(85)35(70-32(5)80)11-7-20-66-56(60)61/h15-18,30-31,35-43,45,81H,7-14,19-29H2,1-6H3,(H2,59,83)(H,69,84)(H,70,80)(H,71,82)(H,72,89)(H,73,88)(H,74,86)(H,75,91)(H,76,90)(H,77,85)(H,78,87)(H4,60,61,66)(H4,62,63,67)(H4,64,65,68) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Biochemistry 41: 6383-90 (2002)
Article DOI: 10.1021/bi0200514
BindingDB Entry DOI: 10.7270/Q23N21ZT |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318697
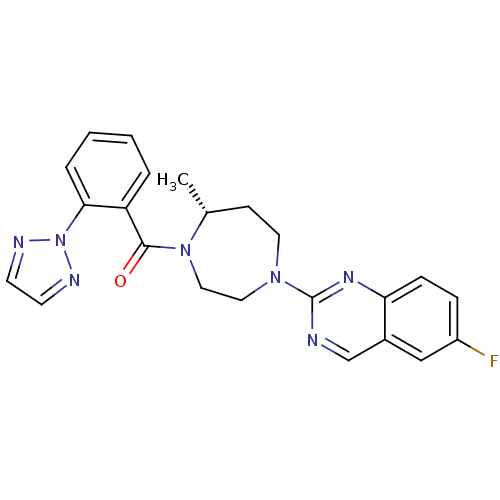
(6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C23H22FN7O/c1-16-8-11-29(23-25-15-17-14-18(24)6-7-20(17)28-23)12-13-30(16)22(32)19-4-2-3-5-21(19)31-26-9-10-27-31/h2-7,9-10,14-16H,8,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85788
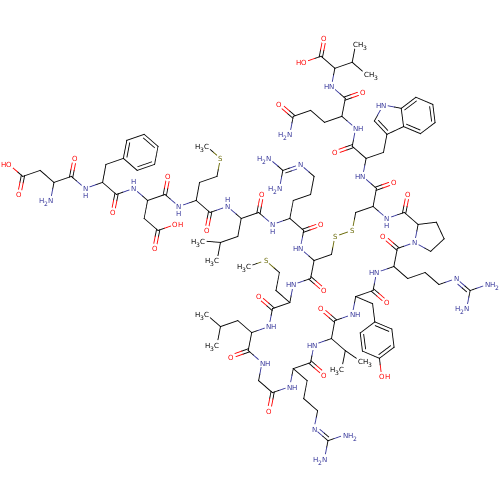
(MCH | hMCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C105H160N30O26S4/c1-53(2)42-70-86(144)118-50-80(138)119-64(24-16-36-114-103(108)109)90(148)133-83(55(5)6)100(158)130-73(45-58-28-30-60(136)31-29-58)93(151)124-69(26-18-38-116-105(112)113)101(159)135-39-19-27-78(135)99(157)132-77(98(156)128-74(46-59-49-117-63-23-15-14-22-61(59)63)95(153)121-66(32-33-79(107)137)91(149)134-84(56(7)8)102(160)161)52-165-164-51-76(97(155)123-68(35-41-163-10)88(146)126-70)131-87(145)65(25-17-37-115-104(110)111)120-92(150)71(43-54(3)4)127-89(147)67(34-40-162-9)122-96(154)75(48-82(141)142)129-94(152)72(44-57-20-12-11-13-21-57)125-85(143)62(106)47-81(139)140/h11-15,20-23,28-31,49,53-56,62,64-78,83-84,117,136H,16-19,24-27,32-48,50-52,106H2,1-10H3,(H2,107,137)(H,118,144)(H,119,138)(H,120,150)(H,121,153)(H,122,154)(H,123,155)(H,124,151)(H,125,143)(H,126,146)(H,127,147)(H,128,156)(H,129,152)(H,130,158)(H,131,145)(H,132,157)(H,133,148)(H,134,149)(H,139,140)(H,141,142)(H,160,161)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Biochemistry 41: 6383-90 (2002)
Article DOI: 10.1021/bi0200514
BindingDB Entry DOI: 10.7270/Q23N21ZT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347351
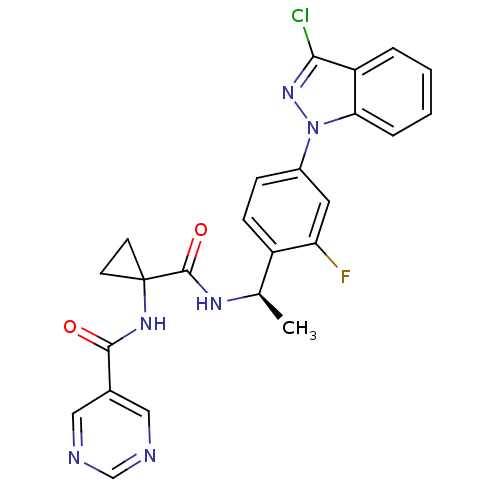
(CHEMBL1801352)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncnc1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C24H20ClFN6O2/c1-14(29-23(34)24(8-9-24)30-22(33)15-11-27-13-28-12-15)17-7-6-16(10-19(17)26)32-20-5-3-2-4-18(20)21(25)31-32/h2-7,10-14H,8-9H2,1H3,(H,29,34)(H,30,33)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347379
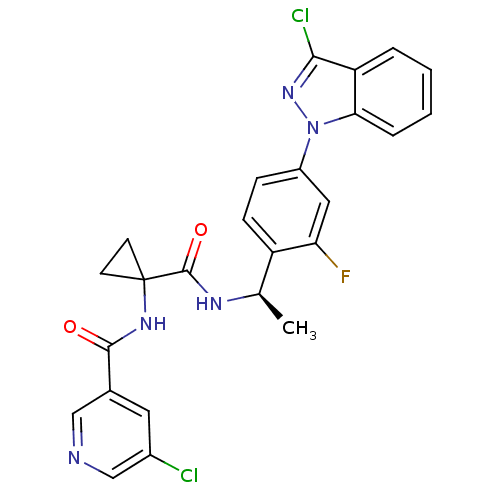
(CHEMBL1801349)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(Cl)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H20Cl2FN5O2/c1-14(30-24(35)25(8-9-25)31-23(34)15-10-16(26)13-29-12-15)18-7-6-17(11-20(18)28)33-21-5-3-2-4-19(21)22(27)32-33/h2-7,10-14H,8-9H2,1H3,(H,30,35)(H,31,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
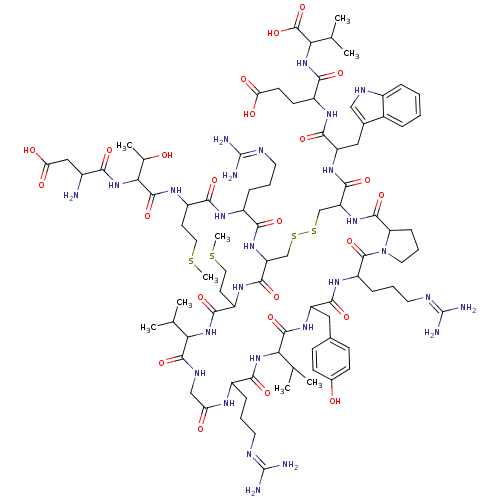
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50068071
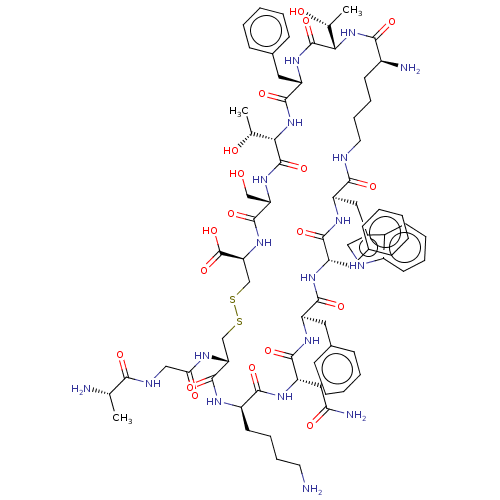
(CHEMBL2370908 | SRIF)Show SMILES [H][C@]1(NC(=O)[C@@H](N)CCCCNC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@]([H])(NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(78)64(100)83-37-61(99)84-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-65(101)49(79)26-16-18-30-81-66(102)55(34-47-36-82-50-27-14-13-25-48(47)50)88-69(105)53(32-45-21-9-5-10-22-45)86-68(104)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-67(103)51(85-73(58)109)28-15-17-29-77/h4-14,19-25,27,36,41-43,49,51-59,62-63,82,95-97H,15-18,26,28-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,81,102)(H,83,100)(H,84,99)(H,85,109)(H,86,104)(H,87,106)(H,88,105)(H,89,103)(H,90,110)(H,91,111)(H,92,108)(H,93,101)(H,94,107)(H,112,113)/t41-,42+,43+,49-,51+,52+,53-,54-,55+,56-,57-,58+,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at somatostatin 2 receptor in transfected HEK 293 cell using [125 I]Tyr11-SRIF-14 as radioligand |
J Med Chem 41: 4693-705 (1998)
Article DOI: 10.1021/jm980118e
BindingDB Entry DOI: 10.7270/Q2TT4RNG |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50371651

(CHEMBL410281)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cc(Cl)no1)c1ncc(cc1F)-c1cc(Cl)cc(Cl)c1OCC(F)F Show InChI InChI=1S/C23H18Cl3F3N4O4/c1-10(31-22(35)23(2-3-23)32-21(34)16-7-17(26)33-37-16)19-15(27)4-11(8-30-19)13-5-12(24)6-14(25)20(13)36-9-18(28)29/h4-8,10,18H,2-3,9H2,1H3,(H,31,35)(H,32,34)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor expressed in rat CNS |
Bioorg Med Chem Lett 18: 682-7 (2008)
BindingDB Entry DOI: 10.7270/Q29024NB |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50371643

(CHEMBL272278)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)C(F)(F)F)c1ccc(cc1F)-c1cc(Cl)cc(Cl)c1OCC(F)F Show InChI InChI=1S/C22H18Cl2F6N2O3/c1-10(31-19(33)21(4-5-21)32-20(34)22(28,29)30)13-3-2-11(6-16(13)25)14-7-12(23)8-15(24)18(14)35-9-17(26)27/h2-3,6-8,10,17H,4-5,9H2,1H3,(H,31,33)(H,32,34)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor expressed in rat CNS |
Bioorg Med Chem Lett 18: 682-7 (2008)
BindingDB Entry DOI: 10.7270/Q29024NB |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318698

(6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1cnc2cc(F)c(F)cc2n1 |r| Show InChI InChI=1S/C23H21F2N7O/c1-15-6-9-30(22-14-26-19-12-17(24)18(25)13-20(19)29-22)10-11-31(15)23(33)16-4-2-3-5-21(16)32-27-7-8-28-32/h2-5,7-8,12-15H,6,9-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50243173
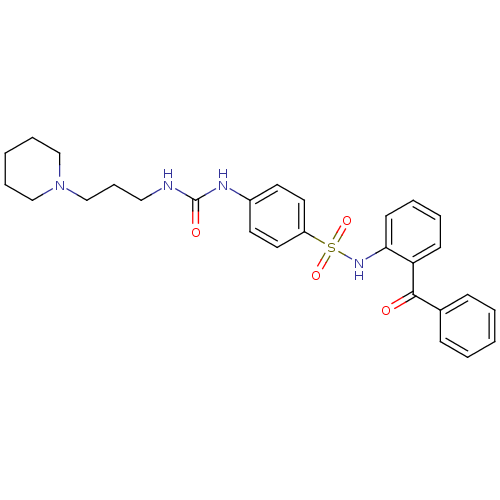
(CHEMBL487445 | N-(2-Benzoyl-phenyl)-4-[3-(3-piperi...)Show SMILES O=C(NCCCN1CCCCC1)Nc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C28H32N4O4S/c33-27(22-10-3-1-4-11-22)25-12-5-6-13-26(25)31-37(35,36)24-16-14-23(15-17-24)30-28(34)29-18-9-21-32-19-7-2-8-20-32/h1,3-6,10-17,31H,2,7-9,18-21H2,(H2,29,30,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor |
J Med Chem 51: 3946-52 (2008)
Article DOI: 10.1021/jm800199h
BindingDB Entry DOI: 10.7270/Q2JH3N33 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549366

(CHEMBL4755248)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NS(=O)(=O)c1cccc(Br)c1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50273367
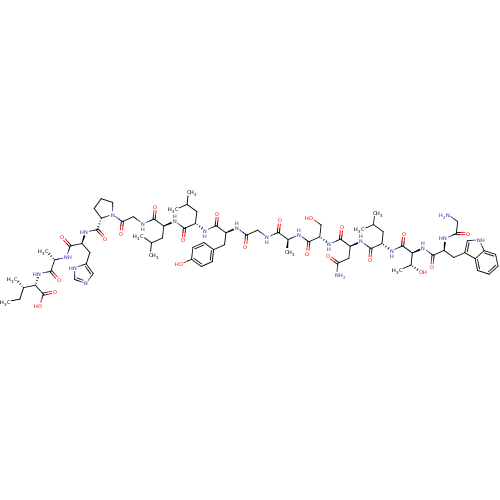
((2S,3S)-2-((S)-2-((S)-2-((S)-1-(2-((S)-2-((S)-2-((...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C78H116N20O21/c1-12-41(8)64(78(118)119)96-67(107)43(10)87-69(109)56(29-47-33-81-37-85-47)93-76(116)59-18-15-23-98(59)63(105)35-84-68(108)51(24-38(2)3)90-70(110)52(25-39(4)5)91-72(112)54(27-45-19-21-48(101)22-20-45)89-62(104)34-83-66(106)42(9)86-75(115)58(36-99)95-73(113)57(30-60(80)102)92-71(111)53(26-40(6)7)94-77(117)65(44(11)100)97-74(114)55(88-61(103)31-79)28-46-32-82-50-17-14-13-16-49(46)50/h13-14,16-17,19-22,32-33,37-44,51-59,64-65,82,99-101H,12,15,18,23-31,34-36,79H2,1-11H3,(H2,80,102)(H,81,85)(H,83,106)(H,84,108)(H,86,115)(H,87,109)(H,88,103)(H,89,104)(H,90,110)(H,91,112)(H,92,111)(H,93,116)(H,94,117)(H,95,113)(H,96,107)(H,97,114)(H,118,119)/t41-,42-,43-,44+,51-,52-,53-,54-,55-,56-,57-,58-,59-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
FEBS Lett 405: 285-90 (1997)
Article DOI: 10.1016/s0014-5793(97)00196-8
BindingDB Entry DOI: 10.7270/Q2VH5MBC |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318699
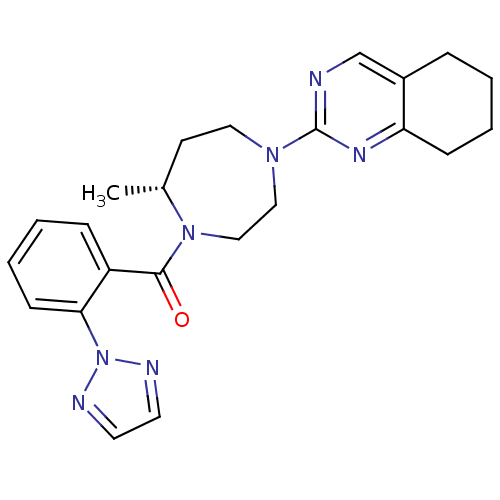
(2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2CCCCc2n1 |r| Show InChI InChI=1S/C23H27N7O/c1-17-10-13-28(23-24-16-18-6-2-4-8-20(18)27-23)14-15-29(17)22(31)19-7-3-5-9-21(19)30-25-11-12-26-30/h3,5,7,9,11-12,16-17H,2,4,6,8,10,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347370

(CHEMBL1801087)Show SMILES COc1ncc(s1)C(=O)NC1(CC1)C(=O)N[C@H](C)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C24H21ClFN5O3S/c1-13(28-22(33)24(9-10-24)29-21(32)19-12-27-23(34-2)35-19)15-8-7-14(11-17(15)26)31-18-6-4-3-5-16(18)20(25)30-31/h3-8,11-13H,9-10H2,1-2H3,(H,28,33)(H,29,32)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347376
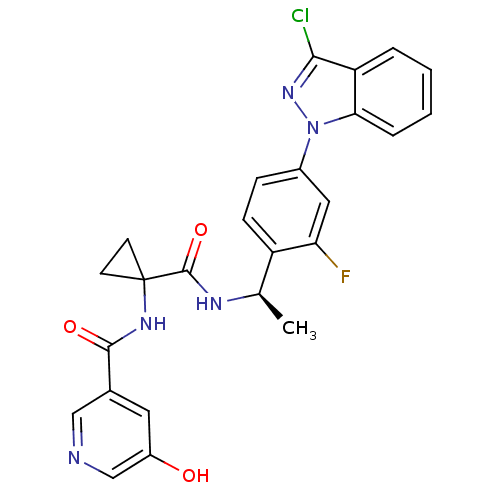
(CHEMBL1801346)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(O)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H21ClFN5O3/c1-14(29-24(35)25(8-9-25)30-23(34)15-10-17(33)13-28-12-15)18-7-6-16(11-20(18)27)32-21-5-3-2-4-19(21)22(26)31-32/h2-7,10-14,33H,8-9H2,1H3,(H,29,35)(H,30,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50068063
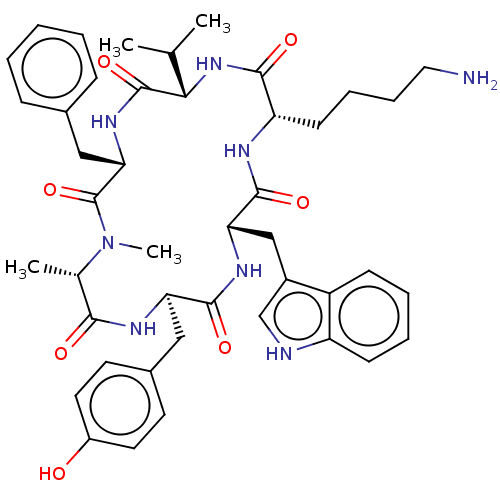
((12S,15R,18S)-9-(4-Amino-butyl)-3-benzyl-15-(4-hyd...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55)/t27-,34-,35+,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at somatostatin 2 receptor in transfected HEK 293 cell using [125 I]Tyr11-SRIF-14 as radioligand |
J Med Chem 41: 4693-705 (1998)
Article DOI: 10.1021/jm980118e
BindingDB Entry DOI: 10.7270/Q2TT4RNG |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50272772

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES C[C@@H](O)[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39-,40+,41+,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at somatostatin 2 receptor in transfected HEK 293 cell using [125 I]Tyr11-SRIF-14 as radioligand |
J Med Chem 41: 4693-705 (1998)
Article DOI: 10.1021/jm980118e
BindingDB Entry DOI: 10.7270/Q2TT4RNG |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
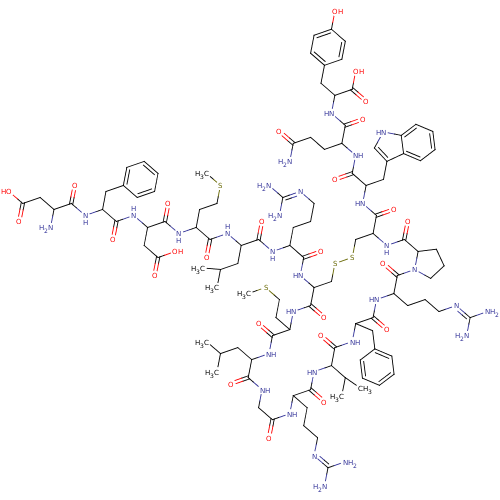
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85976
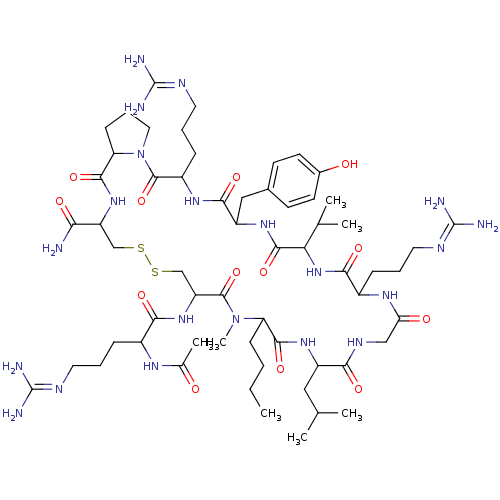
(N-Me-Nle8)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-1-[#7](-[#6])-[#6](=O)-[#6](-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6]-1=O)-[#6](-[#6])-[#6])-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O Show InChI InChI=1S/C60H101N21O13S2/c1-8-9-17-44-53(90)75-40(27-32(2)3)49(86)71-29-46(84)73-38(15-11-24-69-59(64)65)51(88)79-47(33(4)5)55(92)76-41(28-35-19-21-36(83)22-20-35)52(89)74-39(16-12-25-70-60(66)67)57(94)81-26-13-18-45(81)54(91)77-42(48(61)85)30-95-96-31-43(56(93)80(44)7)78-50(87)37(72-34(6)82)14-10-23-68-58(62)63/h19-22,32-33,37-45,47,83H,8-18,23-31H2,1-7H3,(H2,61,85)(H,71,86)(H,72,82)(H,73,84)(H,74,89)(H,75,90)(H,76,92)(H,77,91)(H,78,87)(H,79,88)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Biochemistry 41: 6383-90 (2002)
Article DOI: 10.1021/bi0200514
BindingDB Entry DOI: 10.7270/Q23N21ZT |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(MOUSE) | BDBM50091652
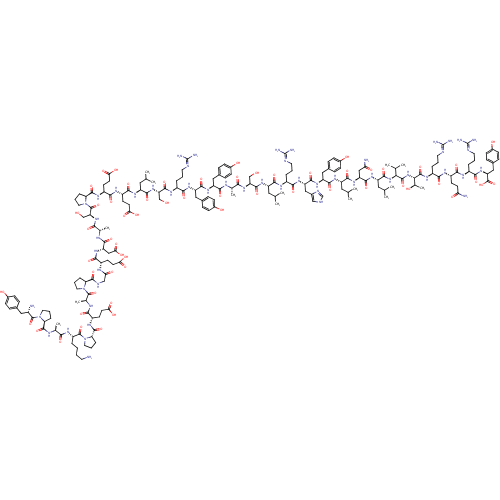
(CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C190H287N53O58/c1-92(2)74-124(166(280)216-114(27-18-66-204-187(195)196)158(272)231-131(83-107-86-203-91-209-107)171(285)230-130(81-105-41-51-111(251)52-42-105)169(283)225-125(75-93(3)4)167(281)232-132(84-143(194)254)172(286)226-127(77-95(7)8)173(287)238-150(96(9)10)180(294)239-151(101(15)247)181(295)222-117(30-21-69-207-190(201)202)156(270)218-119(55-60-142(193)253)161(275)215-116(29-20-68-206-189(199)200)159(273)234-134(186(300)301)82-106-43-53-112(252)54-44-106)227-175(289)135(88-244)235-153(267)97(11)210-164(278)128(79-103-37-47-109(249)48-38-103)229-170(284)129(80-104-39-49-110(250)50-40-104)228-157(271)115(28-19-67-205-188(197)198)217-174(288)136(89-245)236-168(282)126(76-94(5)6)224-163(277)121(58-63-147(260)261)219-162(276)122(59-64-148(262)263)221-179(293)141-34-25-73-243(141)185(299)137(90-246)237-154(268)98(12)211-165(279)133(85-149(264)265)233-160(274)118(56-61-145(256)257)214-144(255)87-208-176(290)138-31-22-70-240(138)182(296)100(14)213-155(269)120(57-62-146(258)259)220-178(292)140-33-24-72-242(140)184(298)123(26-16-17-65-191)223-152(266)99(13)212-177(291)139-32-23-71-241(139)183(297)113(192)78-102-35-45-108(248)46-36-102/h35-54,86,91-101,113-141,150-151,244-252H,16-34,55-85,87-90,191-192H2,1-15H3,(H2,193,253)(H2,194,254)(H,203,209)(H,208,290)(H,210,278)(H,211,279)(H,212,291)(H,213,269)(H,214,255)(H,215,275)(H,216,280)(H,217,288)(H,218,270)(H,219,276)(H,220,292)(H,221,293)(H,222,295)(H,223,266)(H,224,277)(H,225,283)(H,226,286)(H,227,289)(H,228,271)(H,229,284)(H,230,285)(H,231,272)(H,232,281)(H,233,274)(H,234,273)(H,235,267)(H,236,282)(H,237,268)(H,238,287)(H,239,294)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,300,301)(H4,195,196,204)(H4,197,198,205)(H4,199,200,206)(H4,201,202,207)/t97-,98-,99-,100-,101+,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,150-,151-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Biol Chem 271: 16435-8 (1996)
Article DOI: 10.1074/jbc.271.28.16435
BindingDB Entry DOI: 10.7270/Q2XK8D36 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50371333
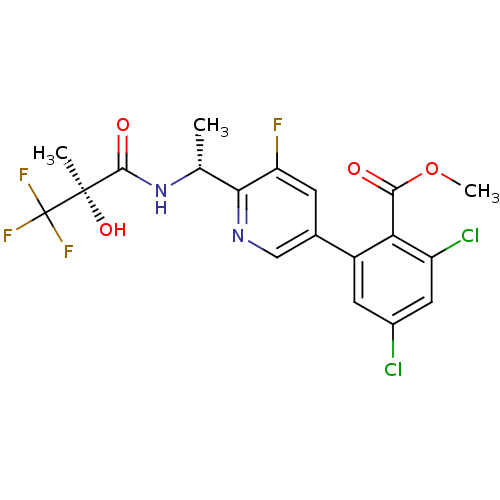
(CHEMBL256671)Show SMILES COC(=O)c1c(Cl)cc(Cl)cc1-c1cnc([C@@H](C)NC(=O)[C@@](C)(O)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C19H16Cl2F4N2O4/c1-8(27-17(29)18(2,30)19(23,24)25)15-13(22)4-9(7-26-15)11-5-10(20)6-12(21)14(11)16(28)31-3/h4-8,30H,1-3H3,(H,27,29)/t8-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradikinin B1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 716-20 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.050
BindingDB Entry DOI: 10.7270/Q2GM8849 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318695
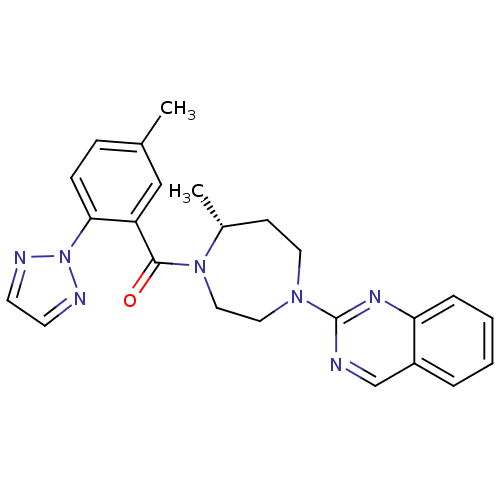
(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513934
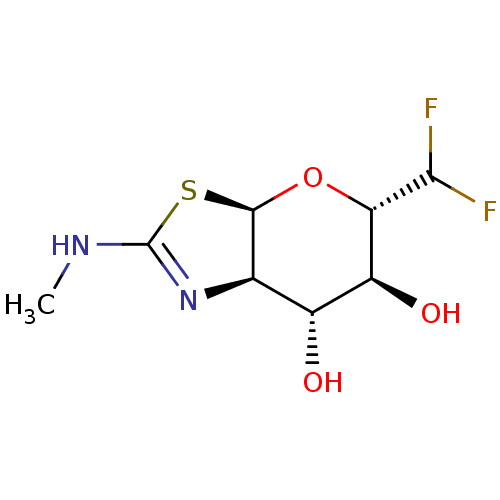
(CHEMBL4443587)Show SMILES [H][C@]12O[C@H](C(F)F)[C@@H](O)[C@H](O)[C@@]1([H])N=C(NC)S2 |r,t:14| Show InChI InChI=1S/C8H12F2N2O3S/c1-11-8-12-2-3(13)4(14)5(6(9)10)15-7(2)16-8/h2-7,13-14H,1H3,(H,11,12)/t2-,3-,4+,5+,7-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347378

(CHEMBL1801348)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cncc(F)c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C25H20ClF2N5O2/c1-14(30-24(35)25(8-9-25)31-23(34)15-10-16(27)13-29-12-15)18-7-6-17(11-20(18)28)33-21-5-3-2-4-19(21)22(26)32-33/h2-7,10-14H,8-9H2,1H3,(H,30,35)(H,31,34)/t14-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50347373
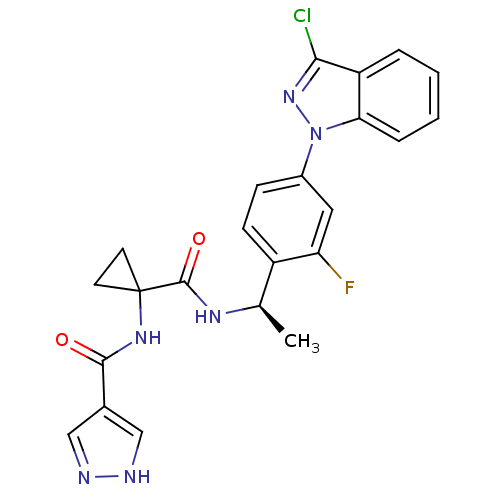
(CHEMBL1801341)Show SMILES C[C@@H](NC(=O)C1(CC1)NC(=O)c1cn[nH]c1)c1ccc(cc1F)-n1nc(Cl)c2ccccc12 |r| Show InChI InChI=1S/C23H20ClFN6O2/c1-13(28-22(33)23(8-9-23)29-21(32)14-11-26-27-12-14)16-7-6-15(10-18(16)25)31-19-5-3-2-4-17(19)20(24)30-31/h2-7,10-13H,8-9H2,1H3,(H,26,27)(H,28,33)(H,29,32)/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 7011-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.121
BindingDB Entry DOI: 10.7270/Q2KS6RX9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50265335
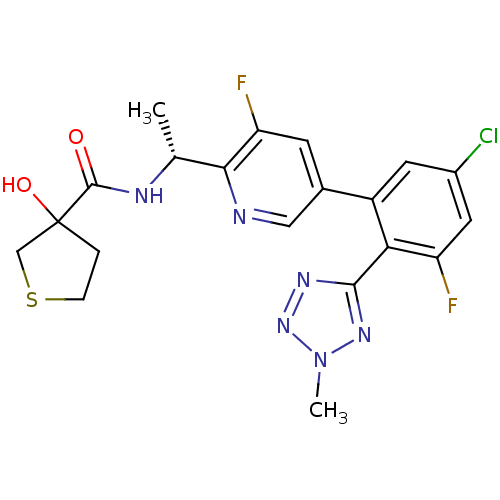
(CHEMBL496530 | N-((R)-1-(5-(5-chloro-3-fluoro-2-(2...)Show SMILES C[C@@H](NC(=O)C1(O)CCSC1)c1ncc(cc1F)-c1cc(Cl)cc(F)c1-c1nnn(C)n1 |r| Show InChI InChI=1S/C20H19ClF2N6O2S/c1-10(25-19(30)20(31)3-4-32-9-20)17-15(23)5-11(8-24-17)13-6-12(21)7-14(22)16(13)18-26-28-29(2)27-18/h5-8,10,31H,3-4,9H2,1-2H3,(H,25,30)/t10-,20?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10leu9kallidin from human bradykinin B1 receptor expressed in CHO cells by Wallac beta-plate scintillation counting |
Bioorg Med Chem Lett 18: 5107-10 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.126
BindingDB Entry DOI: 10.7270/Q2GX4BCV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318695

(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318695

(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50210360
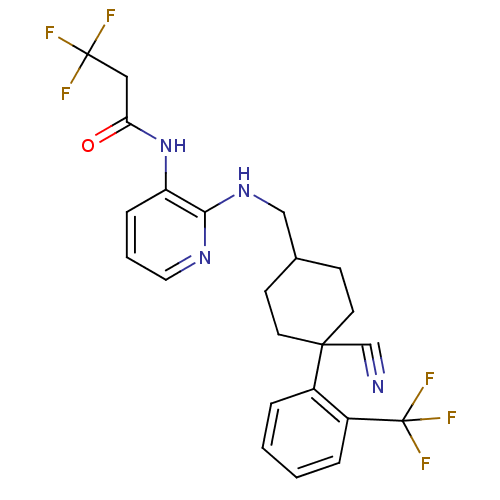
(CHEMBL234096 | N-(2-((4-cyano-4-(2-(trifluoromethy...)Show SMILES FC(F)(F)CC(=O)Nc1cccnc1NCC1CCC(CC1)(C#N)c1ccccc1C(F)(F)F |(-8.05,-29.89,;-6.71,-30.66,;-5.95,-29.33,;-7.48,-31.99,;-5.38,-31.43,;-5.38,-32.96,;-6.71,-33.73,;-4.06,-33.75,;-4.05,-35.29,;-5.39,-36.06,;-5.39,-37.6,;-4.05,-38.36,;-2.73,-37.6,;-2.73,-36.06,;-1.41,-35.27,;-.08,-34.5,;1.27,-35.25,;1.28,-36.8,;2.61,-37.57,;3.96,-36.77,;3.94,-35.22,;2.59,-34.47,;5.36,-36.1,;6.75,-35.44,;4.51,-38.19,;6.05,-38.2,;6.81,-39.54,;6.03,-40.87,;4.48,-40.86,;3.73,-39.52,;2.19,-39.5,;.65,-39.51,;2.13,-37.97,;2.26,-41.04,)| Show InChI InChI=1S/C23H22F6N4O/c24-22(25,26)12-19(34)33-18-6-3-11-31-20(18)32-13-15-7-9-21(14-30,10-8-15)16-4-1-2-5-17(16)23(27,28)29/h1-6,11,15H,7-10,12-13H2,(H,31,32)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 17: 3006-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.059
BindingDB Entry DOI: 10.7270/Q29C6X4F |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50202429
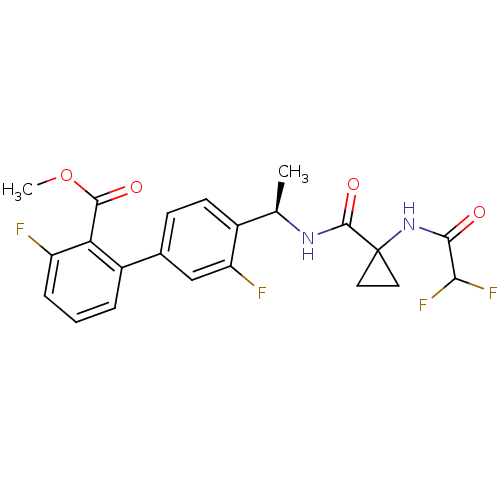
(CHEMBL414962 | methyl 4'-{(1R)-1-[({1-[(difluoroac...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)C(F)F)c(F)c1 |r| Show InChI InChI=1S/C22H20F4N2O4/c1-11(27-21(31)22(8-9-22)28-19(29)18(25)26)13-7-6-12(10-16(13)24)14-4-3-5-15(23)17(14)20(30)32-2/h3-7,10-11,18H,8-9H2,1-2H3,(H,27,31)(H,28,29)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-Arg10 Leu9 kallidin from human bradykinin B1 receptor expressed in CHO cells |
J Med Chem 50: 272-82 (2007)
Article DOI: 10.1021/jm061094b
BindingDB Entry DOI: 10.7270/Q2X63MMQ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157515
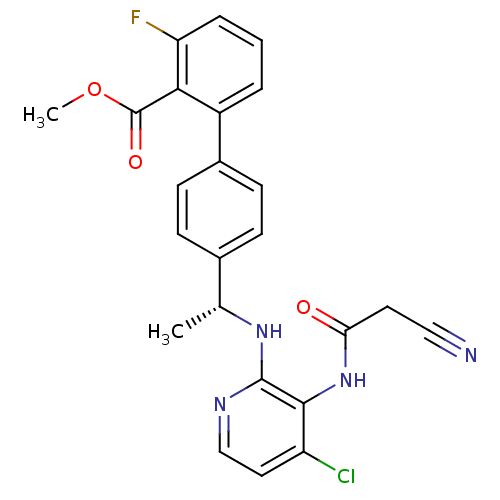
(4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamino)-pyridi...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20ClFN4O3/c1-14(29-23-22(18(25)11-13-28-23)30-20(31)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)24(32)33-2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50371657

(CHEMBL404370)Show SMILES COC(=O)c1c(Cl)cc(Cl)cc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)c2cc(OC)no2)c(F)c1 Show InChI InChI=1S/C25H22Cl2FN3O6/c1-12(29-24(34)25(6-7-25)30-22(32)19-11-20(35-2)31-37-19)15-5-4-13(8-18(15)28)16-9-14(26)10-17(27)21(16)23(33)36-3/h4-5,8-12H,6-7H2,1-3H3,(H,29,34)(H,30,32)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor expressed in rat CNS |
Bioorg Med Chem Lett 18: 682-7 (2008)
BindingDB Entry DOI: 10.7270/Q29024NB |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50321509
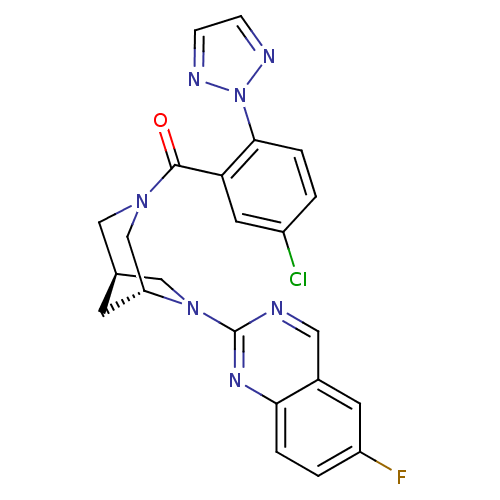
((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)((1R,5R)...)Show SMILES Fc1ccc2nc(ncc2c1)N1C[C@H]2C[C@@H]1CN(C2)C(=O)c1cc(Cl)ccc1-n1nccn1 |r| Show InChI InChI=1S/C23H19ClFN7O/c24-16-1-4-21(32-27-5-6-28-32)19(9-16)22(33)30-11-14-7-18(13-30)31(12-14)23-26-10-15-8-17(25)2-3-20(15)29-23/h1-6,8-10,14,18H,7,11-13H2/t14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R |
Bioorg Med Chem Lett 20: 4201-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.047
BindingDB Entry DOI: 10.7270/Q25Q4X3F |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50212094
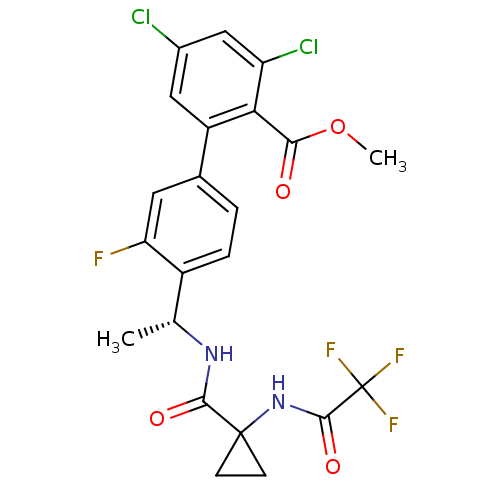
(3,5-dichloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-triflu...)Show SMILES COC(=O)c1c(Cl)cc(Cl)cc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C22H18Cl2F4N2O4/c1-10(29-19(32)21(5-6-21)30-20(33)22(26,27)28)13-4-3-11(7-16(13)25)14-8-12(23)9-15(24)17(14)18(31)34-2/h3-4,7-10H,5-6H2,1-2H3,(H,29,32)(H,30,33)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor expressed in rat CNS |
Bioorg Med Chem Lett 18: 682-7 (2008)
BindingDB Entry DOI: 10.7270/Q29024NB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data