 Found 321 hits with Last Name = 'gardner' and Initial = 'cj'
Found 321 hits with Last Name = 'gardner' and Initial = 'cj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114539
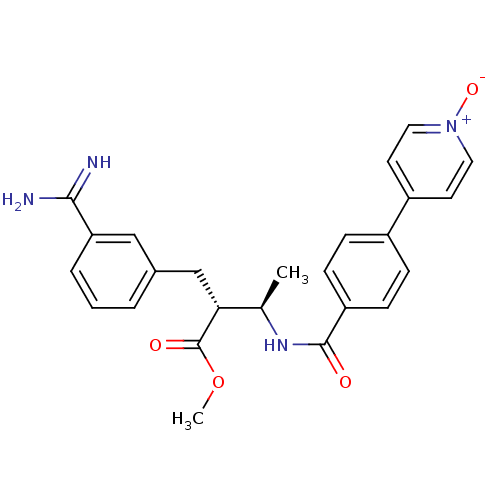
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+]([O-])cc1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)15-17-4-3-5-21(14-17)23(26)27)28-24(30)20-8-6-18(7-9-20)19-10-12-29(32)13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114534
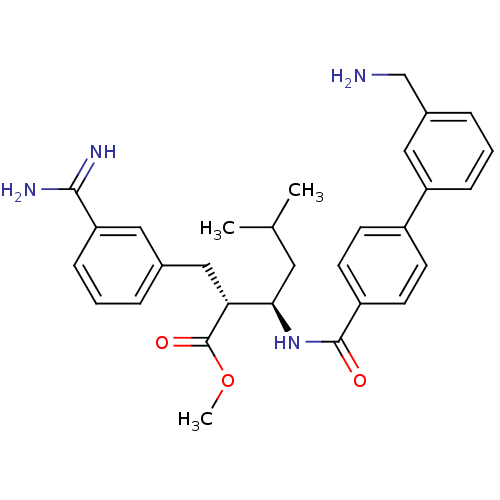
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC(C)C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C30H36N4O3/c1-19(2)14-27(26(30(36)37-3)17-20-6-4-9-25(15-20)28(32)33)34-29(35)23-12-10-22(11-13-23)24-8-5-7-21(16-24)18-31/h4-13,15-16,19,26-27H,14,17-18,31H2,1-3H3,(H3,32,33)(H,34,35)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085393
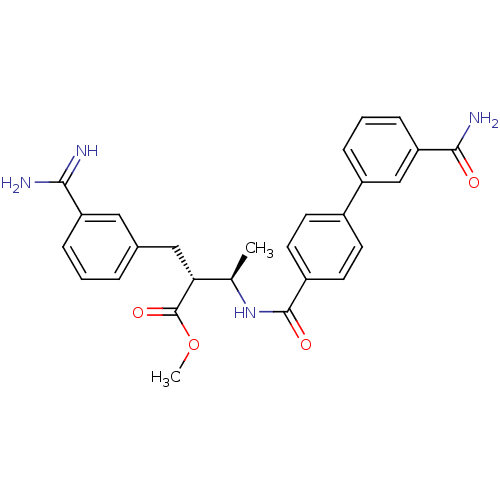
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(c1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)14-17-5-3-7-21(13-17)24(28)29)31-26(33)19-11-9-18(10-12-19)20-6-4-8-22(15-20)25(30)32/h3-13,15-16,23H,14H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114544
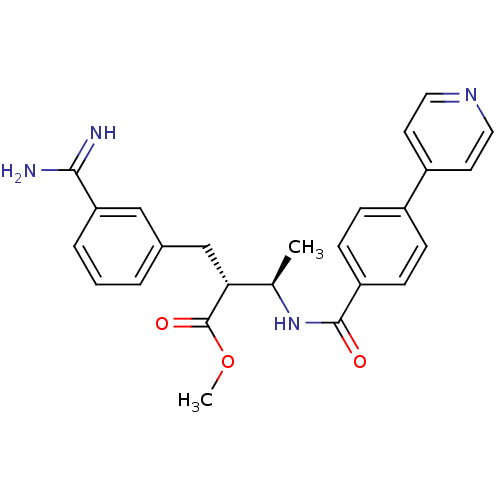
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-4-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C25H26N4O3/c1-16(22(25(31)32-2)15-17-4-3-5-21(14-17)23(26)27)29-24(30)20-8-6-18(7-9-20)19-10-12-28-13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219760
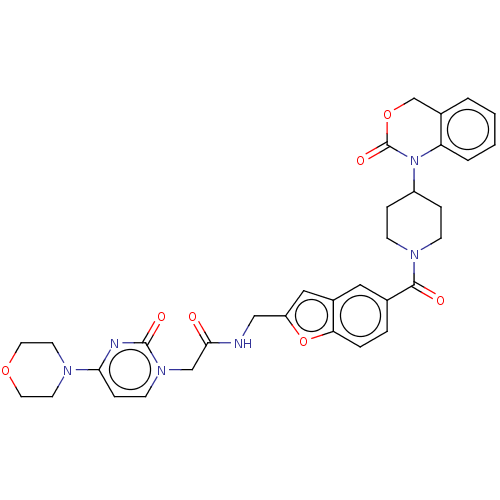
(CHEMBL286895)Show SMILES O=C(Cn1ccc(nc1=O)N1CCOCC1)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C33H34N6O7/c40-30(20-38-12-9-29(35-32(38)42)36-13-15-44-16-14-36)34-19-26-18-24-17-22(5-6-28(24)46-26)31(41)37-10-7-25(8-11-37)39-27-4-2-1-3-23(27)21-45-33(39)43/h1-6,9,12,17-18,25H,7-8,10-11,13-16,19-21H2,(H,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114543

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1[O-] Show InChI InChI=1S/C25H26N4O4/c1-16(21(25(31)33-2)15-17-6-5-7-20(14-17)23(26)27)28-24(30)19-11-9-18(10-12-19)22-8-3-4-13-29(22)32/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114536
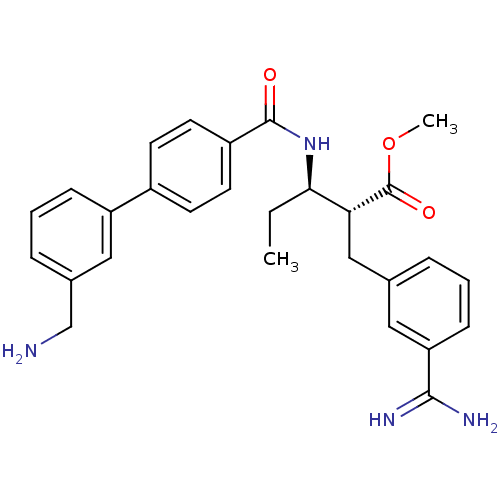
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES CC[C@@H](NC(=O)c1ccc(cc1)-c1cccc(CN)c1)[C@@H](Cc1cccc(c1)C(N)=N)C(=O)OC Show InChI InChI=1S/C28H32N4O3/c1-3-25(24(28(34)35-2)16-18-6-4-9-23(14-18)26(30)31)32-27(33)21-12-10-20(11-13-21)22-8-5-7-19(15-22)17-29/h4-15,24-25H,3,16-17,29H2,1-2H3,(H3,30,31)(H,32,33)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114540

(3-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+](C)c1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)15-18-6-4-7-21(14-18)24(27)28)29-25(31)20-11-9-19(10-12-20)22-8-5-13-30(2)16-22/h4-14,16-17,23H,15H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114537
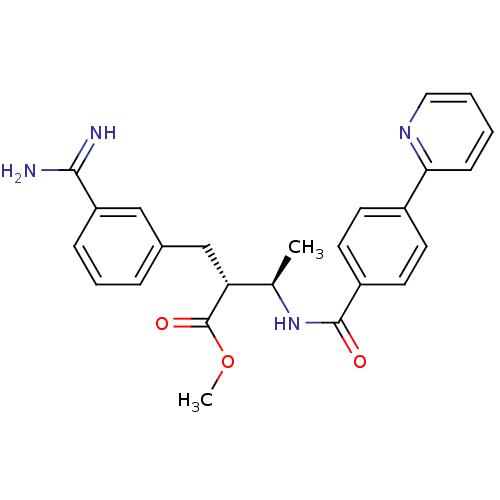
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-2-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccn1 Show InChI InChI=1S/C25H26N4O3/c1-16(21(25(31)32-2)15-17-6-5-7-20(14-17)23(26)27)29-24(30)19-11-9-18(10-12-19)22-8-3-4-13-28-22/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12597
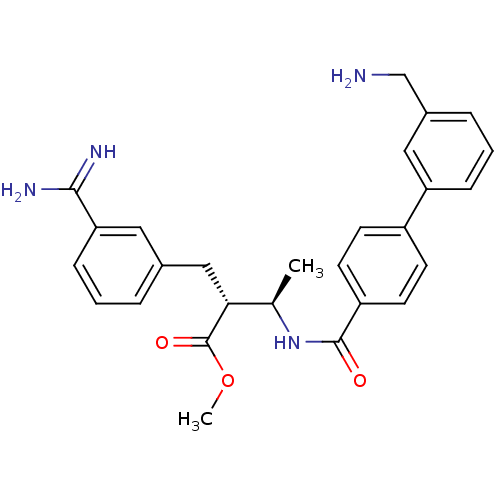
(CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C27H30N4O3/c1-17(24(27(33)34-2)15-18-5-3-8-23(13-18)25(29)30)31-26(32)21-11-9-20(10-12-21)22-7-4-6-19(14-22)16-28/h3-14,17,24H,15-16,28H2,1-2H3,(H3,29,30)(H,31,32)/t17-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114531
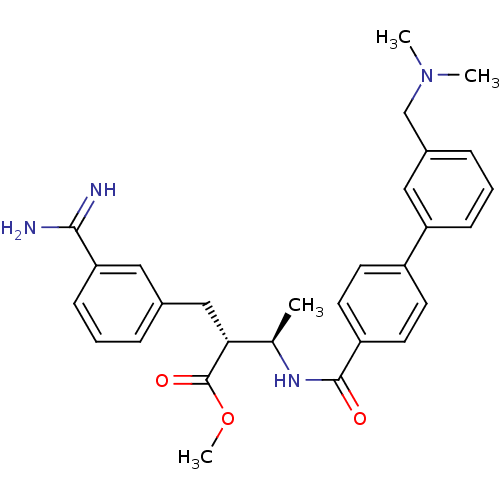
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-dimethyl...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C29H34N4O3/c1-19(26(29(35)36-4)17-20-7-5-10-25(15-20)27(30)31)32-28(34)23-13-11-22(12-14-23)24-9-6-8-21(16-24)18-33(2)3/h5-16,19,26H,17-18H2,1-4H3,(H3,30,31)(H,32,34)/t19-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219779
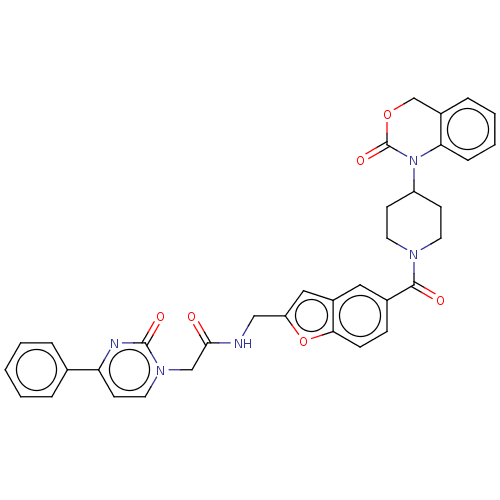
(CHEMBL30510)Show SMILES O=C(Cn1ccc(nc1=O)-c1ccccc1)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C35H31N5O6/c41-32(21-39-17-14-29(37-34(39)43)23-6-2-1-3-7-23)36-20-28-19-26-18-24(10-11-31(26)46-28)33(42)38-15-12-27(13-16-38)40-30-9-5-4-8-25(30)22-45-35(40)44/h1-11,14,17-19,27H,12-13,15-16,20-22H2,(H,36,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114548
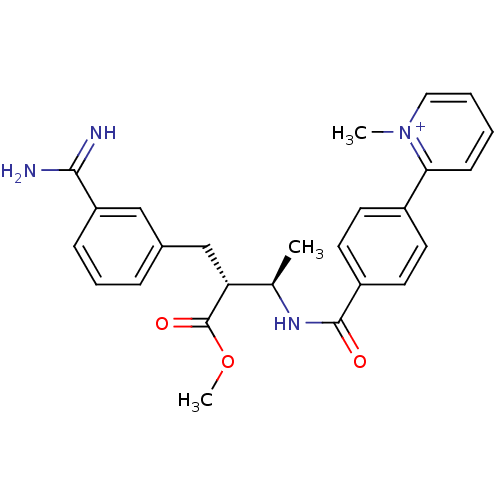
(2-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1C Show InChI InChI=1S/C26H28N4O3/c1-17(22(26(32)33-3)16-18-7-6-8-21(15-18)24(27)28)29-25(31)20-12-10-19(11-13-20)23-9-4-5-14-30(23)2/h4-15,17,22H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219786
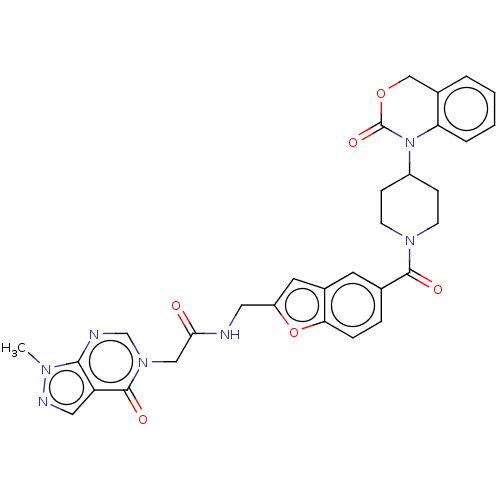
(CHEMBL285820)Show SMILES Cn1ncc2c1ncn(CC(=O)NCc1cc3cc(ccc3o1)C(=O)N1CCC(CC1)N1C(=O)OCc3ccccc13)c2=O Show InChI InChI=1S/C31H29N7O6/c1-35-28-24(15-34-35)30(41)37(18-33-28)16-27(39)32-14-23-13-21-12-19(6-7-26(21)44-23)29(40)36-10-8-22(9-11-36)38-25-5-3-2-4-20(25)17-43-31(38)42/h2-7,12-13,15,18,22H,8-11,14,16-17H2,1H3,(H,32,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219762

(CHEMBL283894)Show SMILES CCOc1ccn(CC(=O)NCc2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c(=O)n1 Show InChI InChI=1S/C31H31N5O7/c1-2-41-28-11-14-35(30(39)33-28)18-27(37)32-17-24-16-22-15-20(7-8-26(22)43-24)29(38)34-12-9-23(10-13-34)36-25-6-4-3-5-21(25)19-42-31(36)40/h3-8,11,14-16,23H,2,9-10,12-13,17-19H2,1H3,(H,32,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50113050
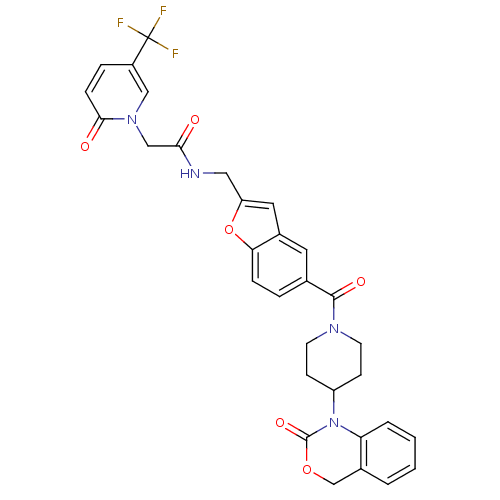
(CHEMBL31065 | N-{5-[4-(2-Oxo-4H-benzo[d][1,3]oxazi...)Show SMILES FC(F)(F)c1ccc(=O)n(CC(=O)NCc2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c1 Show InChI InChI=1S/C31H27F3N4O6/c32-31(33,34)22-6-8-28(40)37(16-22)17-27(39)35-15-24-14-21-13-19(5-7-26(21)44-24)29(41)36-11-9-23(10-12-36)38-25-4-2-1-3-20(25)18-43-30(38)42/h1-8,13-14,16,23H,9-12,15,17-18H2,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114547

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+]([O-])c1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)14-17-5-3-6-20(13-17)23(26)27)28-24(30)19-10-8-18(9-11-19)21-7-4-12-29(32)15-21/h3-13,15-16,22H,14H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114528
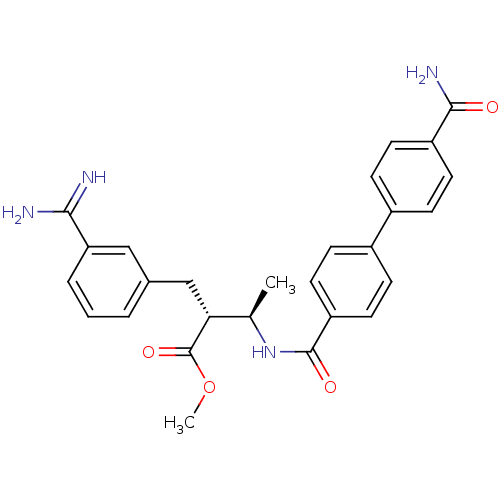
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(4'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)15-17-4-3-5-22(14-17)24(28)29)31-26(33)21-12-8-19(9-13-21)18-6-10-20(11-7-18)25(30)32/h3-14,16,23H,15H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219774

(CHEMBL281243)Show SMILES COc1cccn(CC(=O)NCc2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c1=O Show InChI InChI=1S/C31H30N4O7/c1-40-27-7-4-12-34(30(27)38)18-28(36)32-17-24-16-22-15-20(8-9-26(22)42-24)29(37)33-13-10-23(11-14-33)35-25-6-3-2-5-21(25)19-41-31(35)39/h2-9,12,15-16,23H,10-11,13-14,17-19H2,1H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114542
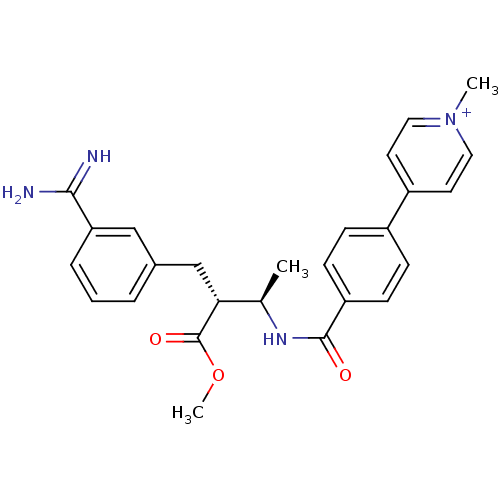
(4-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+](C)cc1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)16-18-5-4-6-22(15-18)24(27)28)29-25(31)21-9-7-19(8-10-21)20-11-13-30(2)14-12-20/h4-15,17,23H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114538
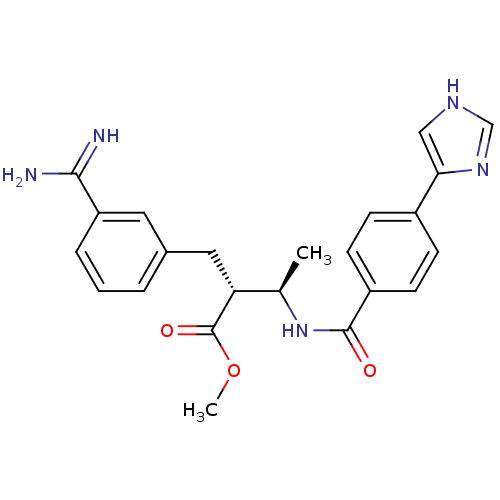
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1H-imidaz...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1c[nH]cn1 Show InChI InChI=1S/C23H25N5O3/c1-14(19(23(30)31-2)11-15-4-3-5-18(10-15)21(24)25)28-22(29)17-8-6-16(7-9-17)20-12-26-13-27-20/h3-10,12-14,19H,11H2,1-2H3,(H3,24,25)(H,26,27)(H,28,29)/t14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219775

(CHEMBL31651)Show SMILES FC(F)(F)c1ccc(=O)n(CC(=O)NCc2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccc(Cl)cc23)c1 Show InChI InChI=1S/C31H26ClF3N4O6/c32-22-4-1-19-17-44-30(43)39(25(19)13-22)23-7-9-37(10-8-23)29(42)18-2-5-26-20(11-18)12-24(45-26)14-36-27(40)16-38-15-21(31(33,34)35)3-6-28(38)41/h1-6,11-13,15,23H,7-10,14,16-17H2,(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50113051
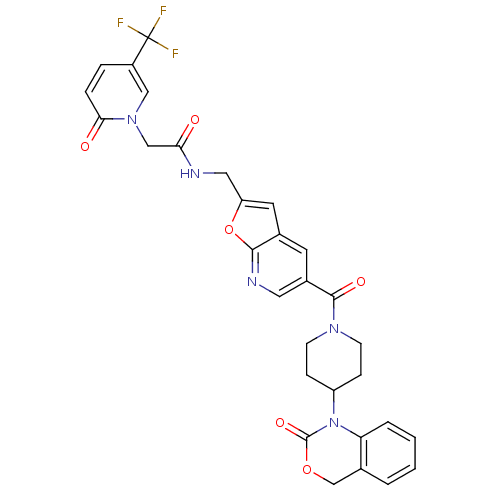
(CHEMBL284442 | N-{5-[4-(2-Oxo-4H-benzo[d][1,3]oxaz...)Show SMILES FC(F)(F)c1ccc(=O)n(CC(=O)NCc2cc3cc(cnc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c1 Show InChI InChI=1S/C30H26F3N5O6/c31-30(32,33)21-5-6-26(40)37(15-21)16-25(39)34-14-23-12-19-11-20(13-35-27(19)44-23)28(41)36-9-7-22(8-10-36)38-24-4-2-1-3-18(24)17-43-29(38)42/h1-6,11-13,15,22H,7-10,14,16-17H2,(H,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219776
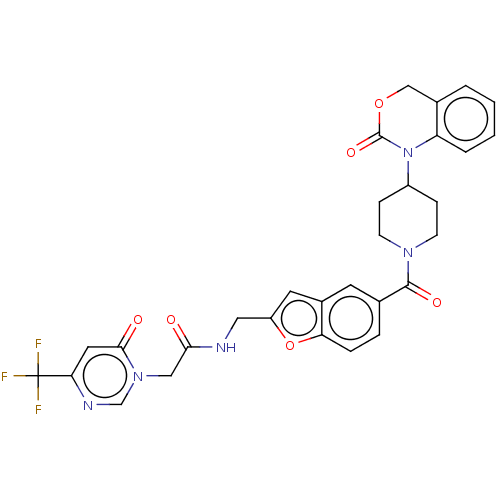
(CHEMBL32404)Show SMILES FC(F)(F)c1cc(=O)n(CC(=O)NCc2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)cn1 Show InChI InChI=1S/C30H26F3N5O6/c31-30(32,33)25-13-27(40)37(17-35-25)15-26(39)34-14-22-12-20-11-18(5-6-24(20)44-22)28(41)36-9-7-21(8-10-36)38-23-4-2-1-3-19(23)16-43-29(38)42/h1-6,11-13,17,21H,7-10,14-16H2,(H,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114530
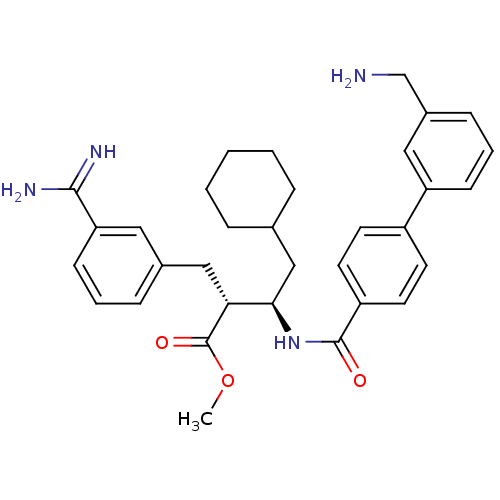
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC1CCCCC1)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C33H40N4O3/c1-40-33(39)29(19-23-9-5-12-28(17-23)31(35)36)30(20-22-7-3-2-4-8-22)37-32(38)26-15-13-25(14-16-26)27-11-6-10-24(18-27)21-34/h5-6,9-18,22,29-30H,2-4,7-8,19-21,34H2,1H3,(H3,35,36)(H,37,38)/t29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085406

((2R,3R)-3-[(Biphenyl-4-carbonyl)-amino]-2-(3-carba...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H27N3O3/c1-17(23(26(31)32-2)16-18-7-6-10-22(15-18)24(27)28)29-25(30)21-13-11-20(12-14-21)19-8-4-3-5-9-19/h3-15,17,23H,16H2,1-2H3,(H3,27,28)(H,29,30)/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085406

((2R,3R)-3-[(Biphenyl-4-carbonyl)-amino]-2-(3-carba...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H27N3O3/c1-17(23(26(31)32-2)16-18-7-6-10-22(15-18)24(27)28)29-25(30)21-13-11-20(12-14-21)19-8-4-3-5-9-19/h3-15,17,23H,16H2,1-2H3,(H3,27,28)(H,29,30)/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114541

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-imidazol-1...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-n1ccnc1 Show InChI InChI=1S/C23H25N5O3/c1-15(20(23(30)31-2)13-16-4-3-5-18(12-16)21(24)25)27-22(29)17-6-8-19(9-7-17)28-11-10-26-14-28/h3-12,14-15,20H,13H2,1-2H3,(H3,24,25)(H,27,29)/t15-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50029649
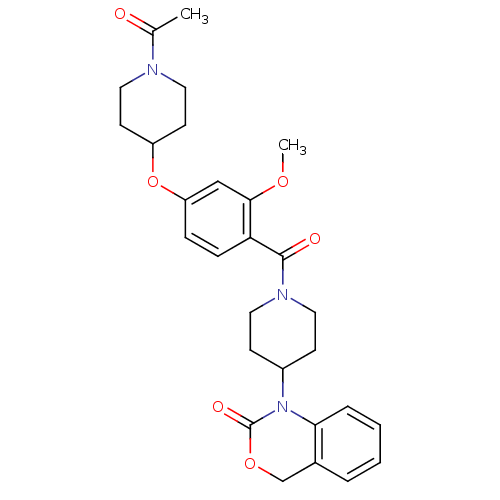
(1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...)Show SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C28H33N3O6/c1-19(32)29-15-11-22(12-16-29)37-23-7-8-24(26(17-23)35-2)27(33)30-13-9-21(10-14-30)31-25-6-4-3-5-20(25)18-36-28(31)34/h3-8,17,21-22H,9-16,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219785

(CHEMBL283039)Show SMILES Fc1ccc2N(C3CCN(CC3)C(=O)c3ccc4oc(CNC(=O)Cn5cc(ccc5=O)C(F)(F)F)cc4c3)C(=O)OCc2c1 Show InChI InChI=1S/C31H26F4N4O6/c32-22-3-4-25-20(12-22)17-44-30(43)39(25)23-7-9-37(10-8-23)29(42)18-1-5-26-19(11-18)13-24(45-26)14-36-27(40)16-38-15-21(31(33,34)35)2-6-28(38)41/h1-6,11-13,15,23H,7-10,14,16-17H2,(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219509
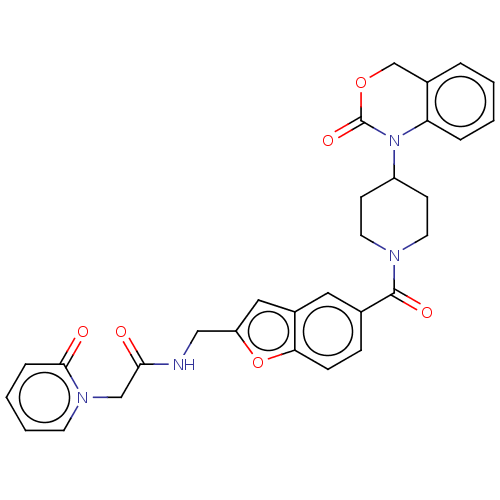
(CHEMBL287325)Show SMILES O=C(Cn1ccccc1=O)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C30H28N4O6/c35-27(18-33-12-4-3-7-28(33)36)31-17-24-16-22-15-20(8-9-26(22)40-24)29(37)32-13-10-23(11-14-32)34-25-6-2-1-5-21(25)19-39-30(34)38/h1-9,12,15-16,23H,10-11,13-14,17-19H2,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219783

(CHEMBL282494)Show SMILES Fc1ccc2COC(=O)N(C3CCN(CC3)C(=O)c3ccc4oc(CNC(=O)Cn5cc(ccc5=O)C(F)(F)F)cc4c3)c2c1 Show InChI InChI=1S/C31H26F4N4O6/c32-22-4-1-19-17-44-30(43)39(25(19)13-22)23-7-9-37(10-8-23)29(42)18-2-5-26-20(11-18)12-24(45-26)14-36-27(40)16-38-15-21(31(33,34)35)3-6-28(38)41/h1-6,11-13,15,23H,7-10,14,16-17H2,(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219764

(CHEMBL281044)Show SMILES O=C(Cn1ccc2ccccc2c1=O)NCc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C34H30N4O6/c39-31(20-37-14-11-22-5-1-3-7-28(22)33(37)41)35-19-27-18-25-17-23(9-10-30(25)44-27)32(40)36-15-12-26(13-16-36)38-29-8-4-2-6-24(29)21-43-34(38)42/h1-11,14,17-18,26H,12-13,15-16,19-21H2,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219781
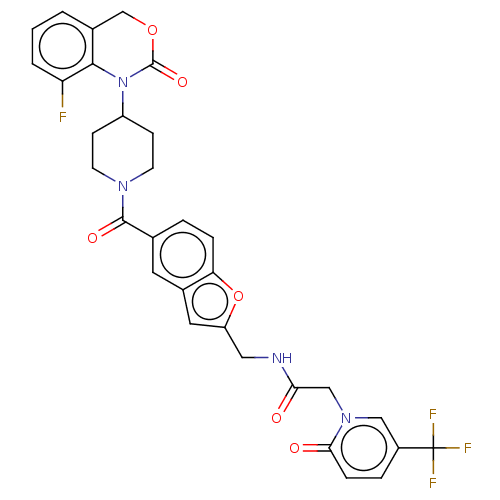
(CHEMBL30910)Show SMILES Fc1cccc2COC(=O)N(C3CCN(CC3)C(=O)c3ccc4oc(CNC(=O)Cn5cc(ccc5=O)C(F)(F)F)cc4c3)c12 Show InChI InChI=1S/C31H26F4N4O6/c32-24-3-1-2-19-17-44-30(43)39(28(19)24)22-8-10-37(11-9-22)29(42)18-4-6-25-20(12-18)13-23(45-25)14-36-26(40)16-38-15-21(31(33,34)35)5-7-27(38)41/h1-7,12-13,15,22H,8-11,14,16-17H2,(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219761
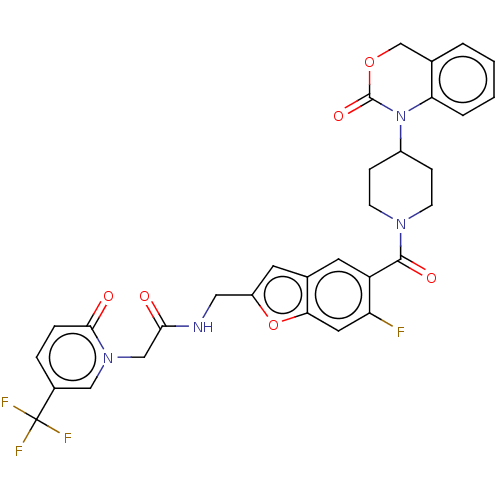
(CHEMBL287101)Show SMILES Fc1cc2oc(CNC(=O)Cn3cc(ccc3=O)C(F)(F)F)cc2cc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C31H26F4N4O6/c32-24-13-26-19(11-22(45-26)14-36-27(40)16-38-15-20(31(33,34)35)5-6-28(38)41)12-23(24)29(42)37-9-7-21(8-10-37)39-25-4-2-1-3-18(25)17-44-30(39)43/h1-6,11-13,15,21H,7-10,14,16-17H2,(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114545

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-3-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C25H26N4O3/c1-16(22(25(31)32-2)14-17-5-3-6-20(13-17)23(26)27)29-24(30)19-10-8-18(9-11-19)21-7-4-12-28-15-21/h3-13,15-16,22H,14H2,1-2H3,(H3,26,27)(H,29,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114526

((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@H](NC(=O)c1ccc(cc1)-c1cccc(CN)c1)C(C)C Show InChI InChI=1S/C29H34N4O3/c1-18(2)26(25(29(35)36-3)16-19-6-4-9-24(14-19)27(31)32)33-28(34)22-12-10-21(11-13-22)23-8-5-7-20(15-23)17-30/h4-15,18,25-26H,16-17,30H2,1-3H3,(H3,31,32)(H,33,34)/t25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114529
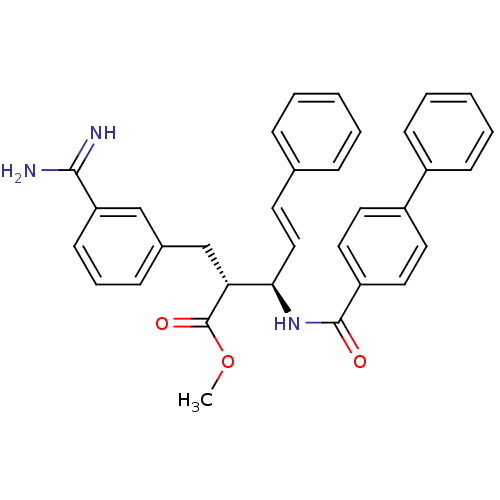
((E)-(2R,3R)-3-[(Biphenyl-4-carbonyl)-amino]-2-(3-c...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)\C=C\c1ccccc1 Show InChI InChI=1S/C33H31N3O3/c1-39-33(38)29(22-24-11-8-14-28(21-24)31(34)35)30(20-15-23-9-4-2-5-10-23)36-32(37)27-18-16-26(17-19-27)25-12-6-3-7-13-25/h2-21,29-30H,22H2,1H3,(H3,34,35)(H,36,37)/b20-15+/t29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219782
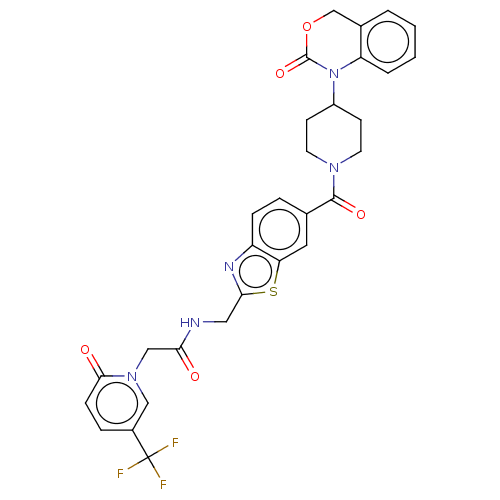
(CHEMBL281081)Show SMILES FC(F)(F)c1ccc(=O)n(CC(=O)NCc2nc3ccc(cc3s2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c1 Show InChI InChI=1S/C30H26F3N5O5S/c31-30(32,33)20-6-8-27(40)37(15-20)16-25(39)34-14-26-35-22-7-5-18(13-24(22)44-26)28(41)36-11-9-21(10-12-36)38-23-4-2-1-3-19(23)17-43-29(38)42/h1-8,13,15,21H,9-12,14,16-17H2,(H,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219770

(CHEMBL283676)Show SMILES [H][C@@]1(Cn2cc(ccc2=O)C(F)(F)F)CCCCN1Cc1cc2cc(ccc2o1)C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C35H35F3N4O5/c36-35(37,38)26-9-11-32(43)41(19-26)20-28-6-3-4-14-40(28)21-29-18-25-17-23(8-10-31(25)47-29)33(44)39-15-12-27(13-16-39)42-30-7-2-1-5-24(30)22-46-34(42)45/h1-2,5,7-11,17-19,27-28H,3-4,6,12-16,20-22H2/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219773

(CHEMBL445875)Show SMILES FC(F)(F)c1ccc(=O)n(Cc2cccc(n2)-c2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)c1 Show InChI InChI=1S/C34H27F3N4O5/c35-34(36,37)24-9-11-31(42)40(18-24)19-25-5-3-6-27(38-25)30-17-23-16-21(8-10-29(23)46-30)32(43)39-14-12-26(13-15-39)41-28-7-2-1-4-22(28)20-45-33(41)44/h1-11,16-18,26H,12-15,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114527

((2R,3R)-3-[(Biphenyl-4-carbonyl)-amino]-2-(3-carba...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](Cc1ccccc1)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C32H31N3O3/c1-38-32(37)28(20-23-11-8-14-27(19-23)30(33)34)29(21-22-9-4-2-5-10-22)35-31(36)26-17-15-25(16-18-26)24-12-6-3-7-13-24/h2-19,28-29H,20-21H2,1H3,(H3,33,34)(H,35,36)/t28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114532

((R)-2-{[(Biphenyl-4-carbonyl)-amino]-methyl}-3-(3-...)Show SMILES COC(=O)[C@@H](CNC(=O)c1ccc(cc1)-c1ccccc1)Cc1cccc(c1)C(N)=N Show InChI InChI=1S/C25H25N3O3/c1-31-25(30)22(15-17-6-5-9-21(14-17)23(26)27)16-28-24(29)20-12-10-19(11-13-20)18-7-3-2-4-8-18/h2-14,22H,15-16H2,1H3,(H3,26,27)(H,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50062714
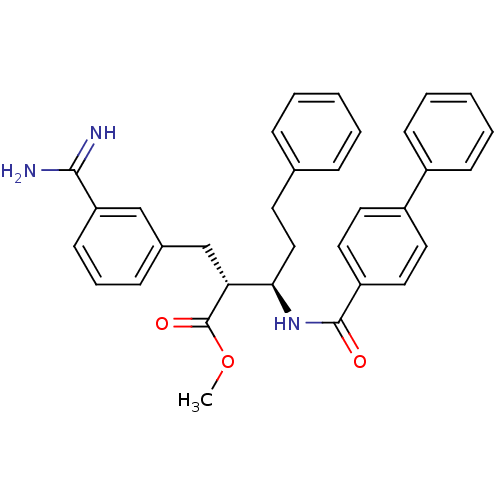
((2R,3R)-3-[(Biphenyl-4-carbonyl)-amino]-2-(3-carba...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CCc1ccccc1)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C33H33N3O3/c1-39-33(38)29(22-24-11-8-14-28(21-24)31(34)35)30(20-15-23-9-4-2-5-10-23)36-32(37)27-18-16-26(17-19-27)25-12-6-3-7-13-25/h2-14,16-19,21,29-30H,15,20,22H2,1H3,(H3,34,35)(H,36,37)/t29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219777

(CHEMBL281988)Show SMILES FC(F)(F)c1ccc(=O)n(c1)[C@@H]1CCCN(Cc2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)OCc3ccccc23)C1 Show InChI InChI=1S/C34H33F3N4O5/c35-34(36,37)25-8-10-31(42)40(18-25)27-5-3-13-38(19-27)20-28-17-24-16-22(7-9-30(24)46-28)32(43)39-14-11-26(12-15-39)41-29-6-2-1-4-23(29)21-45-33(41)44/h1-2,4,6-10,16-18,26-27H,3,5,11-15,19-21H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50219780

(CHEMBL287629)Show SMILES FC(F)(F)c1ccc(=O)n(CC(=O)NCc2cc3cc(ccc3o2)C(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c1 Show InChI InChI=1S/C32H29F3N4O5/c33-32(34,35)23-7-10-29(41)38(18-23)19-28(40)36-17-25-16-22-15-21(5-8-27(22)44-25)31(43)37-13-11-24(12-14-37)39-26-4-2-1-3-20(26)6-9-30(39)42/h1-5,7-8,10,15-16,18,24H,6,9,11-14,17,19H2,(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3[H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 12: 1405-11 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VRF |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50114548

(2-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1C Show InChI InChI=1S/C26H28N4O3/c1-17(22(26(32)33-3)16-18-7-6-8-21(15-18)24(27)28)29-25(31)20-12-10-19(11-13-20)23-9-4-5-14-30(23)2/h4-15,17,22H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency against Trypsin |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50114543

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1[O-] Show InChI InChI=1S/C25H26N4O4/c1-16(21(25(31)33-2)15-17-6-5-7-20(14-17)23(26)27)28-24(30)19-11-9-18(10-12-19)22-8-3-4-13-29(22)32/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency against Trypsin |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50114530

((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC1CCCCC1)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C33H40N4O3/c1-40-33(39)29(19-23-9-5-12-28(17-23)31(35)36)30(20-22-7-3-2-4-8-22)37-32(38)26-15-13-25(14-16-26)27-11-6-10-24(18-27)21-34/h5-6,9-18,22,29-30H,2-4,7-8,19-21,34H2,1H3,(H3,35,36)(H,37,38)/t29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human trypsin |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50114534

((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC(C)C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C30H36N4O3/c1-19(2)14-27(26(30(36)37-3)17-20-6-4-9-25(15-20)28(32)33)34-29(35)23-12-10-22(11-13-23)24-8-5-7-21(16-24)18-31/h4-13,15-16,19,26-27H,14,17-18,31H2,1-3H3,(H3,32,33)(H,34,35)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human trypsin |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data