

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255960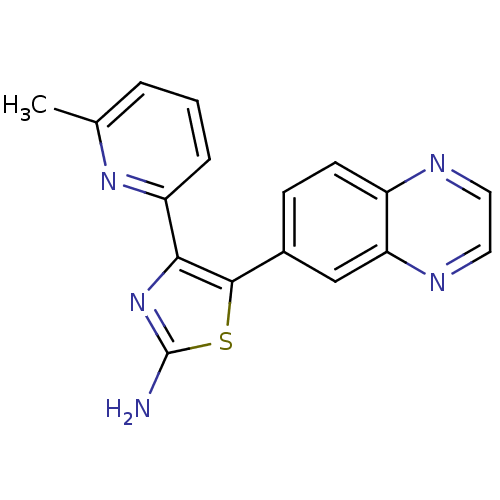 (4-(6-methylpyridin-2-yl)-5-(quinoxalin-6-yl)thiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255959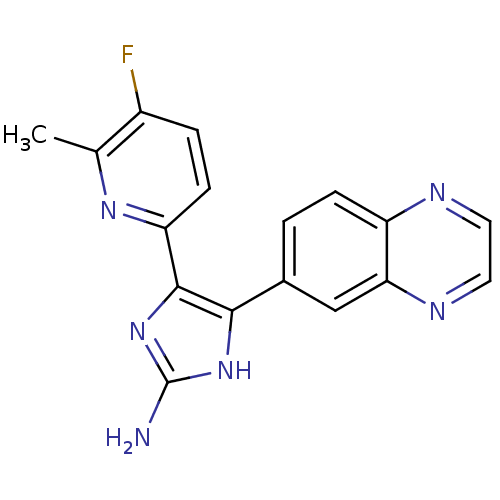 (4-(5-fluoro-6-methylpyridin-2-yl)-5-(quinoxalin-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255228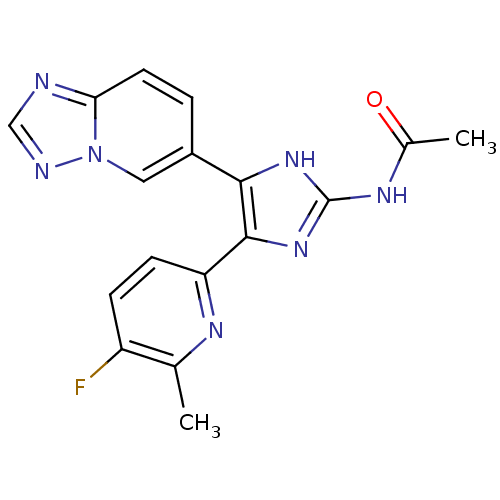 (CHEMBL517068 | N-(5-([1,2,4]triazolo[1,5-a]pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255227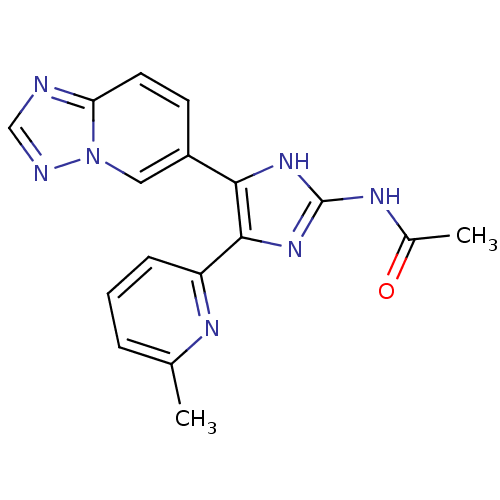 (CHEMBL450161 | N-(5-([1,2,4]triazolo[1,5-a]pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255958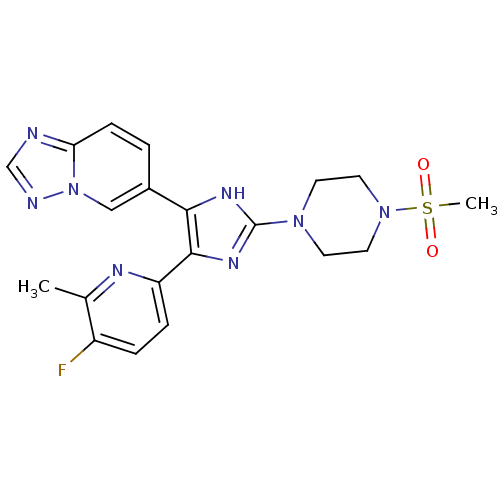 (6-(4-(5-fluoro-6-methylpyridin-2-yl)-2-(4-(methyls...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255179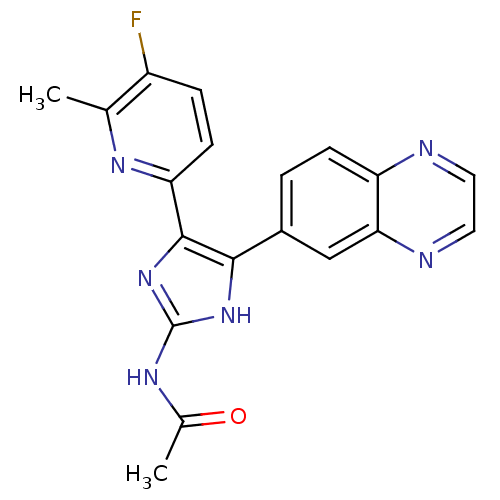 (CHEMBL519939 | N-(4-(5-fluoro-6-methylpyridin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255231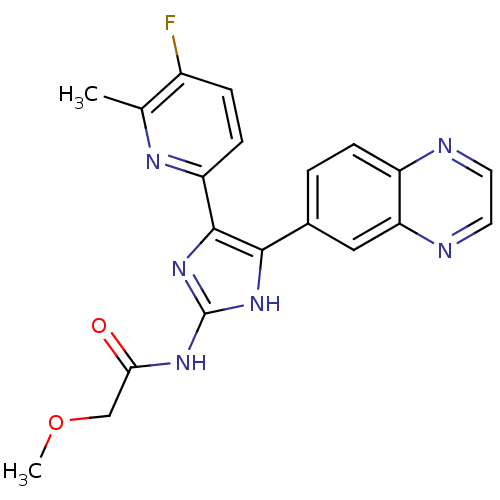 (CHEMBL479262 | N-(4-(5-fluoro-6-methylpyridin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255230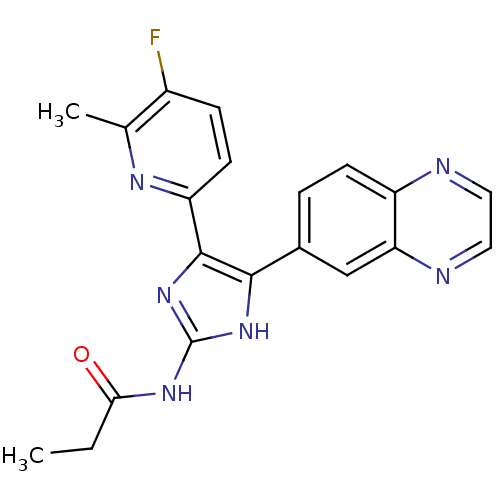 (CHEMBL521441 | N-(4-(5-fluoro-6-methylpyridin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255908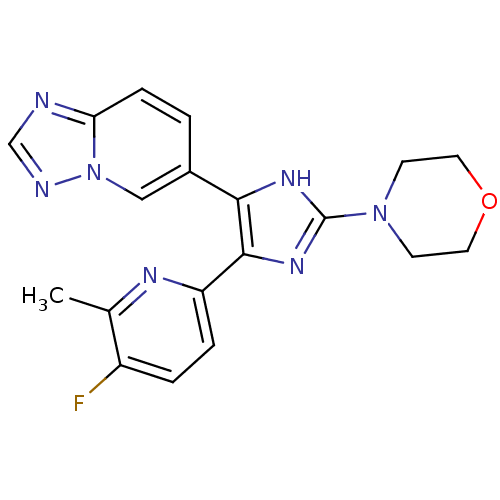 (4-(5-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-4-(5-flu...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255957 (1-(4-(5-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-4-(5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255909 (6-(4-(5-fluoro-6-methylpyridin-2-yl)-2-(4-methylpi...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255906 (CHEMBL480731 | N-(4-(5-fluoro-6-methylpyridin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255907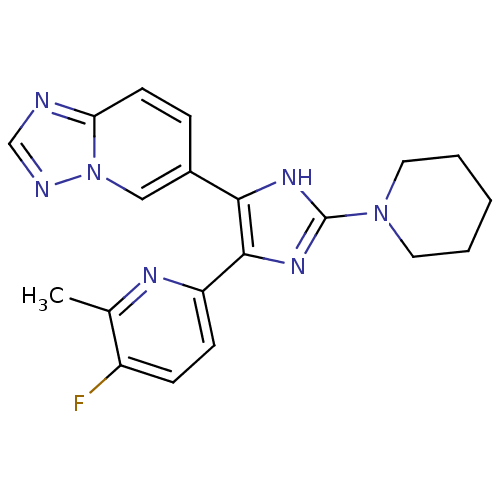 (6-(4-(5-fluoro-6-methylpyridin-2-yl)-2-(piperidin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255229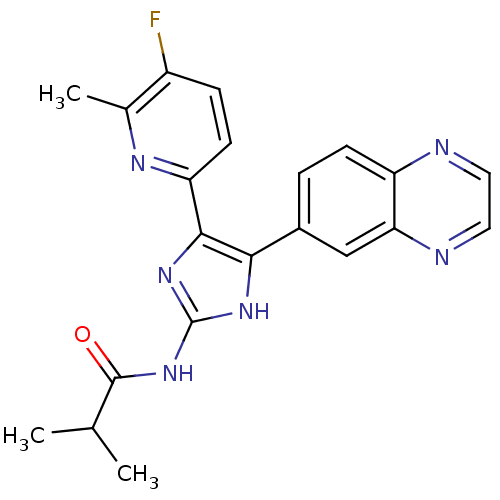 (CHEMBL482207 | N-(4-(5-fluoro-6-methylpyridin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Binding affinity to human TGFBR1 | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50338159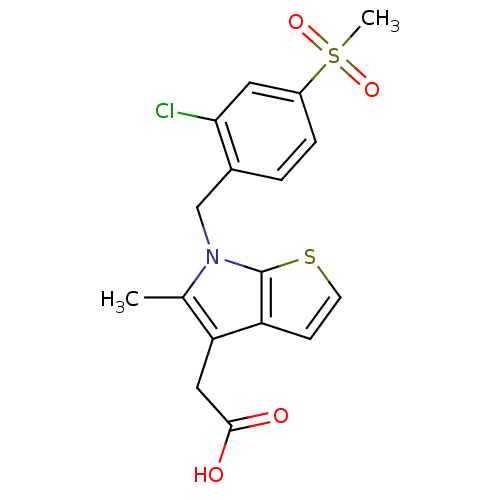 (2-(6-(2-chloro-4-(methylsulfonyl)benzyl)-5-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cell membranes | Bioorg Med Chem Lett 21: 1861-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.008 BindingDB Entry DOI: 10.7270/Q2BV7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308587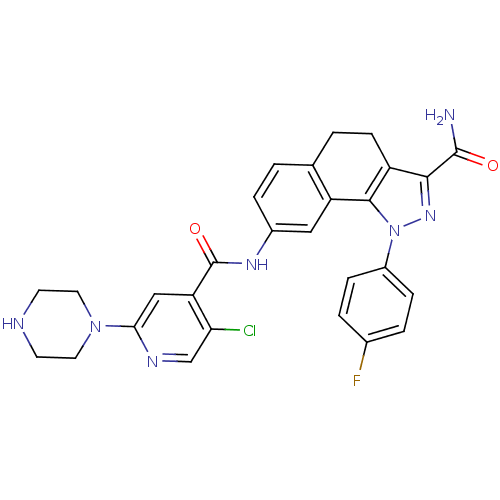 (8-[(5-Chloro-2-piperazin-1-ylisonicotinoyl)amino]-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308599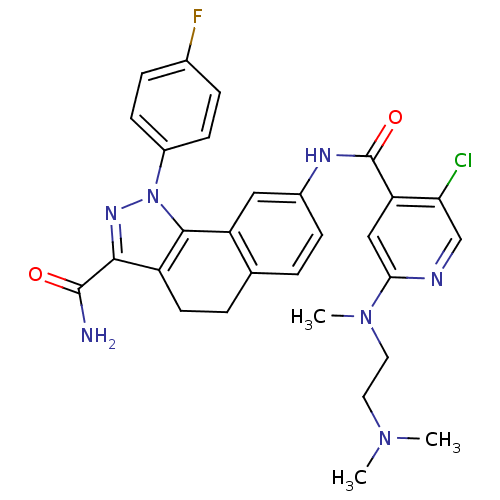 (8-({5-Chloro-2-[[2-(dimethylamino)ethyl]-(methyl)a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50338157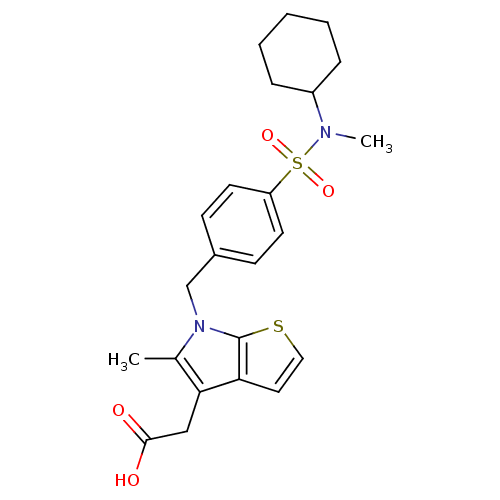 (2-(6-(4-(N-cyclohexyl-N-methylsulfamoyl)benzyl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cell membranes | Bioorg Med Chem Lett 21: 1861-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.008 BindingDB Entry DOI: 10.7270/Q2BV7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308585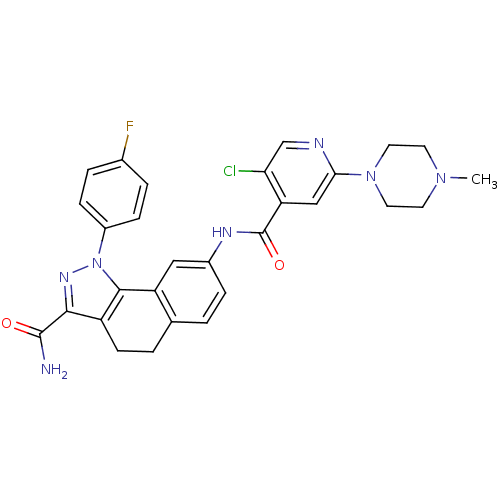 (8-{[5-Chloro-2-(4-methylpiperazin-1-yl)isonicotino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308598 (8-{[5-Chloro-2-(1,4-diazepan-1-yl)isonicotinoyl]am...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308597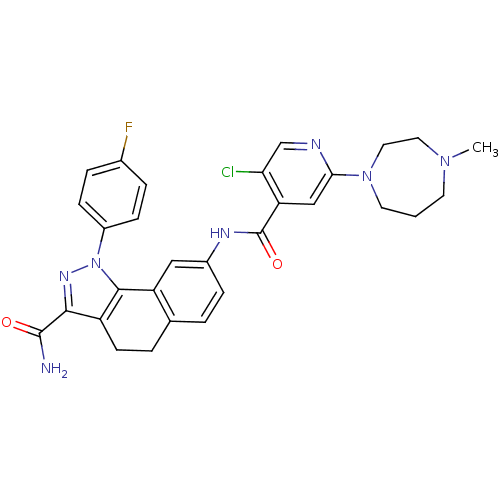 (8-{[5-Chloro-2-(4-methyl-1,4-diazepan-1-yl)isonico...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308584 (1-(1,3-Benzodioxol-5-yl)-8-{[5-chloro-2-(4-methylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50255959 (4-(5-fluoro-6-methylpyridin-2-yl)-5-(quinoxalin-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbot Laboratories Curated by ChEMBL | Assay Description Inhibition of TGFBR1 (unknown origin) transfected in human HepG2 cells after 24 hrs by plasminogen activator inhibitor-luciferase reporter gene assay | Bioorg Med Chem Lett 19: 912-6 (2009) Article DOI: 10.1016/j.bmcl.2008.11.119 BindingDB Entry DOI: 10.7270/Q2FX799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308569 (1-(benzo[d][1,3]dioxol-5-yl)-8-(2-chloronicotinami...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308586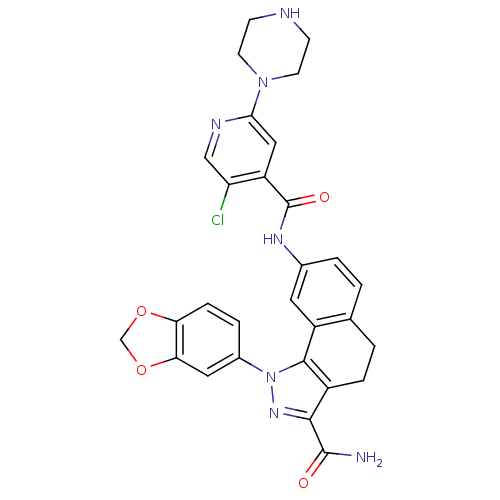 (1-(1,3-Benzodioxol-5-yl)-8-[(5-chloro-2-piperazin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308595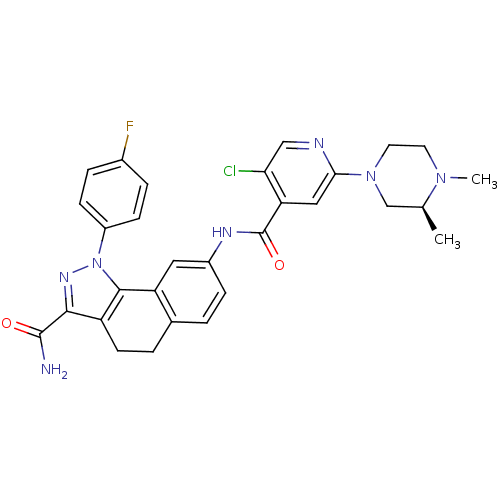 (8-({5-Chloro-2-[(3S)-3,4-dimethylpiperazin-1-yl]is...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308592 (8-({5-Chloro-2-[(3R,5S)-3,5-dimethylpiperazin-1-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308574 (8-(2-chloronicotinamido)-1-(4-fluorophenyl)-4,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308593 (8-({5-Chloro-2-[(3R)-3,4-dimethylpiperazin-1-yl]is...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50338158 (2-(5-methyl-6-(4-(methylsulfonyl)benzyl)-6H-thieno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cell membranes | Bioorg Med Chem Lett 21: 1861-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.008 BindingDB Entry DOI: 10.7270/Q2BV7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308596 (8-({5-Chloro-2-[(3S)-3-methylpiperazin-1-yl]-isoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308588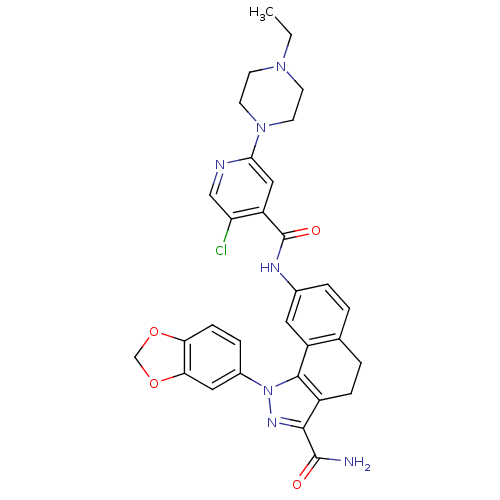 (8-{[5-Chloro-2-(4-ethylpiperazin-1-yl)isonicotinoy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308594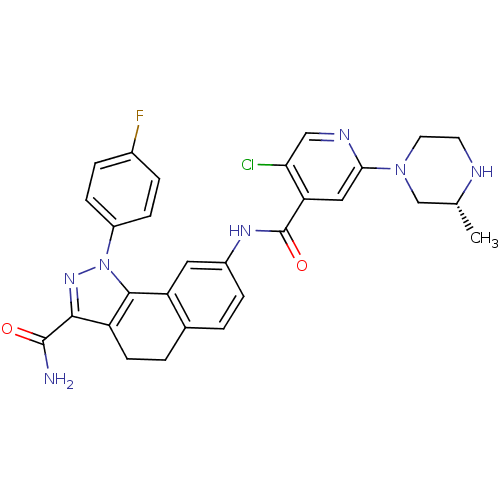 (8-({5-Chloro-2-[(3R)-3-methylpiperazin-1-yl]isonic...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308590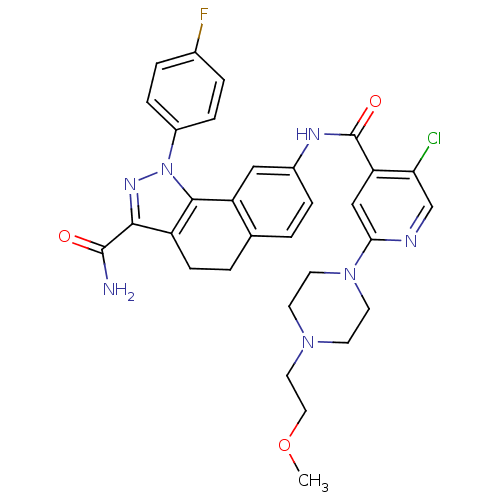 (8-({5-Chloro-2-[4-(2-methoxyethyl)piperazin-1-yl]i...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50338156 (2-(5-methyl-6-(4-(morpholinosulfonyl)benzyl)-6H-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cell membranes | Bioorg Med Chem Lett 21: 1861-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.008 BindingDB Entry DOI: 10.7270/Q2BV7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308600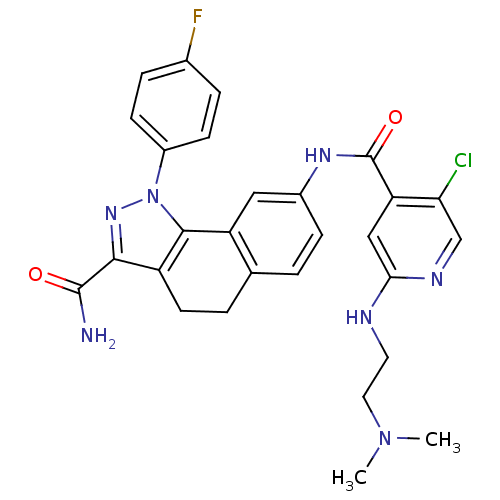 (8-[(5-Chloro-2-{[2-(dimethylamino)ethyl]amino}-iso...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50338152 (2-(5-methyl-6-(4-(methylsulfonyl)phenylsulfonyl)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cell membranes | Bioorg Med Chem Lett 21: 1861-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.008 BindingDB Entry DOI: 10.7270/Q2BV7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308601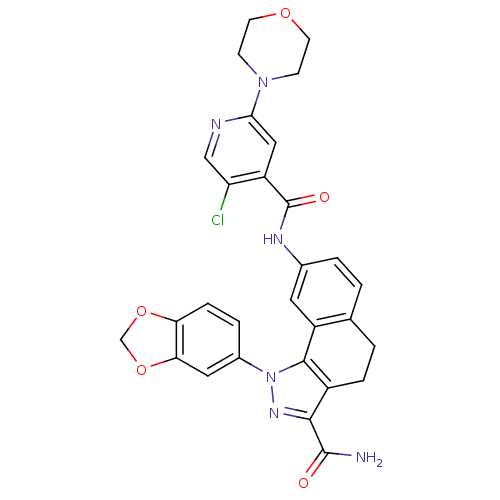 (1-(1,3-Benzodioxol-5-yl)-8-[(5-chloro-2-morpholin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50308591 (8-({5-Chloro-2-[(3R,5S)-3,4,5-trimethylpiperazin-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant IKK2-mediated transfer of [gamma33P]ATP to biotinylated IkappaBalpha after 30 mins by scintillation counting | Bioorg Med Chem 18: 403-14 (2010) Article DOI: 10.1016/j.bmc.2009.10.040 BindingDB Entry DOI: 10.7270/Q2M61KCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287470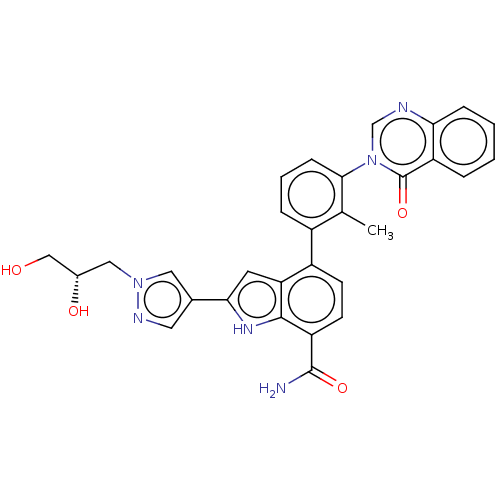 (US9567339, Example K.1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287471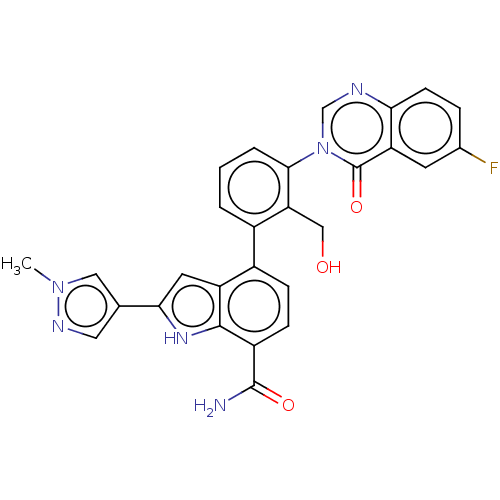 (US9567339, Example M.1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287472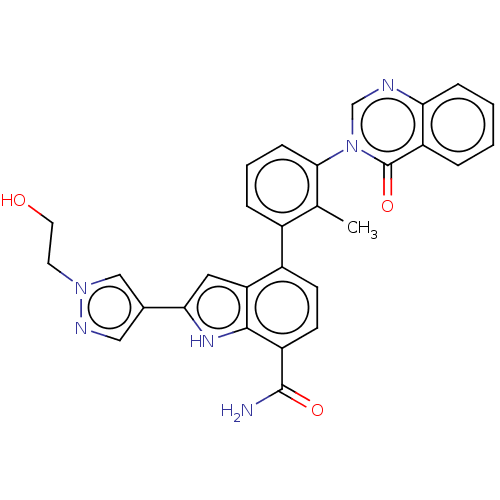 (US9567339, Example M.1.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287475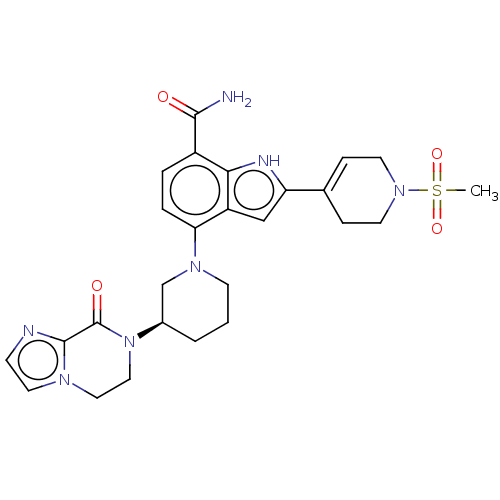 (US9567339, Example O.1.3*) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287476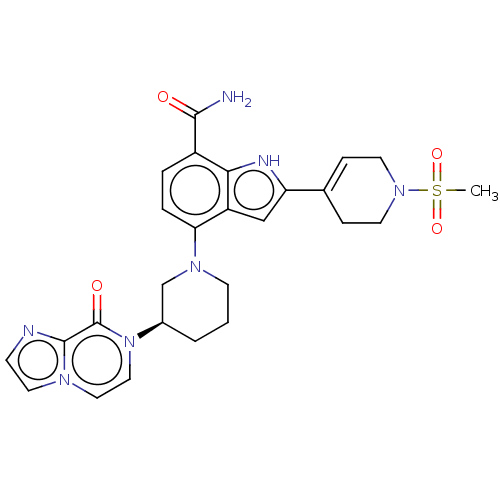 (US9567339, Example O.1.4*) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287477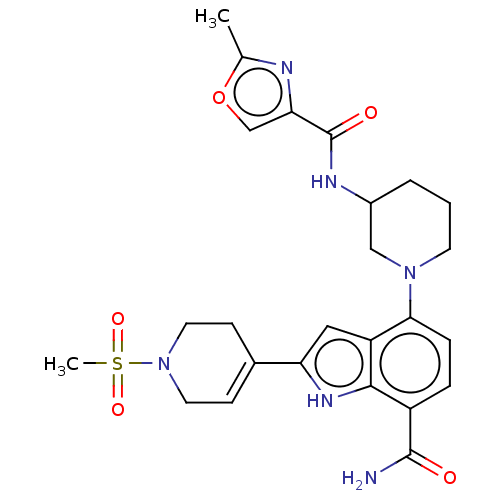 (US9567339, Example O.1.5*) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287489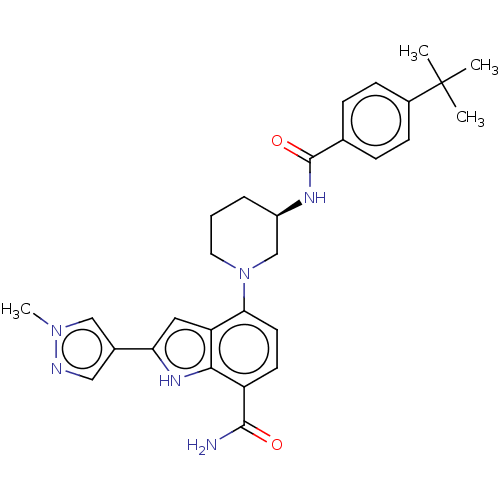 (US9567339, Example O.1.19*) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287495 (US9567339, Example O.1.25*) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287496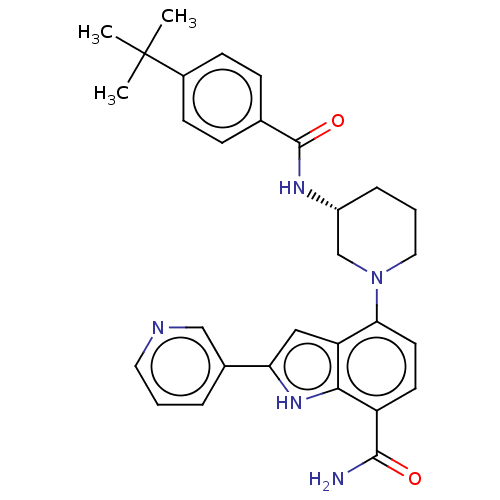 (US9567339, Example O.1.26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287500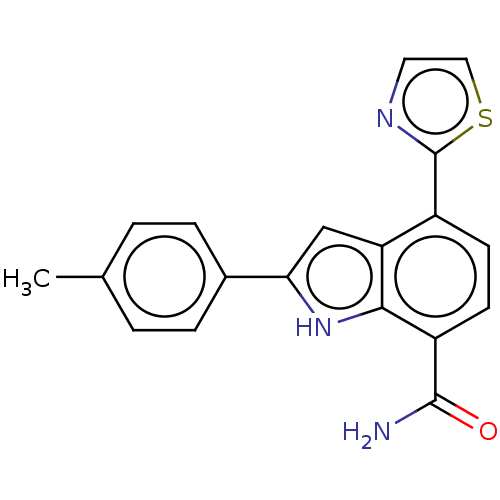 (US9567339, Example U.1.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [393-659] (Homo sapiens (Human)) | BDBM287503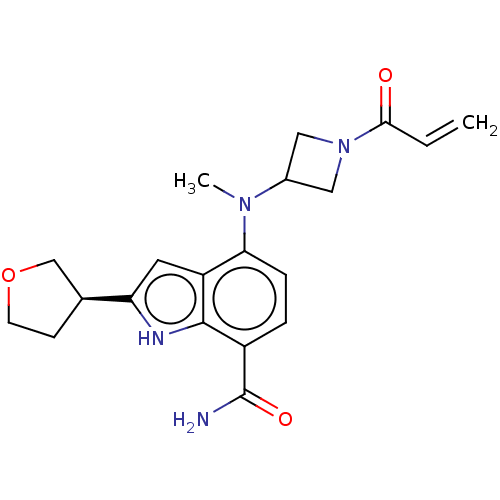 (US9567339, Example 5.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc. US Patent | Assay Description The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... | US Patent US9567339 (2017) BindingDB Entry DOI: 10.7270/Q2P2715T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 517 total ) | Next | Last >> |