 Found 2209 hits with Last Name = 'fry' and Initial = 'd'
Found 2209 hits with Last Name = 'fry' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM86311
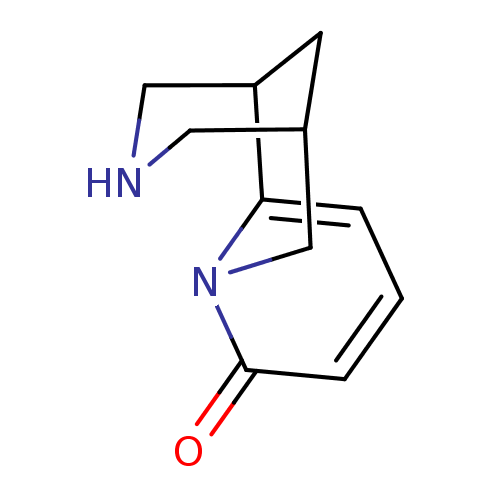
(CAS_485-35-8 | Cytisine | Cytisine-(-) | NSC_22407...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50047021
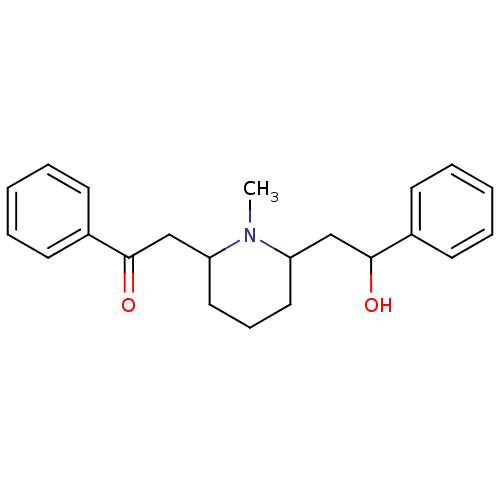
(2-(6-(2-hydroxy-2-phenylethyl)-1-methylpiperidin-2...)Show InChI InChI=1S/C22H27NO2/c1-23-19(15-21(24)17-9-4-2-5-10-17)13-8-14-20(23)16-22(25)18-11-6-3-7-12-18/h2-7,9-12,19-21,24H,8,13-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108
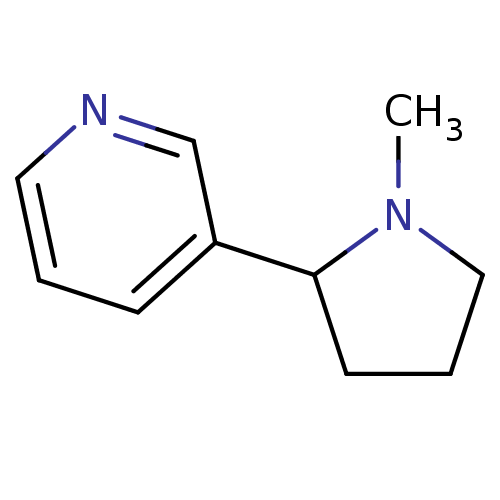
((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8296
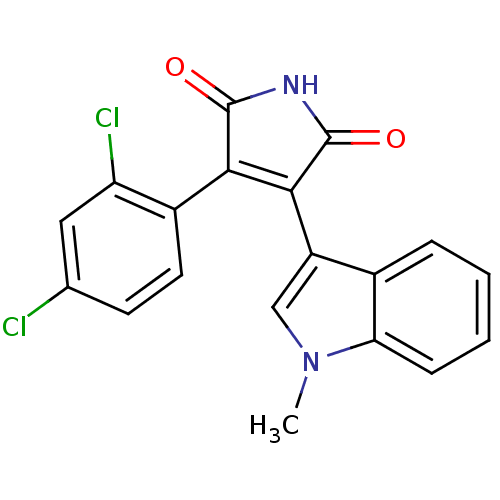
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human GSK-3alpha expressed in baculovirus infected insect Sf9 cells using GS-2 peptide as substrate... |
ACS Med Chem Lett 6: 548-52 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00044
BindingDB Entry DOI: 10.7270/Q2348N35 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM85207
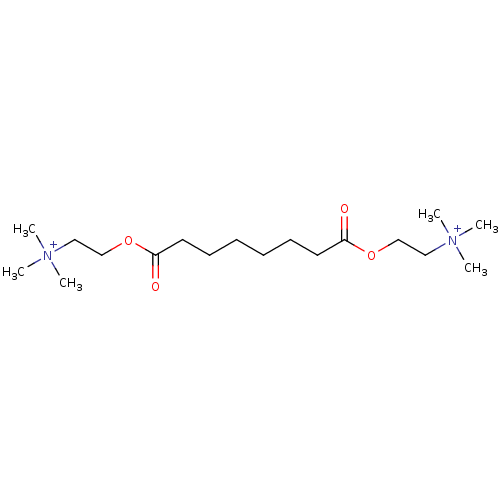
(CAS_123990 | NSC_123990 | Suberyldicholine)Show SMILES C[N+](C)(C)CCOC(=O)CCCCCCC(=O)OCC[N+](C)(C)C Show InChI InChI=1S/C18H38N2O4/c1-19(2,3)13-15-23-17(21)11-9-7-8-10-12-18(22)24-16-14-20(4,5)6/h7-16H2,1-6H3/q+2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50061567
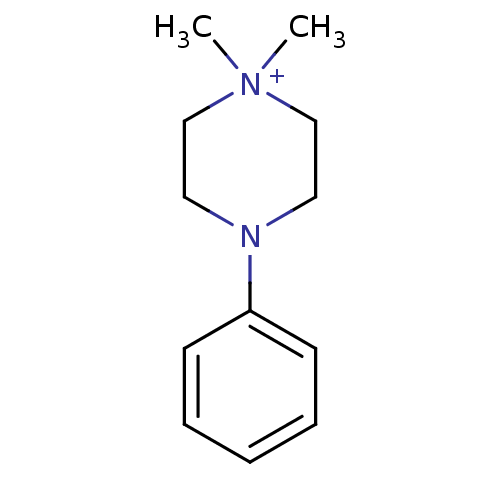
(1,1-Dimethyl-4-phenyl-piperazin-1-ium | CHEMBL1347...)Show InChI InChI=1S/C12H19N2/c1-14(2)10-8-13(9-11-14)12-6-4-3-5-7-12/h3-7H,8-11H2,1-2H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004656
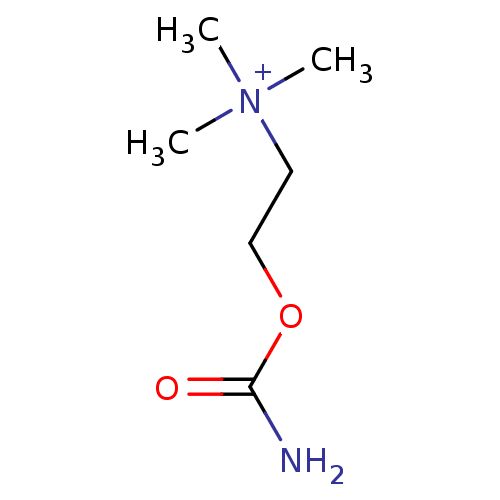
((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM8959

((2-Mercaptoethyl)trimethylammonium iodide acetate ...)Show InChI InChI=1S/C7H16NOS/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50060582
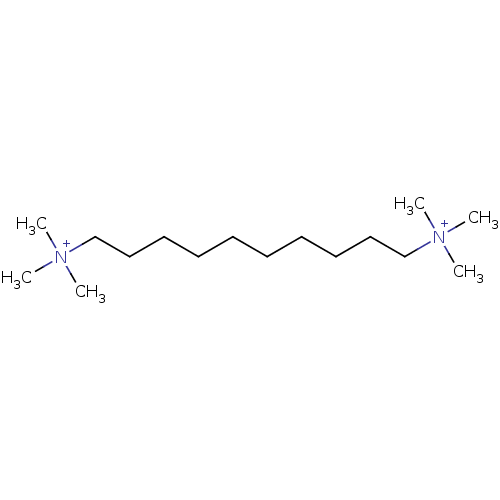
(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM81458
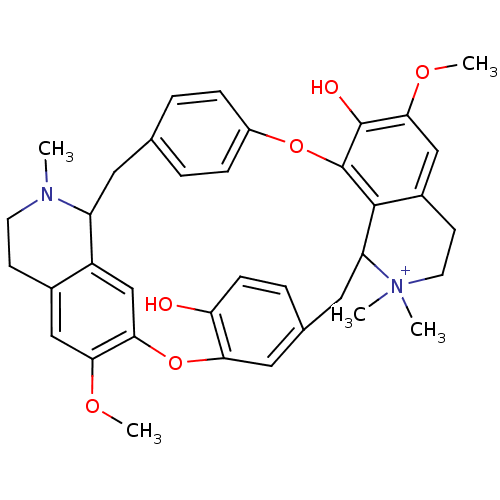
(CAS_57-94-3 | NSC_6000 | d-Tubocurarine)Show SMILES COc1cc2CC[N+](C)(C)C3Cc4ccc(O)c(Oc5cc6C(Cc7ccc(Oc(c1O)c23)cc7)N(C)CCc6cc5OC)c4 Show InChI InChI=1S/C37H40N2O6/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM86309
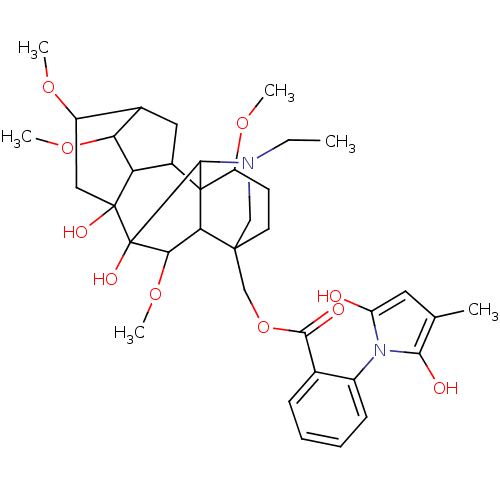
(CAS_21019-30-7 | Methyllycaconitine | NSC_5311278)Show SMILES CCN1CC2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CCC(OC)C34C5CC6C(OC)C5C(O)(CC6OC)C(O)(C(OC)C23)C14 |TLB:1:2:28:44.42,1:2:23.24.25:47,4:47:48:36.29.35,23:4:28:44.42,25:28:2.3.4:44.42,38:36:47.44:48,37:36:47.44:48,45:44:2.3.4:28,43:42:2.3.4:28,THB:2:48:47.44:36.29.35,42:48:23.24.25:47,36:42:2.3.4:28,30:29:47.44:48,29:28:2.3.4:44.42,33:32:36.38.39:30.29,45:44:48:36.29.35,26:25:47:2.3.48,5:4:28:44.42,(5.58,4.18,;4.03,3.67,;2.52,2.79,;2.59,.56,;1.98,-.55,;1.93,-2.19,;2.56,-3.67,;4.07,-4.23,;5.58,-3.65,;4.27,-5.76,;3.07,-6.74,;3.32,-8.26,;4.76,-8.8,;5.95,-7.83,;5.71,-6.31,;6.9,-5.33,;8.39,-5.72,;9.3,-7.02,;9.22,-4.43,;8.24,-3.24,;8.64,-1.75,;6.81,-3.79,;5.89,-2.31,;3.31,.22,;3.31,1.76,;1.98,2.53,;2.34,4.26,;2.78,5.82,;.65,1.76,;-.46,2.76,;-.27,4.29,;-1.66,4.95,;-2.72,3.83,;-4.4,3.79,;-6.02,3.54,;-1.98,2.48,;-2.7,1.12,;-3.96,.07,;-4.18,1.83,;-4.04,4.66,;-5.38,5.7,;-5.73,7.25,;-2.08,-.3,;-2.98,-1.63,;-.59,-.69,;-1.08,-2.25,;-1.69,-3.73,;.65,.22,;.65,2.85,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50061568

(2,2'-[(1,4-dioxobutane-1,4-diyl)bis(oxy)]bis(N,N,N...)Show InChI InChI=1S/C14H30N2O4/c1-15(2,3)9-11-19-13(17)7-8-14(18)20-12-10-16(4,5)6/h7-12H2,1-6H3/q+2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50026220
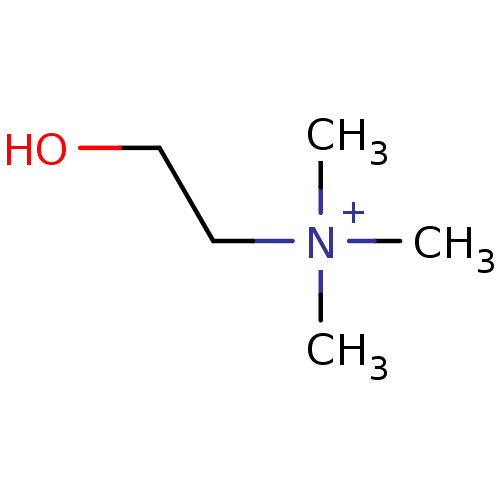
(2-hydroxy-N,N,N-trimethylethanaminium | CHEMBL2824...)Show InChI InChI=1S/C5H14NO/c1-6(2,3)4-5-7/h7H,4-5H2,1-3H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM86424

(CAS_39764 | NSC_39764 | Vecuronium)Show SMILES CC(=O)OC1C(CC2C3CCC4CC(OC(C)=O)C(CC4(C)C3CCC12C)N1CCCCC1)[N+]1(C)CCCCC1 Show InChI InChI=1S/C34H57N2O4/c1-23(37)39-31-20-25-12-13-26-27(34(25,4)22-29(31)35-16-8-6-9-17-35)14-15-33(3)28(26)21-30(32(33)40-24(2)38)36(5)18-10-7-11-19-36/h25-32H,6-22H2,1-5H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM86425

(CAS_27350 | NSC_27350 | Pancuronium)Show SMILES CC(=O)OC1C(CC2C3CCC4CC(OC(C)=O)C(CC4(C)C3CCC12C)[N+]1(C)CCCCC1)[N+]1(C)CCCCC1 Show InChI InChI=1S/C35H60N2O4/c1-24(38)40-32-21-26-13-14-27-28(35(26,4)23-31(32)37(6)19-11-8-12-20-37)15-16-34(3)29(27)22-30(33(34)41-25(2)39)36(5)17-9-7-10-18-36/h26-33H,7-23H2,1-6H3/q+2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50038416
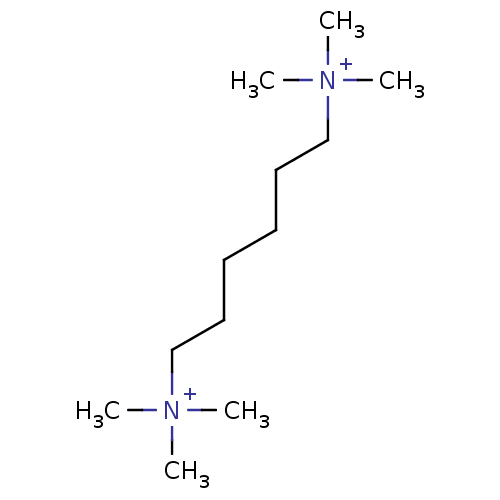
((1,6-trimethyl ammonium)hexane; dibromide | CHEMBL...)Show InChI InChI=1S/C12H30N2/c1-13(2,3)11-9-7-8-10-12-14(4,5)6/h7-12H2,1-6H3/q+2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM10709
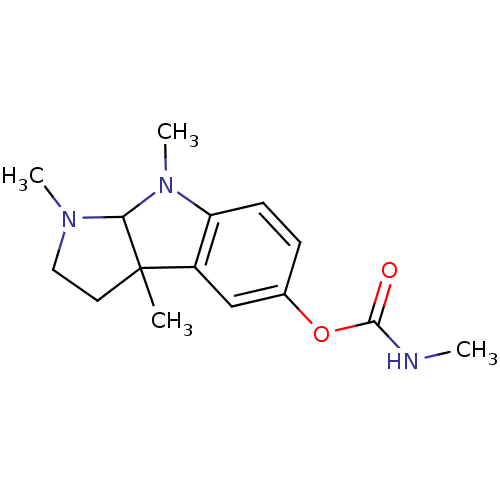
(1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b...)Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50143313

((12S,13aS)-12-Methoxy-1,4,5,6,9,11,12,13-octahydro...)Show SMILES CO[C@H]1CC=C2CCN3CCC4=C(CC(=O)OC4)[C@]23C1 |t:4,11| Show InChI InChI=1S/C16H21NO3/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13/h2,13H,3-10H2,1H3/t13-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM86423
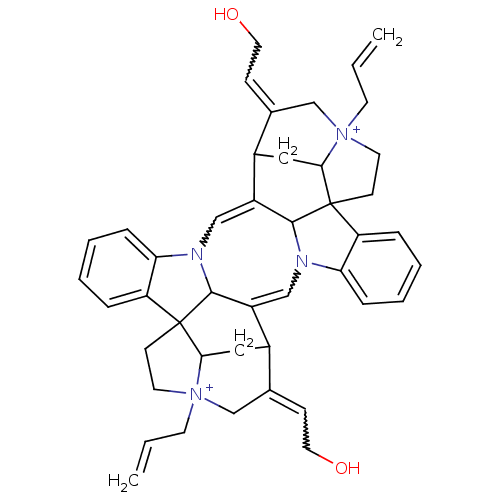
(Alcuronium | CAS_5311001 | NSC_5311001)Show SMILES OCC=C1C[N+]2(CC=C)CCC34C2CC1C1=CN2C5C(=CN(C31)c1ccccc41)C1CC3C5(CC[N+]3(CC=C)CC1=CCO)c1ccccc21 |w:2.1,16.18,20.22,41.50,TLB:41:40:30:18.19.32,20:19:30:35.39.40,10:11:13:5.3.4,2:3:13:11.15.22,28:11:13:5.3.4,THB:34:35:30:18.19.32,16:15:13:5.3.4,6:5:13:11.15.22,33:32:30:35.39.40,9:5:13:11.15.22,36:35:30:18.19.32,44:32:30:35.39.40| Show InChI InChI=1S/C44H50N4O2/c1-3-17-47-19-15-43-35-9-5-7-11-37(35)45-26-34-32-24-40-44(16-20-48(40,18-4-2)28-30(32)14-22-50)36-10-6-8-12-38(36)46(42(34)44)25-33(41(43)45)31(23-39(43)47)29(27-47)13-21-49/h3-14,25-26,31-32,39-42,49-50H,1-2,15-24,27-28H2/q+2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3585
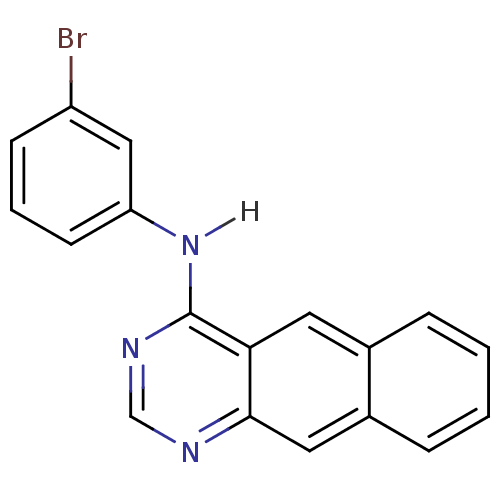
(4-[(3-Bromophenyl)amino]benzo[g]quinazoline | Benz...)Show InChI InChI=1S/C18H12BrN3/c19-14-6-3-7-15(10-14)22-18-16-8-12-4-1-2-5-13(12)9-17(16)20-11-21-18/h1-11H,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3604
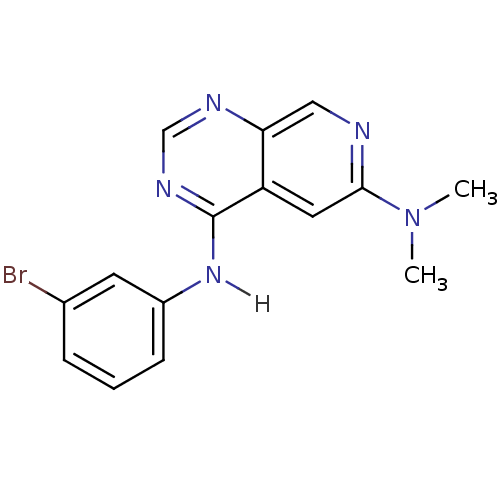
(4-N-(3-bromophenyl)-6-N,6-N-dimethylpyrido[3,4-d]p...)Show InChI InChI=1S/C15H14BrN5/c1-21(2)14-7-12-13(8-17-14)18-9-19-15(12)20-11-5-3-4-10(16)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3604

(4-N-(3-bromophenyl)-6-N,6-N-dimethylpyrido[3,4-d]p...)Show InChI InChI=1S/C15H14BrN5/c1-21(2)14-7-12-13(8-17-14)18-9-19-15(12)20-11-5-3-4-10(16)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 41: 742-51 (1998)
Article DOI: 10.1021/jm970641d
BindingDB Entry DOI: 10.7270/Q2DB800T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3556
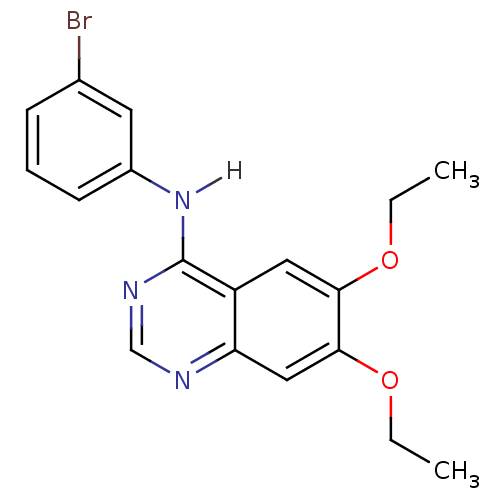
(4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...)Show InChI InChI=1S/C18H18BrN3O2/c1-3-23-16-9-14-15(10-17(16)24-4-2)20-11-21-18(14)22-13-7-5-6-12(19)8-13/h5-11H,3-4H2,1-2H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3603

(4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-11-12(7-17-13)18-8-19-14(11)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3570
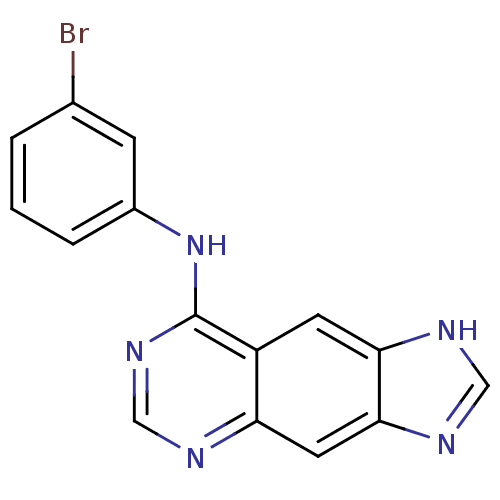
(8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...)Show InChI InChI=1S/C15H10BrN5/c16-9-2-1-3-10(4-9)21-15-11-5-13-14(19-7-18-13)6-12(11)17-8-20-15/h1-8H,(H,18,19)(H,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 42: 5464-74 (1999)
Article DOI: 10.1021/jm9903949
BindingDB Entry DOI: 10.7270/Q28K7783 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3603

(4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-11-12(7-17-13)18-8-19-14(11)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 42: 5464-74 (1999)
Article DOI: 10.1021/jm9903949
BindingDB Entry DOI: 10.7270/Q28K7783 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3603

(4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-11-12(7-17-13)18-8-19-14(11)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 41: 742-51 (1998)
Article DOI: 10.1021/jm970641d
BindingDB Entry DOI: 10.7270/Q2DB800T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3570

(8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...)Show InChI InChI=1S/C15H10BrN5/c16-9-2-1-3-10(4-9)21-15-11-5-13-14(19-7-18-13)6-12(11)17-8-20-15/h1-8H,(H,18,19)(H,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3572
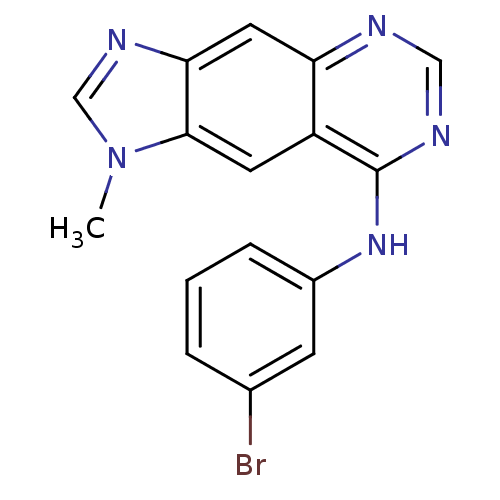
(8-[(3-Bromophenyl)amino]-1-methyl-1H-imidazo[4,5-g...)Show InChI InChI=1S/C16H12BrN5/c1-22-9-20-14-7-13-12(6-15(14)22)16(19-8-18-13)21-11-4-2-3-10(17)5-11/h2-9H,1H3,(H,18,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032
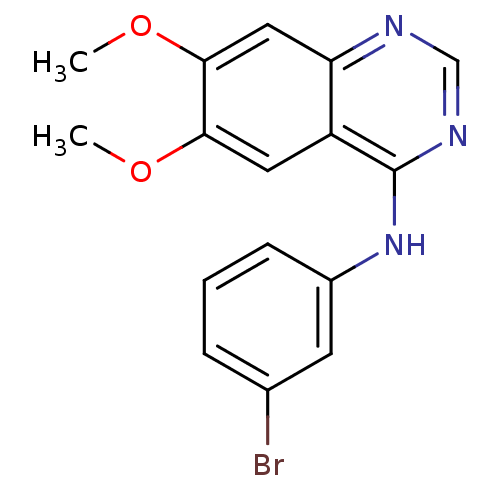
(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3574
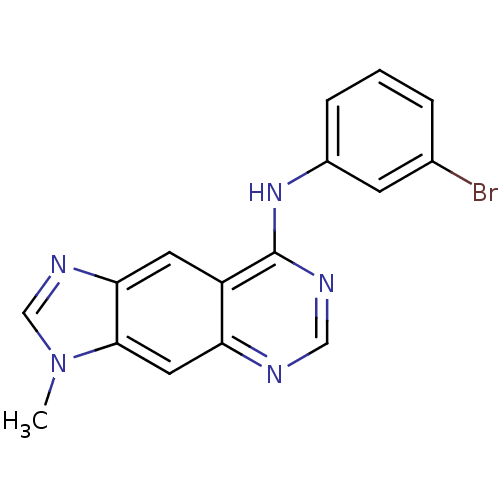
(8-[(3-Bromophenyl)amino]-3-methyl-3H-imidazo[4,5-g...)Show InChI InChI=1S/C16H12BrN5/c1-22-9-20-14-6-12-13(7-15(14)22)18-8-19-16(12)21-11-4-2-3-10(17)5-11/h2-9H,1H3,(H,18,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
| Assay Description
Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50003666

(3-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...)Show SMILES CN([C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCNC(=O)Nc1ccccc1C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C Show InChI InChI=1S/C44H56N8O9/c1-27-15-9-11-19-31(27)50-42(59)46-22-14-13-21-33(39(56)49-35(25-37(53)54)41(58)52(5)36(38(45)55)23-28-16-7-6-8-17-28)48-40(57)34(51-43(60)61-44(2,3)4)24-29-26-47-32-20-12-10-18-30(29)32/h6-12,15-20,26,33-36,47H,13-14,21-25H2,1-5H3,(H2,45,55)(H,48,57)(H,49,56)(H,51,60)(H,53,54)(H2,46,50,59)/t33?,34-,35?,36?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assay |
J Med Chem 35: 4249-52 (1992)
BindingDB Entry DOI: 10.7270/Q2Z60N07 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50003669

(Ac-Tyr(SO3H)-Met-Gly-Trp-Met-R-Dtc-Phe-NH2 | CHEMB...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N1CSC(C)(C)[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H63N9O11S4/c1-29(59)53-39(24-31-15-17-33(18-16-31)73(67,68)69)45(63)55-36(19-21-70-4)44(62)52-27-41(60)54-40(25-32-26-51-35-14-10-9-13-34(32)35)46(64)56-37(20-22-71-5)48(66)58-28-72-49(2,3)42(58)47(65)57-38(43(50)61)23-30-11-7-6-8-12-30/h6-18,26,36-40,42,51H,19-25,27-28H2,1-5H3,(H2,50,61)(H,52,62)(H,53,59)(H,54,60)(H,55,63)(H,56,64)(H,57,65)(H,67,68,69)/t36-,37-,38-,39-,40-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. |
J Med Chem 35: 4249-52 (1992)
BindingDB Entry DOI: 10.7270/Q2Z60N07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3568

(CHEMBL327307 | N-(3,4-dibromophenyl)-6,7-dimethoxy...)Show InChI InChI=1S/C16H13Br2N3O2/c1-22-14-6-10-13(7-15(14)23-2)19-8-20-16(10)21-9-3-4-11(17)12(18)5-9/h3-8H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3595
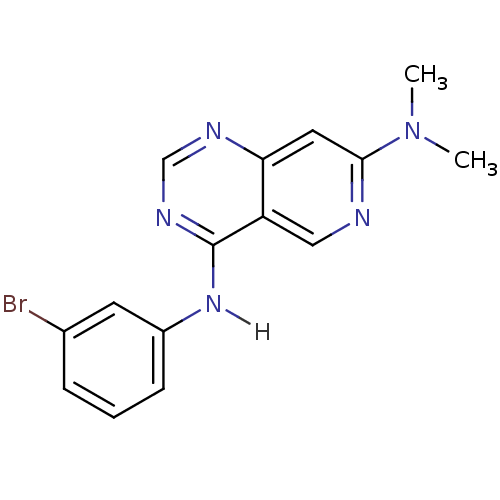
(4-N-(3-bromophenyl)-7-N,7-N-dimethylpyrido[4,3-d]p...)Show InChI InChI=1S/C15H14BrN5/c1-21(2)14-7-13-12(8-17-14)15(19-9-18-13)20-11-5-3-4-10(16)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 40: 3915-25 (1997)
Article DOI: 10.1021/jm970366v
BindingDB Entry DOI: 10.7270/Q2NS0S2V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3595

(4-N-(3-bromophenyl)-7-N,7-N-dimethylpyrido[4,3-d]p...)Show InChI InChI=1S/C15H14BrN5/c1-21(2)14-7-13-12(8-17-14)15(19-9-18-13)20-11-5-3-4-10(16)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3297
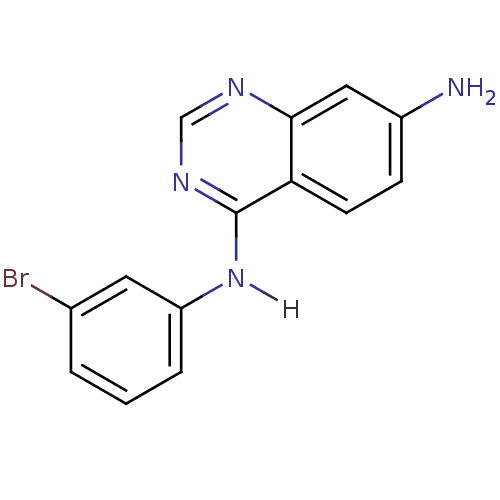
(4-Anilinoquinazoline deriv. 48 | 4-N-(3-bromopheny...)Show InChI InChI=1S/C14H11BrN4/c15-9-2-1-3-11(6-9)19-14-12-5-4-10(16)7-13(12)17-8-18-14/h1-8H,16H2,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 38: 3482-7 (1995)
Article DOI: 10.1021/jm00018a008
BindingDB Entry DOI: 10.7270/Q2319T3K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50049566

(CHEMBL55729 | Quinazolin-7-ylamine)Show InChI InChI=1S/C8H7N3/c9-7-2-1-6-4-10-5-11-8(6)3-7/h1-5H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
| Assay Description
Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3297

(4-Anilinoquinazoline deriv. 48 | 4-N-(3-bromopheny...)Show InChI InChI=1S/C14H11BrN4/c15-9-2-1-3-11(6-9)19-14-12-5-4-10(16)7-13(12)17-8-18-14/h1-8H,16H2,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3303
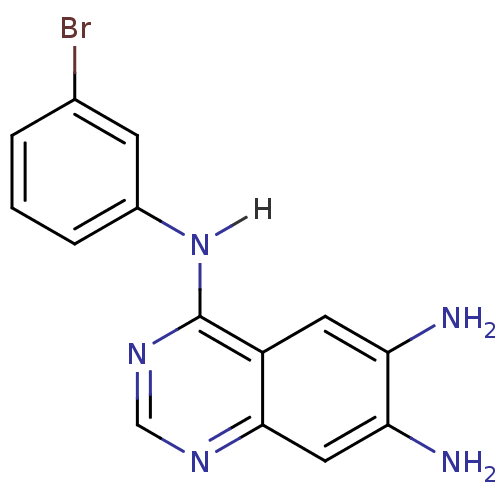
(4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...)Show InChI InChI=1S/C14H12BrN5/c15-8-2-1-3-9(4-8)20-14-10-5-11(16)12(17)6-13(10)18-7-19-14/h1-7H,16-17H2,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 38: 3482-7 (1995)
Article DOI: 10.1021/jm00018a008
BindingDB Entry DOI: 10.7270/Q2319T3K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3303

(4-Anilinoquinazoline deriv. 54 | 4-N-(3-bromopheny...)Show InChI InChI=1S/C14H12BrN5/c15-8-2-1-3-9(4-8)20-14-10-5-11(16)12(17)6-13(10)18-7-19-14/h1-7H,16-17H2,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3600

(4-N-(3-bromophenyl)pyrido[3,4-d]pyrimidine-4,6-dia...)Show InChI InChI=1S/C13H10BrN5/c14-8-2-1-3-9(4-8)19-13-10-5-12(15)16-6-11(10)17-7-18-13/h1-7H,(H2,15,16)(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3594
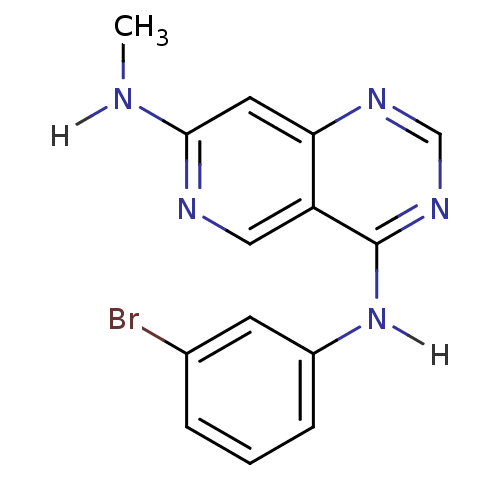
(4-N-(3-bromophenyl)-7-N-methylpyrido[4,3-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-12-11(7-17-13)14(19-8-18-12)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3594

(4-N-(3-bromophenyl)-7-N-methylpyrido[4,3-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-12-11(7-17-13)14(19-8-18-12)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 40: 3915-25 (1997)
Article DOI: 10.1021/jm970366v
BindingDB Entry DOI: 10.7270/Q2NS0S2V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3600

(4-N-(3-bromophenyl)pyrido[3,4-d]pyrimidine-4,6-dia...)Show InChI InChI=1S/C13H10BrN5/c14-8-2-1-3-9(4-8)19-13-10-5-12(15)16-6-11(10)17-7-18-13/h1-7H,(H2,15,16)(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 41: 742-51 (1998)
Article DOI: 10.1021/jm970641d
BindingDB Entry DOI: 10.7270/Q2DB800T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3302
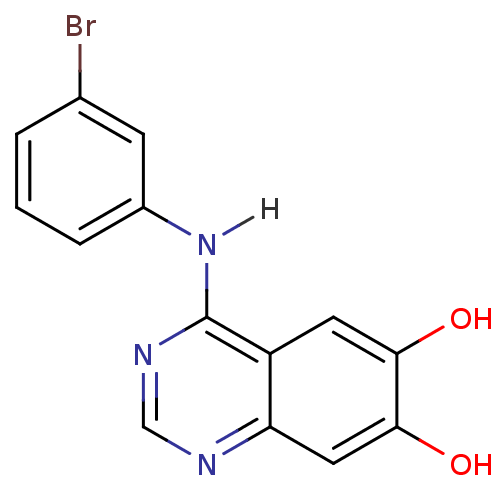
(4-Anilinoquinazoline deriv. 53 | 4-[(3-bromophenyl...)Show InChI InChI=1S/C14H10BrN3O2/c15-8-2-1-3-9(4-8)18-14-10-5-12(19)13(20)6-11(10)16-7-17-14/h1-7,19-20H,(H,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 38: 3482-7 (1995)
Article DOI: 10.1021/jm00018a008
BindingDB Entry DOI: 10.7270/Q2319T3K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3557
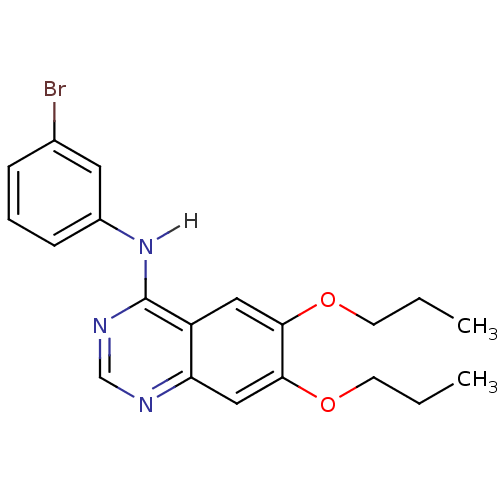
(CHEMBL418967 | N-(3-bromophenyl)-6,7-dipropoxyquin...)Show InChI InChI=1S/C20H22BrN3O2/c1-3-8-25-18-11-16-17(12-19(18)26-9-4-2)22-13-23-20(16)24-15-7-5-6-14(21)10-15/h5-7,10-13H,3-4,8-9H2,1-2H3,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3302

(4-Anilinoquinazoline deriv. 53 | 4-[(3-bromophenyl...)Show InChI InChI=1S/C14H10BrN3O2/c15-8-2-1-3-9(4-8)18-14-10-5-12(19)13(20)6-11(10)16-7-17-14/h1-7,19-20H,(H,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data