

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394591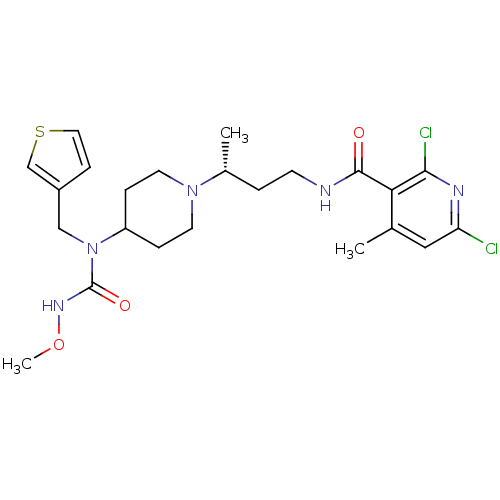 (CHEMBL2164207) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359481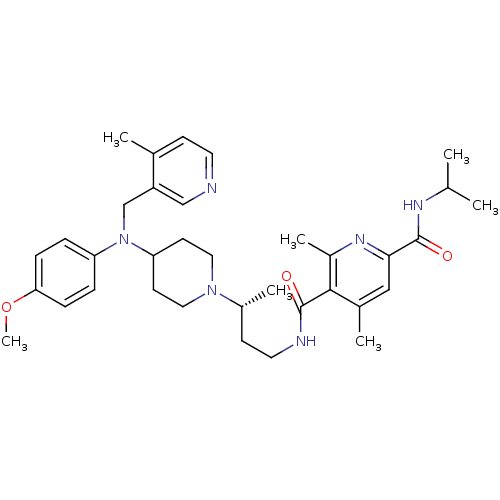 (CHEMBL1926899) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394596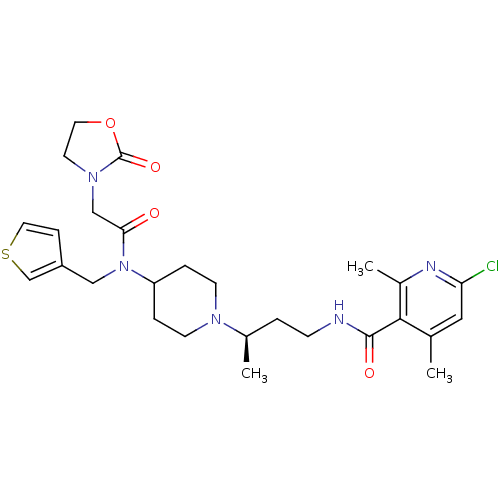 (CHEMBL2164202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394588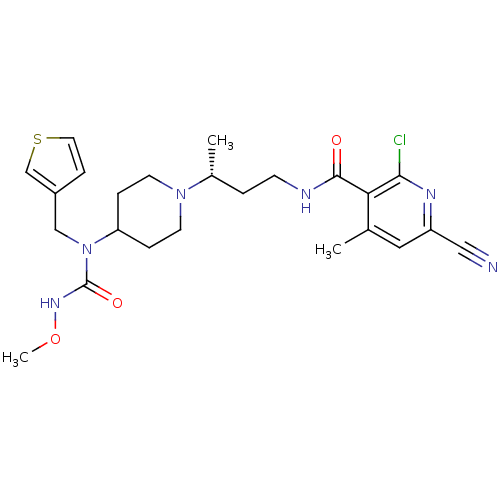 (CHEMBL2164210) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394601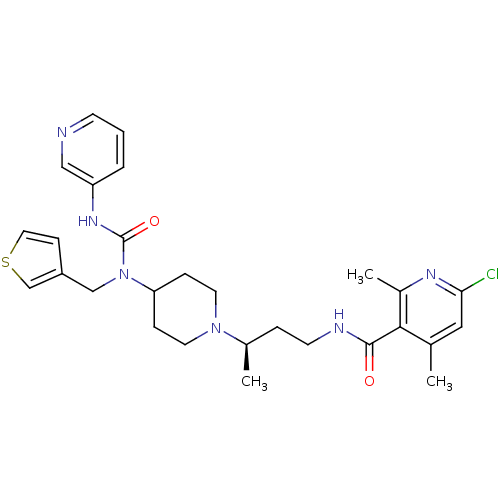 (CHEMBL2164217) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359487 (CHEMBL1926893) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359478 (CHEMBL1927009) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359482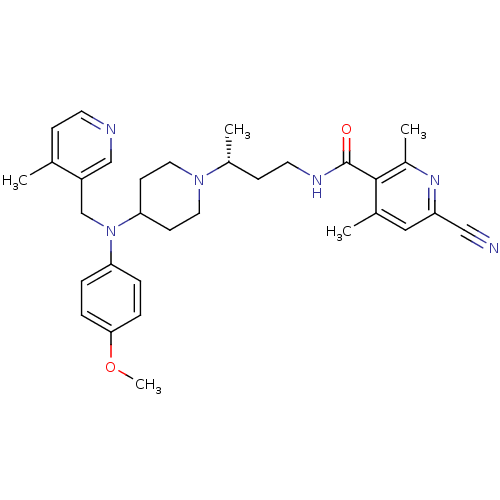 (CHEMBL1926898) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359485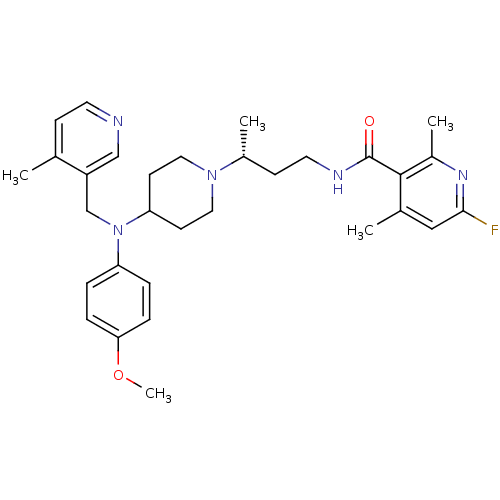 (CHEMBL1926896) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394592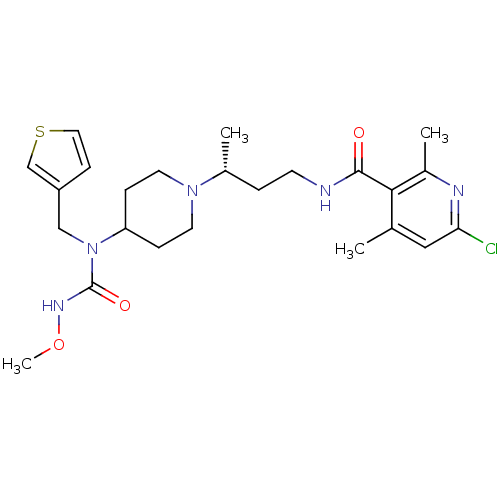 (CHEMBL2164206) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394595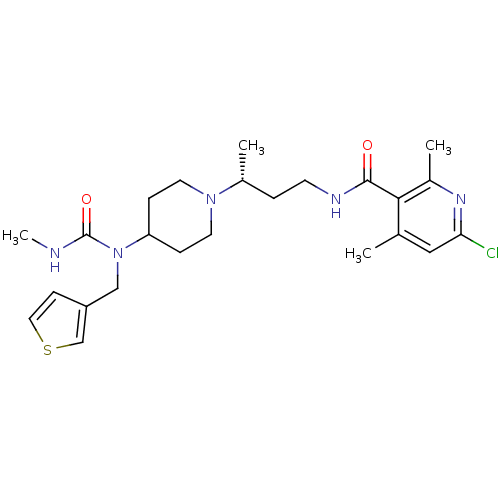 (CHEMBL2164203) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394593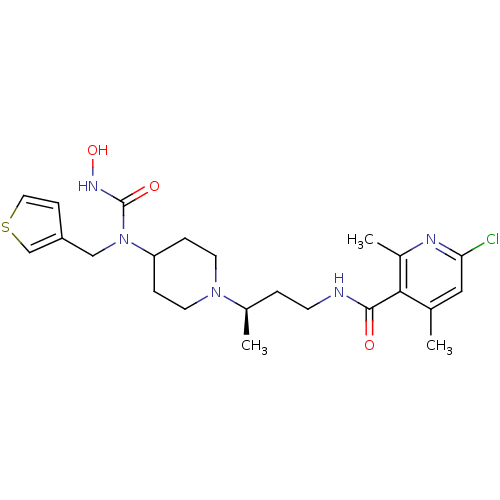 (CHEMBL2164205) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394587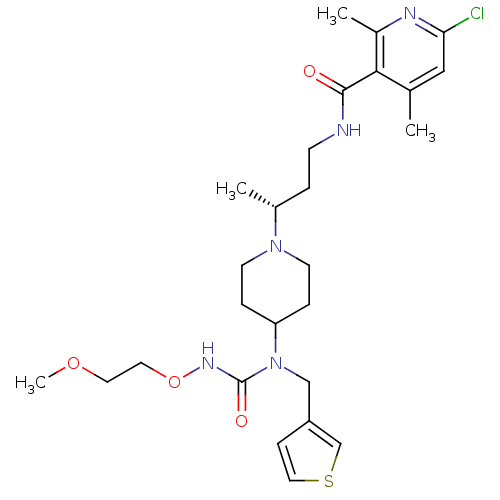 (CHEMBL2164211) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359459 (CHEMBL1927008) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394589 (CHEMBL2164209) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394590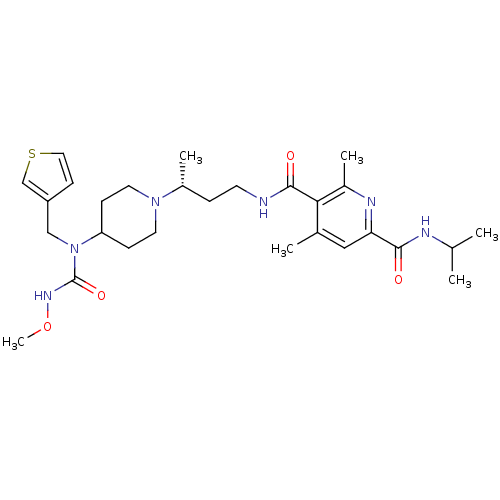 (CHEMBL2164208) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394599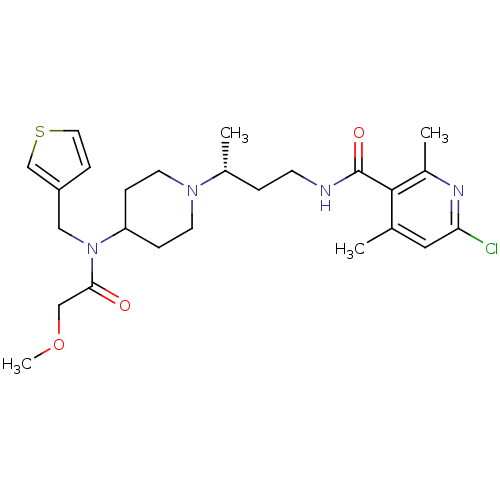 (CHEMBL2164219) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394598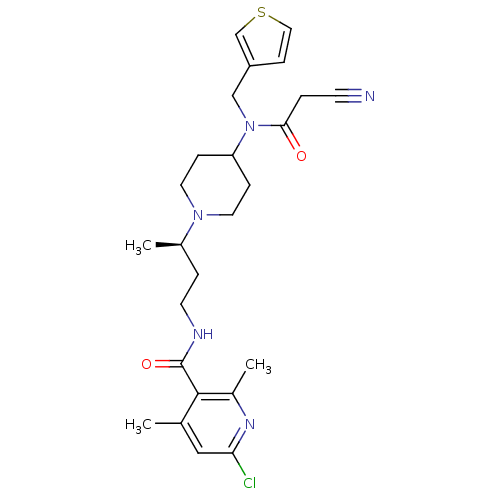 (CHEMBL2164220) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394586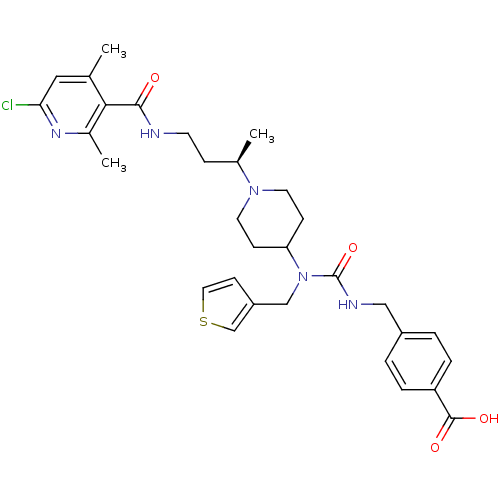 (CHEMBL2164212) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359480 (CHEMBL1926900) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359489 (CHEMBL1926891) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359479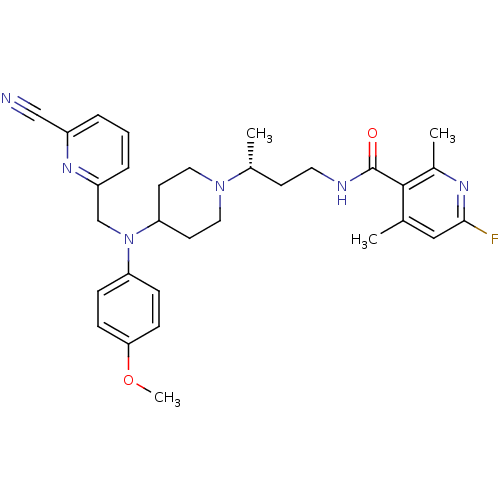 (CHEMBL1927010) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394603 (CHEMBL2164215) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394597 (CHEMBL2164201) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359464 (CHEMBL1926901) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394600 (CHEMBL2164218) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359461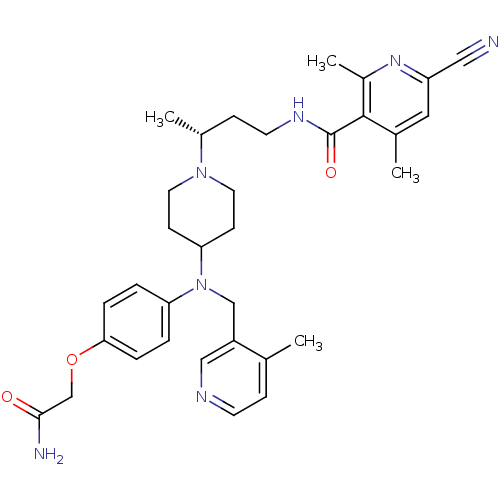 (CHEMBL1926904) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50394605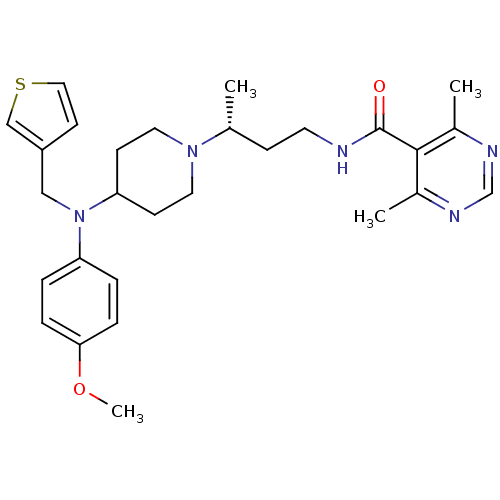 (CHEMBL2164213) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359463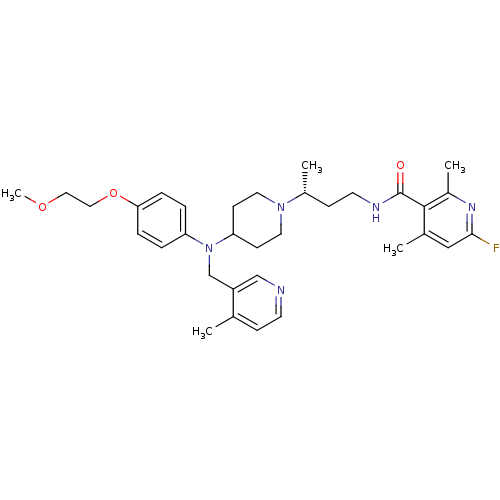 (CHEMBL1926902) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50333890 (CHEMBL1644092 | N-((1H-benzo[d]imidazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells expressing CD4 assessed as inhibition of SDF-1-induced Ca2+ signaling | Bioorg Med Chem Lett 21: 262-6 (2010) Article DOI: 10.1016/j.bmcl.2010.11.023 BindingDB Entry DOI: 10.7270/Q21N81D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359486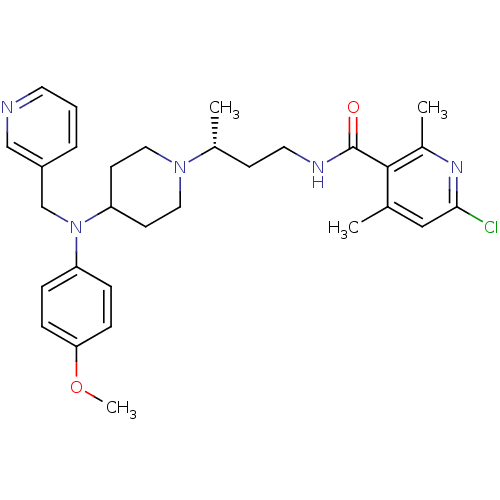 (CHEMBL1926894) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359483 (CHEMBL1926897) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359466 (CHEMBL1926885) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359490 (CHEMBL1926890) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359465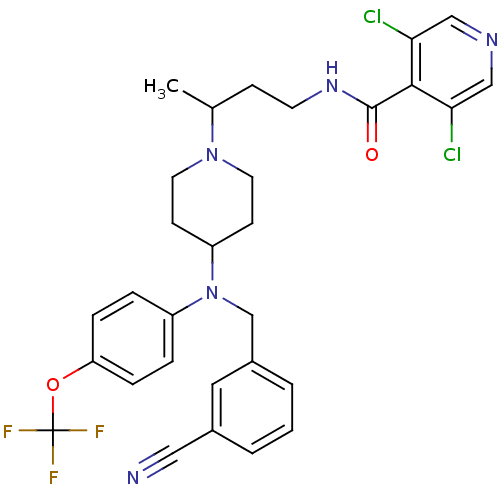 (CHEMBL1926886) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359467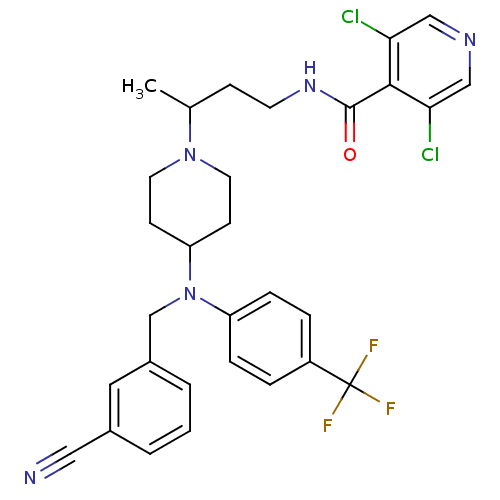 (CHEMBL1926884) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.67 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Osnabr£ck Curated by ChEMBL | Assay Description Antagonist activity against CXCR5 expressed in U87.CD.CXCR5 cells assessed as inhibition of CCL3L1-induced calcium signaling incubated for 10 mins by... | J Med Chem 55: 10405-13 (2012) Article DOI: 10.1021/jm301337y BindingDB Entry DOI: 10.7270/Q26M39QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50333893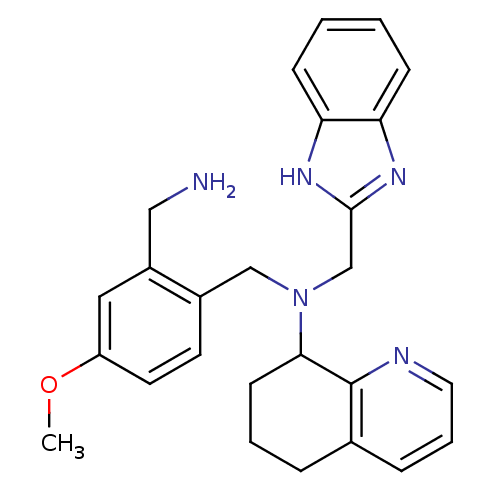 (CHEMBL1644072 | N-((1H-benzo[d]imidazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells expressing CD4 assessed as inhibition of SDF-1-induced Ca2+ signaling | Bioorg Med Chem Lett 21: 262-6 (2010) Article DOI: 10.1016/j.bmcl.2010.11.023 BindingDB Entry DOI: 10.7270/Q21N81D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50333882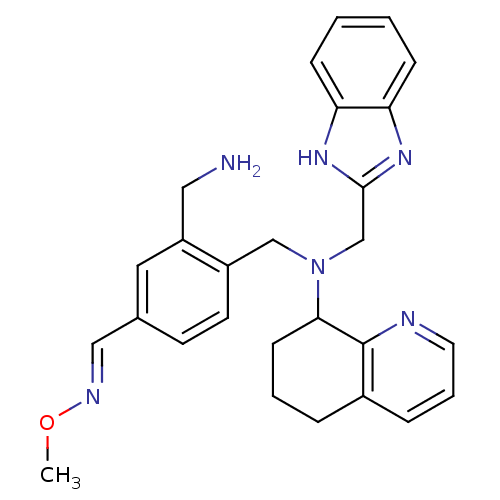 (4-((((1H-benzo[d]imidazol-2-yl)methyl)(5,6,7,8-tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells expressing CD4 assessed as inhibition of SDF-1-induced Ca2+ signaling | Bioorg Med Chem Lett 21: 262-6 (2010) Article DOI: 10.1016/j.bmcl.2010.11.023 BindingDB Entry DOI: 10.7270/Q21N81D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359491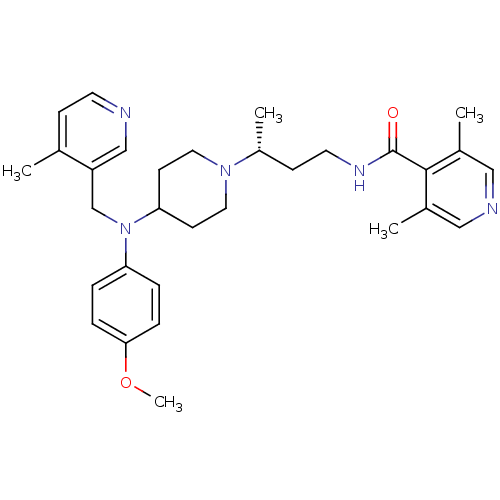 (CHEMBL1926889) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359460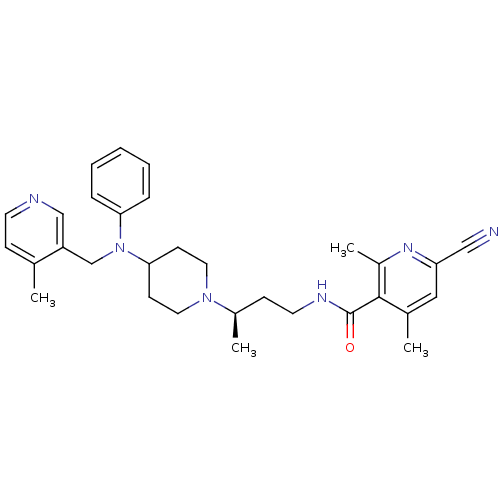 (CHEMBL1926905) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359468 (CHEMBL1926883) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50337581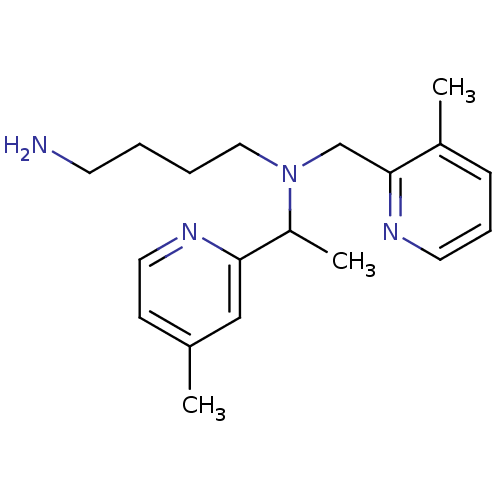 (CHEMBL1682993 | rac-N1-(1-(4-Methylpyridin-2-yl)et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium signaling | Bioorg Med Chem Lett 21: 1414-8 (2011) Article DOI: 10.1016/j.bmcl.2011.01.021 BindingDB Entry DOI: 10.7270/Q2FT8M9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50333894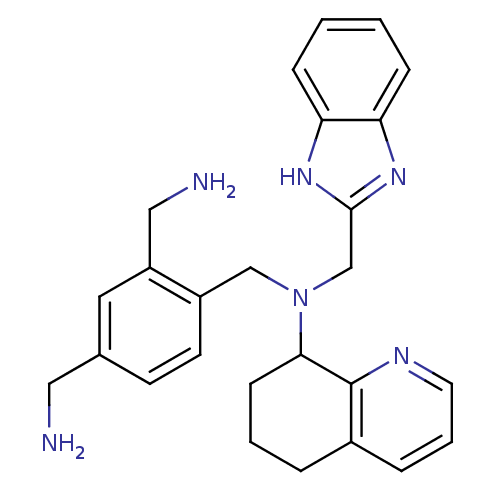 (CHEMBL1644073 | [4-((((1H-benzo[d]imidazol-2-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells expressing CD4 assessed as inhibition of SDF-1-induced Ca2+ signaling | Bioorg Med Chem Lett 21: 262-6 (2010) Article DOI: 10.1016/j.bmcl.2010.11.023 BindingDB Entry DOI: 10.7270/Q21N81D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339989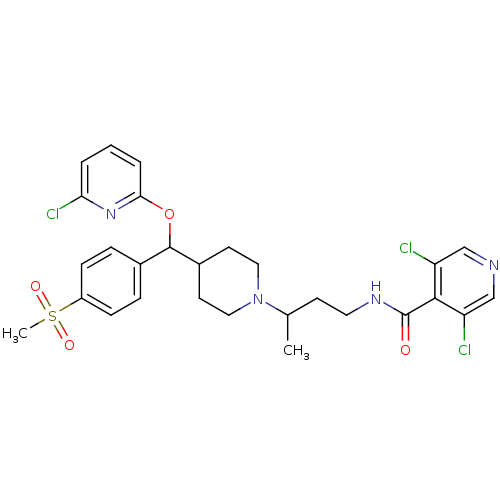 (3,5-dichloro-N-(3-(4-((6-chloropyridin-2-yloxy)(4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [128I]-RANTES from CCR5 expressed in HEK293F cells | Bioorg Med Chem Lett 21: 2450-5 (2011) Article DOI: 10.1016/j.bmcl.2011.02.058 BindingDB Entry DOI: 10.7270/Q2F76CV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359492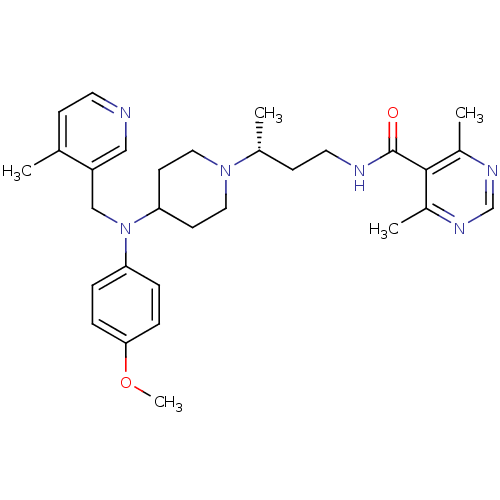 (CHEMBL1926888) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in P4R5 cells co-expressing CD4, and LTR-beta-gal construct assessed as inhibition of infusion to HIV ... | ACS Med Chem Lett 3: 216-221 (2012) Article DOI: 10.1021/ml2002604 BindingDB Entry DOI: 10.7270/Q2T43V6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359492 (CHEMBL1926888) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50337584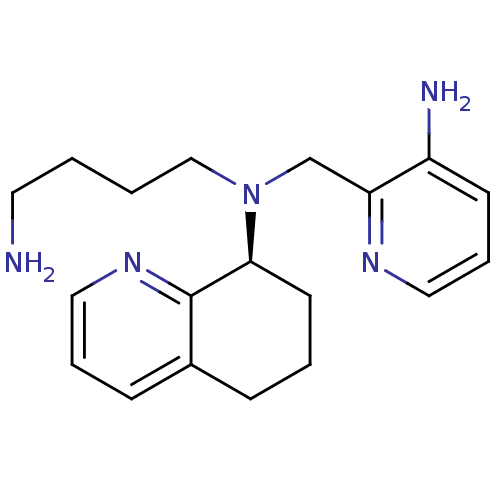 ((S)-N1-((3-aminopyridin-2-yl)methyl)-N1-(5,6,7,8-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human CEM-CCRF cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 1414-8 (2011) Article DOI: 10.1016/j.bmcl.2011.01.021 BindingDB Entry DOI: 10.7270/Q2FT8M9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50333895 (CHEMBL1644074 | [4-((((1H-benzo[d]imidazol-2-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells expressing CD4 assessed as inhibition of SDF-1-induced Ca2+ signaling | Bioorg Med Chem Lett 21: 262-6 (2010) Article DOI: 10.1016/j.bmcl.2010.11.023 BindingDB Entry DOI: 10.7270/Q21N81D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50359471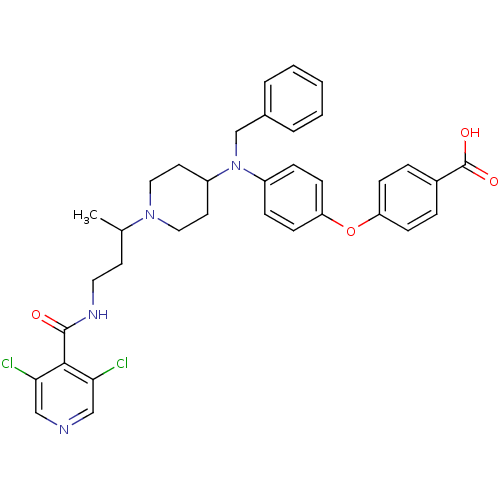 (CHEMBL1926878) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 assessed as inhibition of HIV1 gp120-induced cell-cell fusion between human HeLa P4/R5 cells and CHO-tat10 cel... | Bioorg Med Chem Lett 21: 6950-4 (2011) Article DOI: 10.1016/j.bmcl.2011.09.133 BindingDB Entry DOI: 10.7270/Q2GM87RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 209 total ) | Next | Last >> |