

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50504750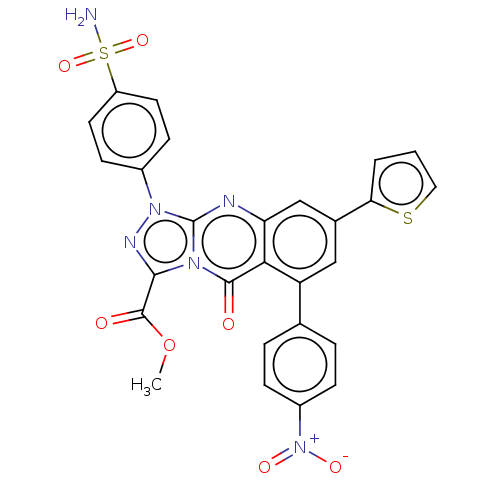 (CHEMBL4536202) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Egyptian Russian University Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111843 BindingDB Entry DOI: 10.7270/Q2WW7N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916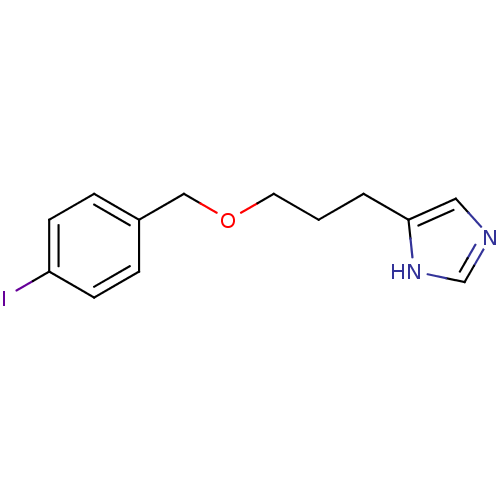 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50504776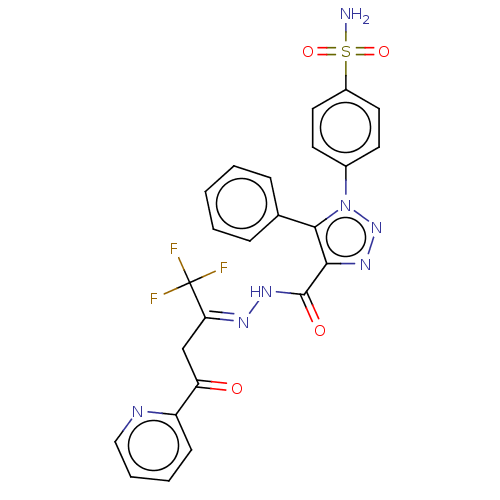 (CHEMBL4441461) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Egyptian Russian University Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111843 BindingDB Entry DOI: 10.7270/Q2WW7N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22910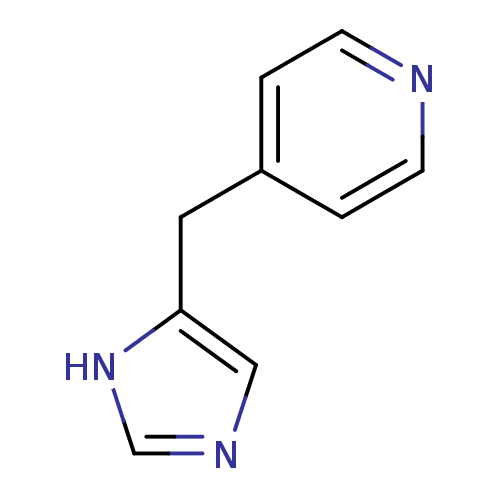 (4-(1H-imidazol-5-ylmethyl)pyridine | Immethridine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22909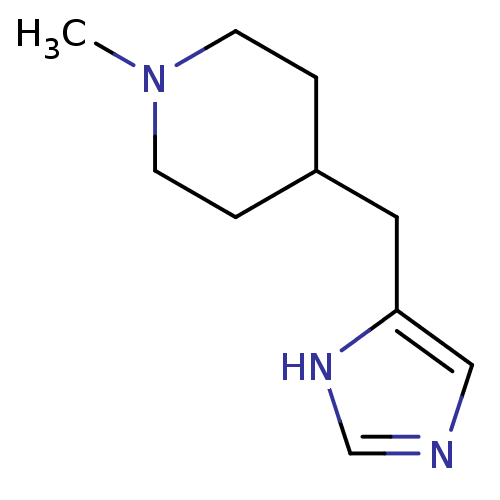 (4-(1H-imidazol-5-ylmethyl)-1-methylpiperidine | Me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419448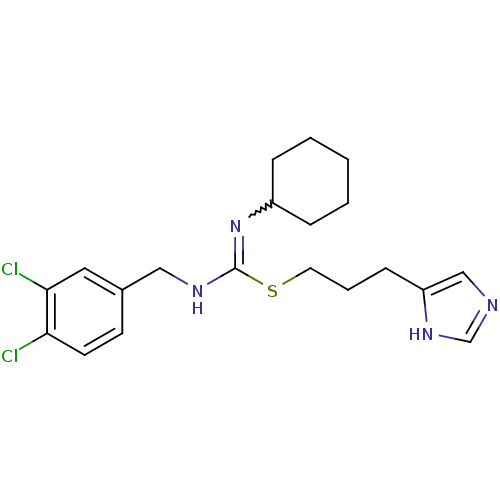 (CHEMBL1923026) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419444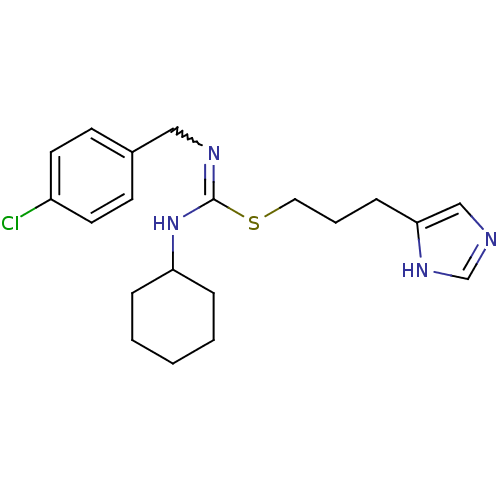 (CHEMBL43934 | VUF-5228) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419456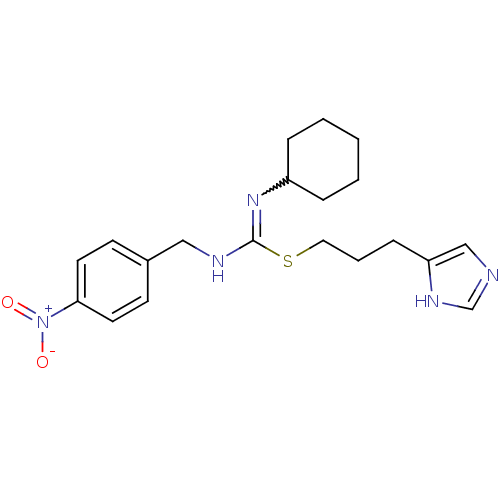 (CHEMBL1923034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50414391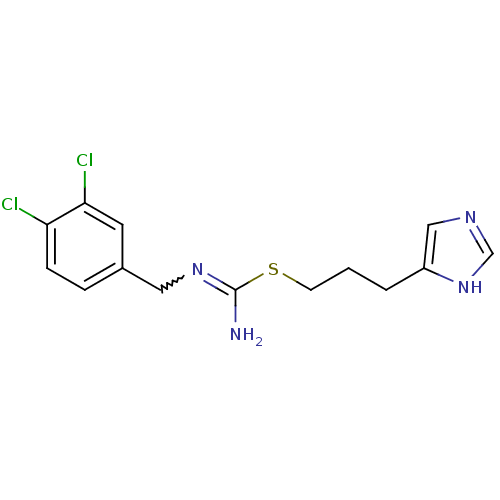 (CHEMBL1202332 | CHEMBL553423) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50419455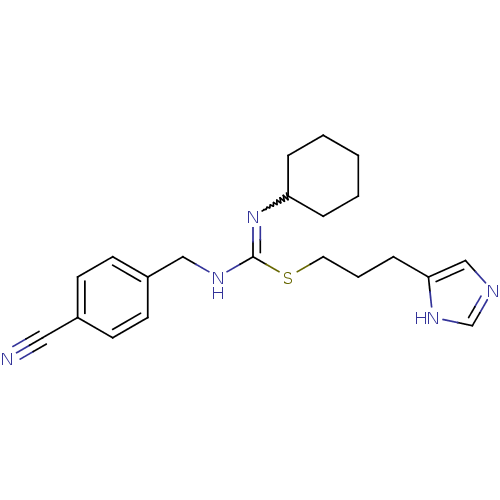 (CHEMBL1923033) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50212876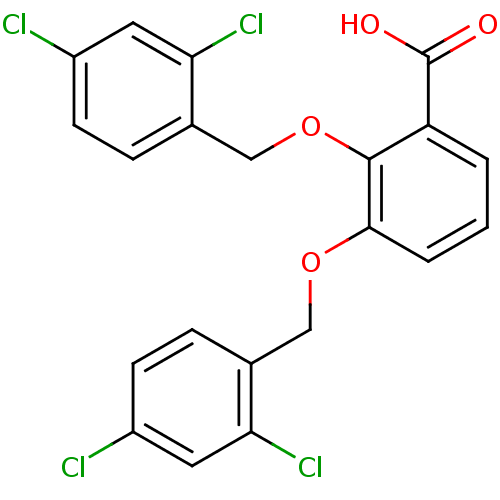 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from eFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50377859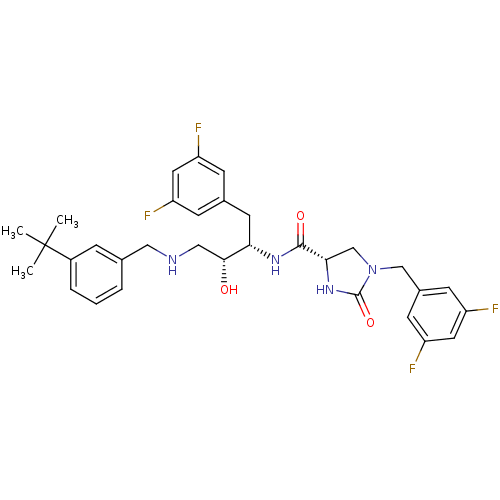 (CHEMBL260621) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human BACE1 | Bioorg Med Chem Lett 18: 2900-4 (2008) Article DOI: 10.1016/j.bmcl.2008.03.081 BindingDB Entry DOI: 10.7270/Q2RV0PKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212876 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414387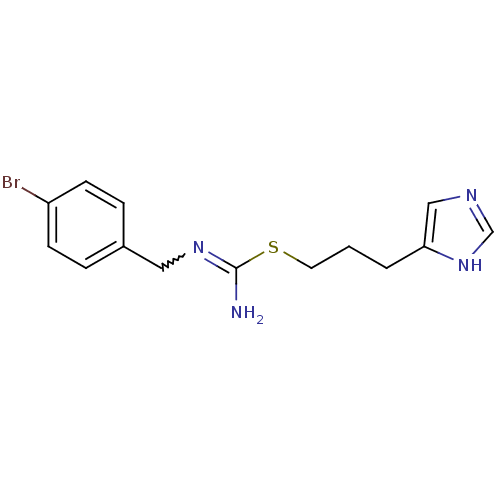 (CHEMBL1743881) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22912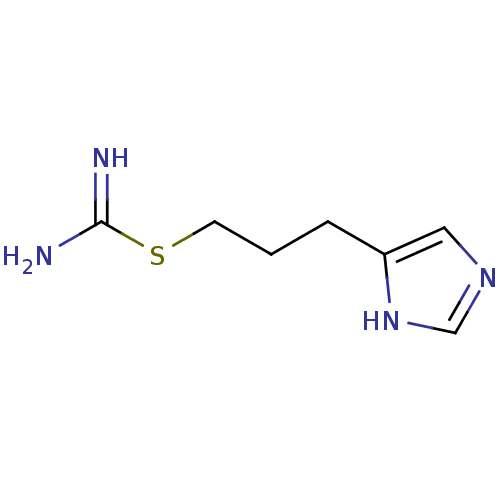 (VUF 8328 | VUF8328 | {[3-(1H-imidazol-4-yl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233626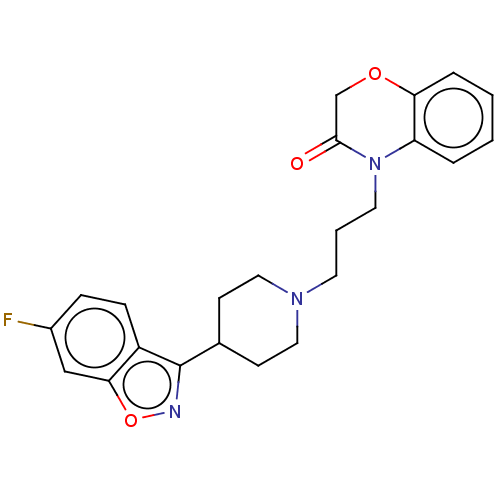 (CHEMBL4062602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233626 (CHEMBL4062602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212885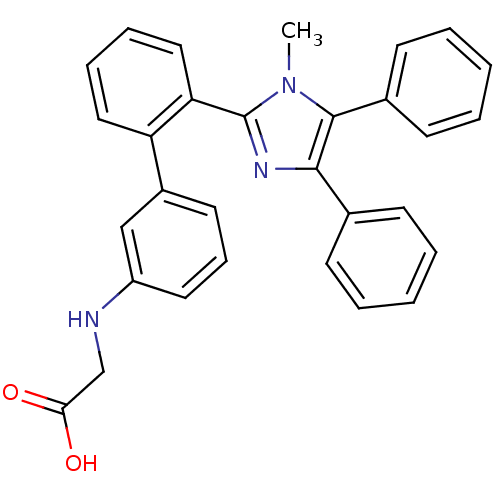 (CHEMBL245282 | [2'-(1-methyl-4,5-diphenyl-1H-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22912 (VUF 8328 | VUF8328 | {[3-(1H-imidazol-4-yl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50361010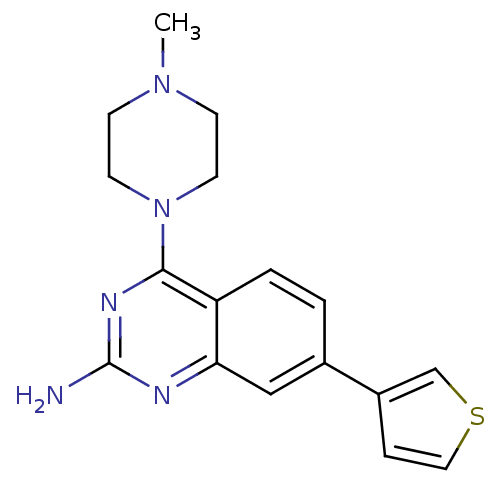 (CHEMBL1935572) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414388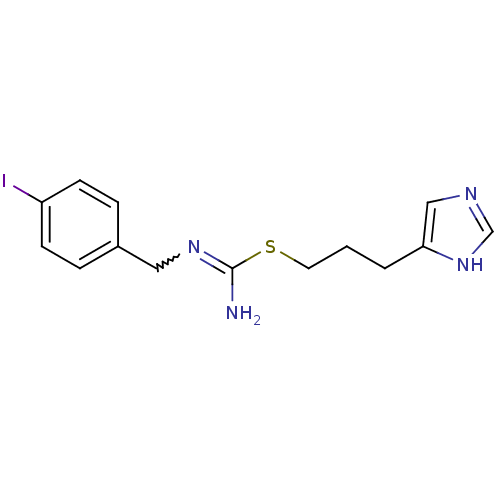 (CHEMBL1202321) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting | Bioorg Med Chem 17: 3987-94 (2009) Article DOI: 10.1016/j.bmc.2009.04.007 BindingDB Entry DOI: 10.7270/Q2SQ91MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233626 (CHEMBL4062602) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233626 (CHEMBL4062602) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50504771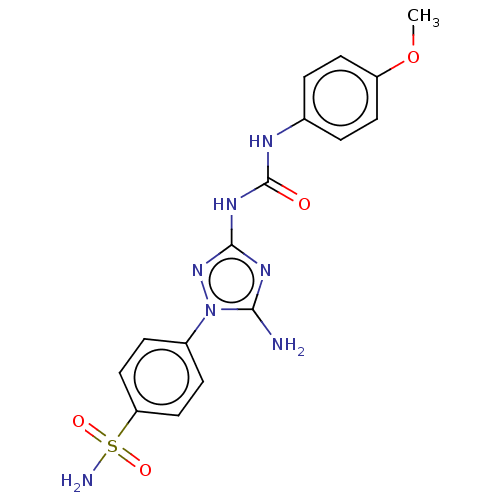 (CHEMBL4529889) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Egyptian Russian University Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111843 BindingDB Entry DOI: 10.7270/Q2WW7N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50582974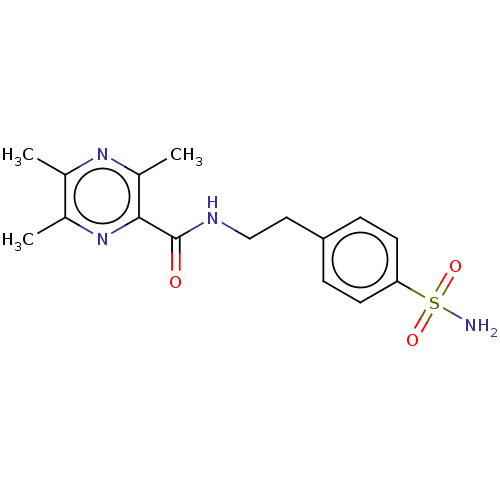 (CHEMBL5082587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CA12 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114008 BindingDB Entry DOI: 10.7270/Q2988BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212884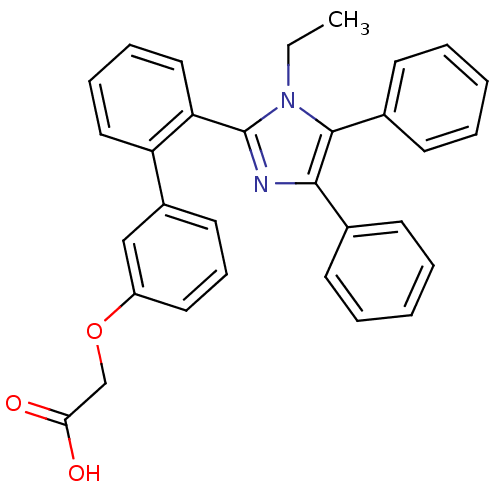 (CHEMBL245284 | [2'-(1-ethyl-4,5-diphenyl-1H-imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid from aFABP | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212877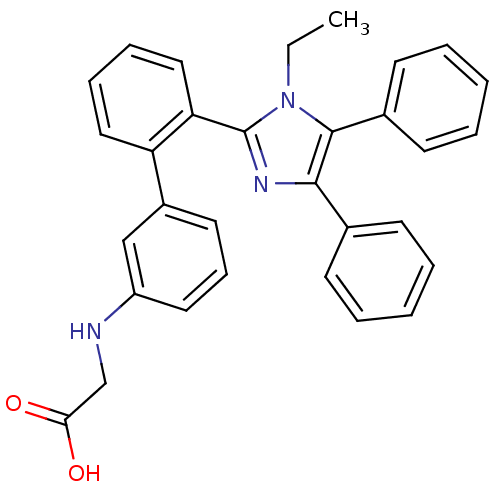 (CHEMBL245653 | [2'-(1-ethyl-4,5-diphenyl-1H-imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212886 (CHEMBL247529 | [2'-(3-ethyl-4,5-diphenyl-furan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50504755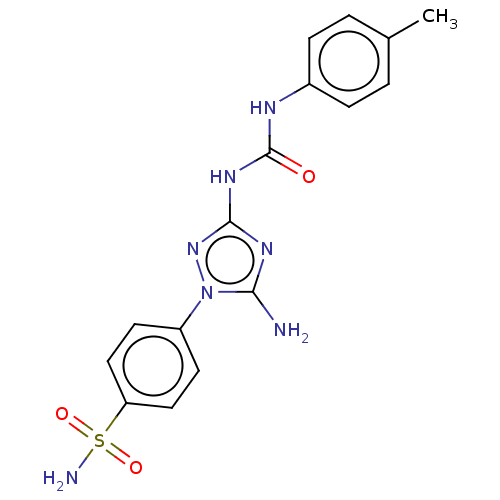 (CHEMBL4552517) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Egyptian Russian University Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111843 BindingDB Entry DOI: 10.7270/Q2WW7N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, heart (Homo sapiens (Human)) | BDBM50212876 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from mFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22530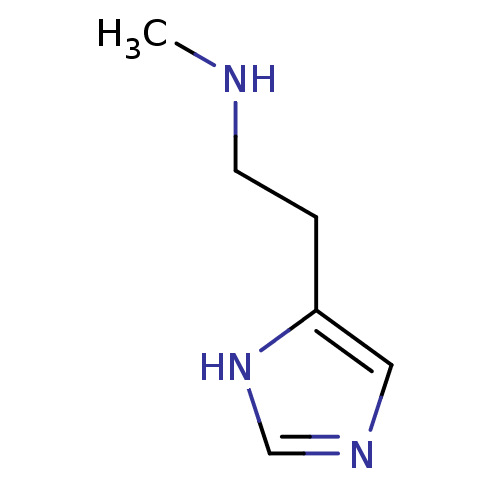 (N(alpha)-Methylhistamine | N-alpha-methylhistamine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50361035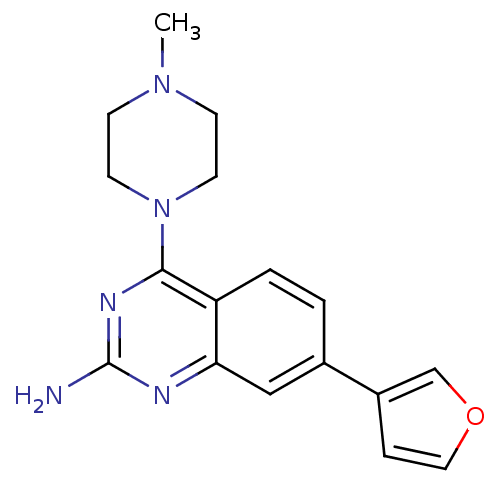 (CHEMBL1935571) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50361010 (CHEMBL1935572) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50361015 (CHEMBL592379) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22907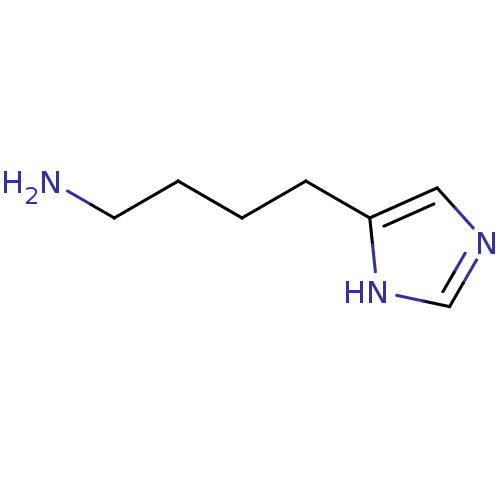 (4-(1H-imidazol-5-yl)butan-1-amine | Imbutamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50582963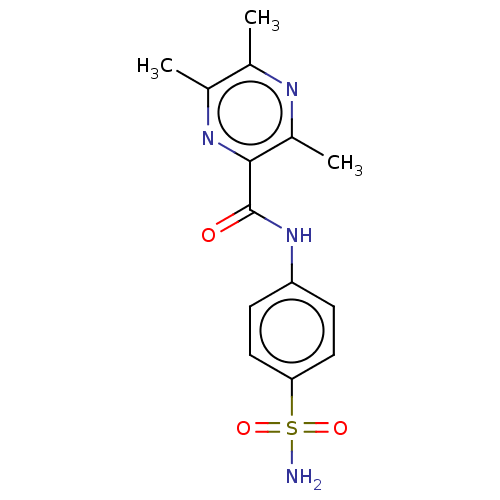 (CHEMBL5075785) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CA12 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114008 BindingDB Entry DOI: 10.7270/Q2988BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50504759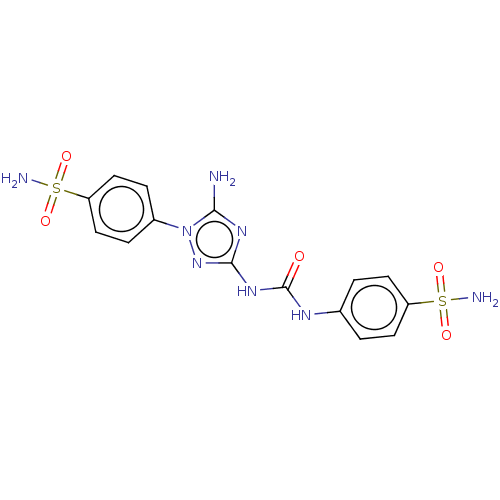 (CHEMBL4587956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Egyptian Russian University Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-12 incubated for 15 mins by stopped flow CO2 hydrase assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111843 BindingDB Entry DOI: 10.7270/Q2WW7N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50334354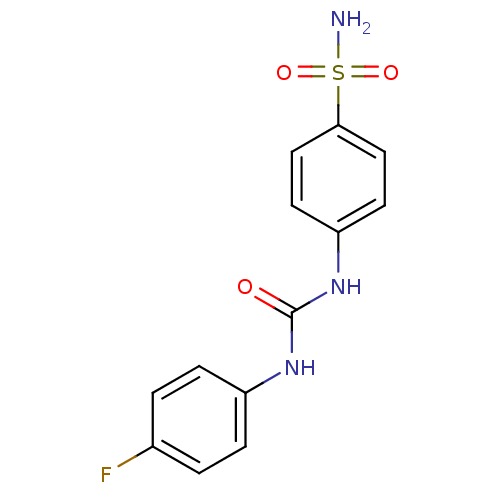 (4-(3-(4-fluorophenyl)ureido)benzenesulfonamide | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CA12 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114008 BindingDB Entry DOI: 10.7270/Q2988BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3322 total ) | Next | Last >> |