 Found 4824 hits with Last Name = 'tan' and Initial = 'd'
Found 4824 hits with Last Name = 'tan' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50170654
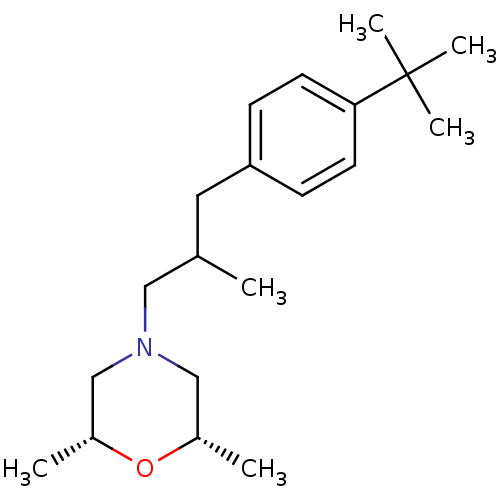
((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...)Show SMILES CC(CN1C[C@H](C)O[C@H](C)C1)Cc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3/t15?,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin School of Medicine and Public Health
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in guinea pig brain microsomes |
Bioorg Med Chem 18: 4397-404 (2010)
Article DOI: 10.1016/j.bmc.2010.04.078
BindingDB Entry DOI: 10.7270/Q20C4VZT |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50170654

((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...)Show SMILES CC(CN1C[C@H](C)O[C@H](C)C1)Cc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3/t15?,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin School of Medicine and Public Health
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in guinea pig liver microsomes |
Bioorg Med Chem 18: 4397-404 (2010)
Article DOI: 10.1016/j.bmc.2010.04.078
BindingDB Entry DOI: 10.7270/Q20C4VZT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384817
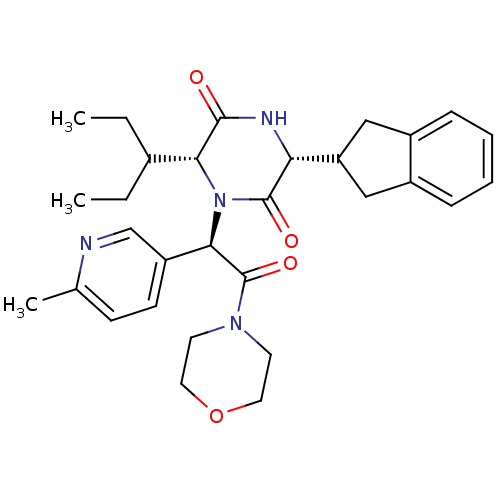
(CHEMBL2037514)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-4-20(5-2)26-28(35)32-25(24-16-21-8-6-7-9-22(21)17-24)29(36)34(26)27(23-11-10-19(3)31-18-23)30(37)33-12-14-38-15-13-33/h6-11,18,20,24-27H,4-5,12-17H2,1-3H3,(H,32,35)/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384800
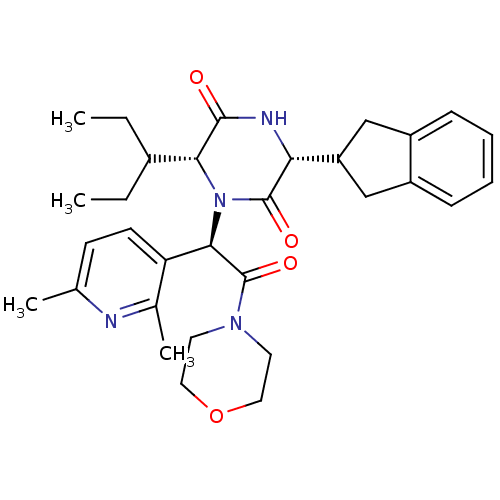
(CHEMBL2037517)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C31H40N4O4/c1-5-21(6-2)27-29(36)33-26(24-17-22-9-7-8-10-23(22)18-24)30(37)35(27)28(25-12-11-19(3)32-20(25)4)31(38)34-13-15-39-16-14-34/h7-12,21,24,26-28H,5-6,13-18H2,1-4H3,(H,33,36)/t26-,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384816

(CHEMBL2037516)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H38N4O3/c1-7-19(8-2)25-27(34)31-24(22-15-20-11-9-10-12-21(20)16-22)28(35)33(25)26(29(36)32(5)6)23-14-13-17(3)30-18(23)4/h9-14,19,22,24-26H,7-8,15-16H2,1-6H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384823
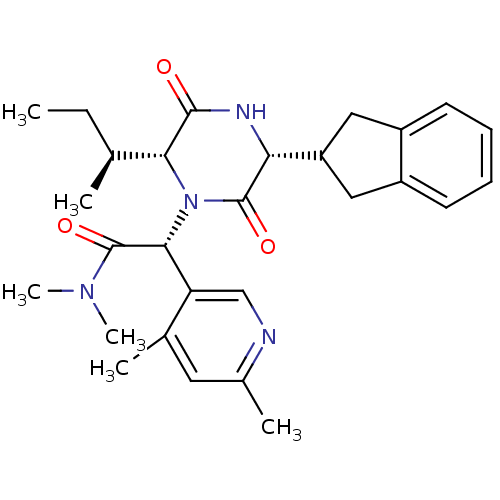
(CHEMBL2037507)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-15-29-18(4)12-17(22)3/h8-12,15-16,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50408664
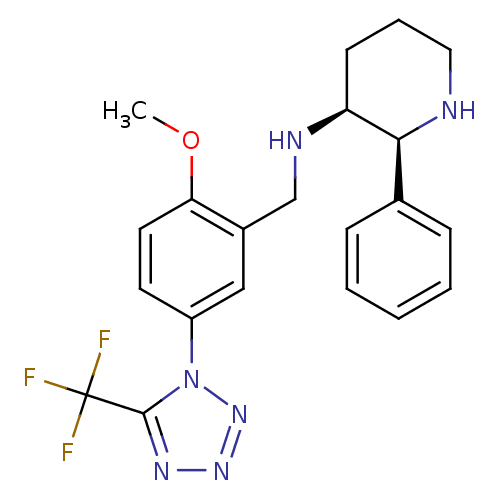
(GR-205171 | VOFOPITANT)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1nnnc1C(F)(F)F Show InChI InChI=1S/C21H23F3N6O/c1-31-18-10-9-16(30-20(21(22,23)24)27-28-29-30)12-15(18)13-26-17-8-5-11-25-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,25-26H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human recombinant NK1 receptor expressed in CHO cells |
J Med Chem 52: 3238-47 (2009)
Article DOI: 10.1021/jm900023b
BindingDB Entry DOI: 10.7270/Q2BP0425 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384837

(CHEMBL2037515)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-27(34)31-23(21-14-19-10-8-9-11-20(19)15-21)28(35)32(24)25(26(33)29-5)22-13-12-16(3)30-17(22)4/h8-13,18,21,23-25H,6-7,14-15H2,1-5H3,(H,29,33)(H,31,34)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384822
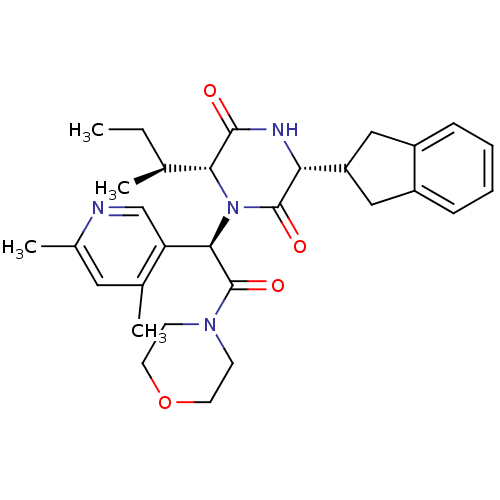
(CHEMBL2037508)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-17-31-20(4)14-19(24)3)30(37)33-10-12-38-13-11-33/h6-9,14,17-18,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384818
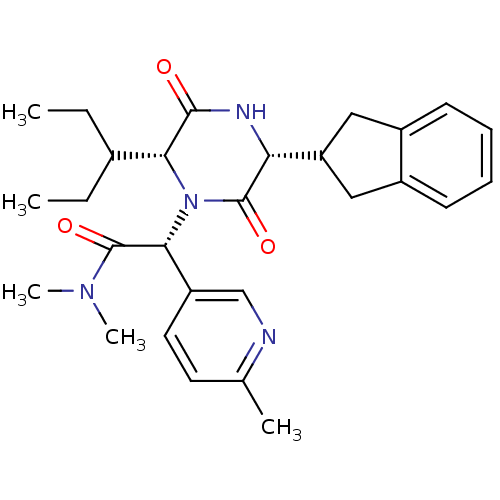
(CHEMBL2037513)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-26(33)30-23(22-14-19-10-8-9-11-20(19)15-22)27(34)32(24)25(28(35)31(4)5)21-13-12-17(3)29-16-21/h8-13,16,18,22-25H,6-7,14-15H2,1-5H3,(H,30,33)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384824
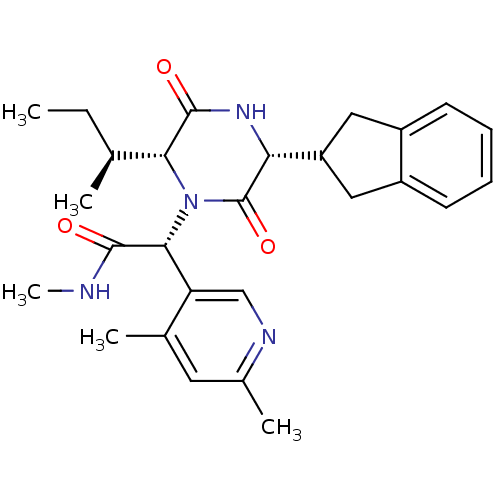
(CHEMBL2037506)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-14-29-17(4)11-16(21)3/h7-11,14-15,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384815
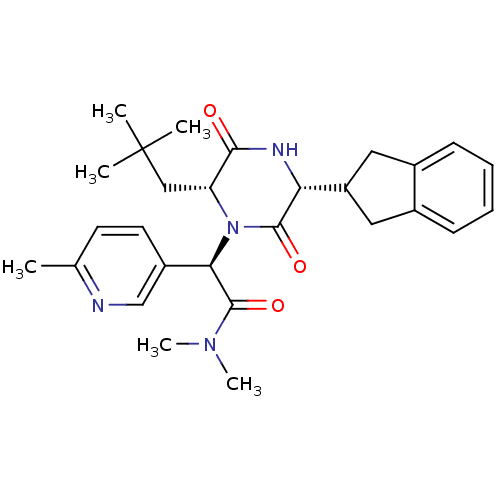
(CHEMBL2037496)Show SMILES CN(C)C(=O)[C@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)c1ccc(C)nc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-17-11-12-20(16-29-17)24(27(35)31(5)6)32-22(15-28(2,3)4)25(33)30-23(26(32)34)21-13-18-9-7-8-10-19(18)14-21/h7-12,16,21-24H,13-15H2,1-6H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50190528
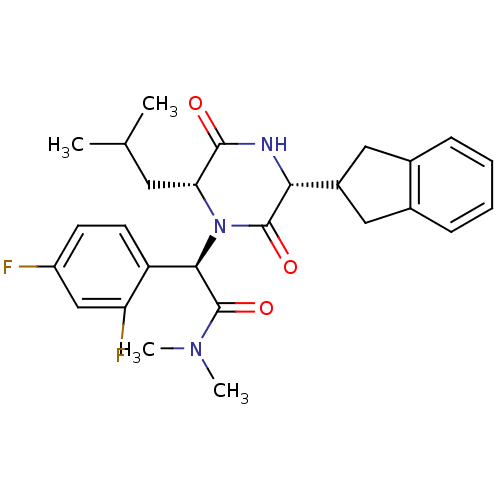
((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H31F2N3O3/c1-15(2)11-22-25(33)30-23(18-12-16-7-5-6-8-17(16)13-18)26(34)32(22)24(27(35)31(3)4)20-10-9-19(28)14-21(20)29/h5-10,14-15,18,22-24H,11-13H2,1-4H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384834
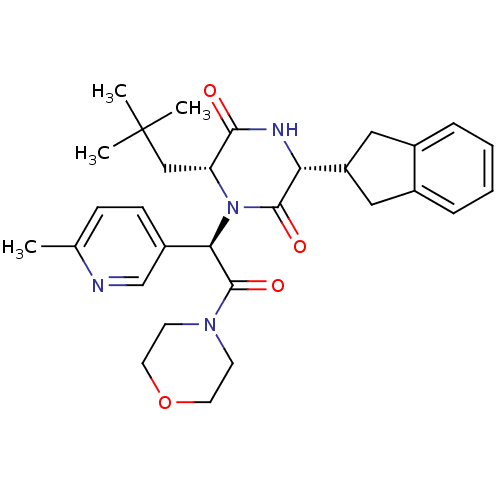
(CHEMBL2037497)Show SMILES Cc1ccc(cn1)[C@@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C30H38N4O4/c1-19-9-10-22(18-31-19)26(29(37)33-11-13-38-14-12-33)34-24(17-30(2,3)4)27(35)32-25(28(34)36)23-15-20-7-5-6-8-21(20)16-23/h5-10,18,23-26H,11-17H2,1-4H3,(H,32,35)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384838
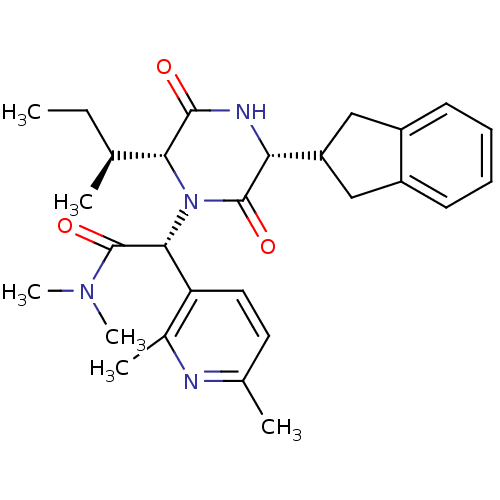
(CHEMBL2037510)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-14-19-10-8-9-11-20(19)15-21)27(34)32(24)25(28(35)31(5)6)22-13-12-17(3)29-18(22)4/h8-13,16,21,23-25H,7,14-15H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417977
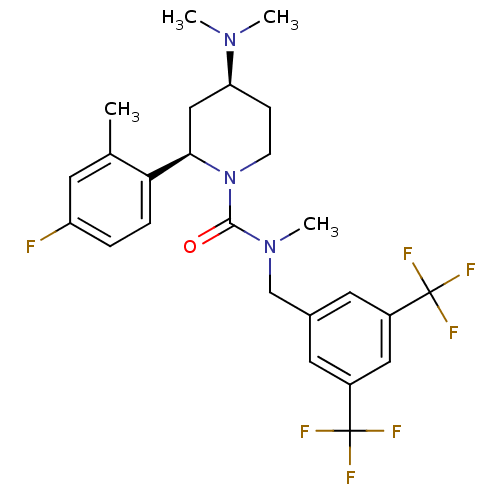
(CHEMBL1672044)Show SMILES CN(C)[C@H]1CCN([C@H](C1)c1ccc(F)cc1C)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H28F7N3O/c1-15-9-19(26)5-6-21(15)22-13-20(33(2)3)7-8-35(22)23(36)34(4)14-16-10-17(24(27,28)29)12-18(11-16)25(30,31)32/h5-6,9-12,20,22H,7-8,13-14H2,1-4H3/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50170654

((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...)Show SMILES CC(CN1C[C@H](C)O[C@H](C)C1)Cc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3/t15?,16-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin School of Medicine and Public Health
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor expressed in Saccharomyces cerevisiae WA0 |
Bioorg Med Chem 18: 4397-404 (2010)
Article DOI: 10.1016/j.bmc.2010.04.078
BindingDB Entry DOI: 10.7270/Q20C4VZT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417978
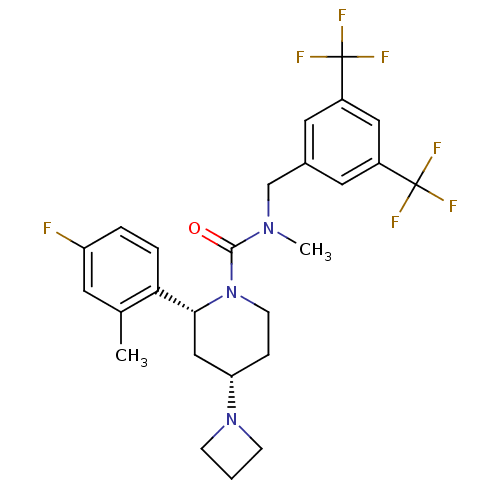
(CHEMBL1672047)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCC1 |r| Show InChI InChI=1S/C26H28F7N3O/c1-16-10-20(27)4-5-22(16)23-14-21(35-7-3-8-35)6-9-36(23)24(37)34(2)15-17-11-18(25(28,29)30)13-19(12-17)26(31,32)33/h4-5,10-13,21,23H,3,6-9,14-15H2,1-2H3/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212159
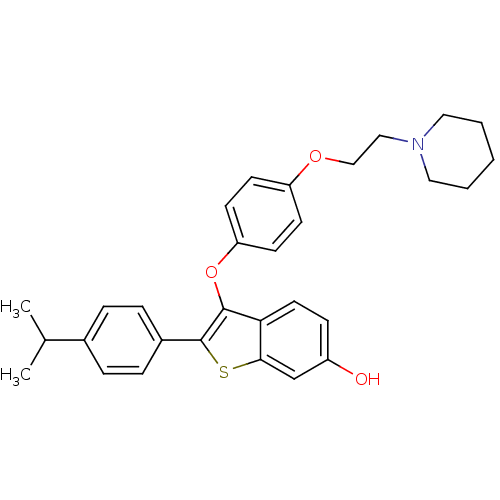
(2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...)Show SMILES CC(C)c1ccc(cc1)-c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H33NO3S/c1-21(2)22-6-8-23(9-7-22)30-29(27-15-10-24(32)20-28(27)35-30)34-26-13-11-25(12-14-26)33-19-18-31-16-4-3-5-17-31/h6-15,20-21,32H,3-5,16-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384819
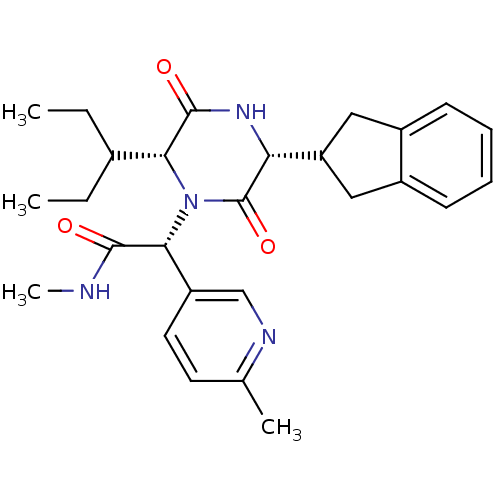
(CHEMBL2037512)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-5-17(6-2)23-26(33)30-22(21-13-18-9-7-8-10-19(18)14-21)27(34)31(23)24(25(32)28-4)20-12-11-16(3)29-15-20/h7-12,15,17,21-24H,5-6,13-14H2,1-4H3,(H,28,32)(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417973
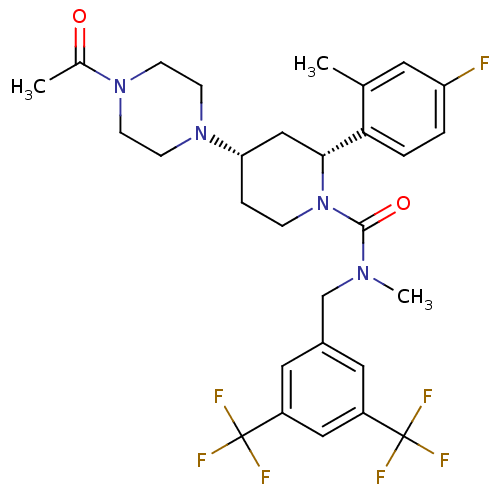
(CHEMBL1672053)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O |r| Show InChI InChI=1S/C29H33F7N4O2/c1-18-12-23(30)4-5-25(18)26-16-24(39-10-8-38(9-11-39)19(2)41)6-7-40(26)27(42)37(3)17-20-13-21(28(31,32)33)15-22(14-20)29(34,35)36/h4-5,12-15,24,26H,6-11,16-17H2,1-3H3/t24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM30704
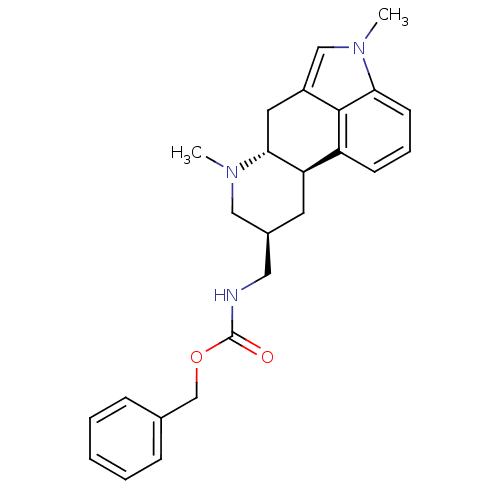
((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50336575
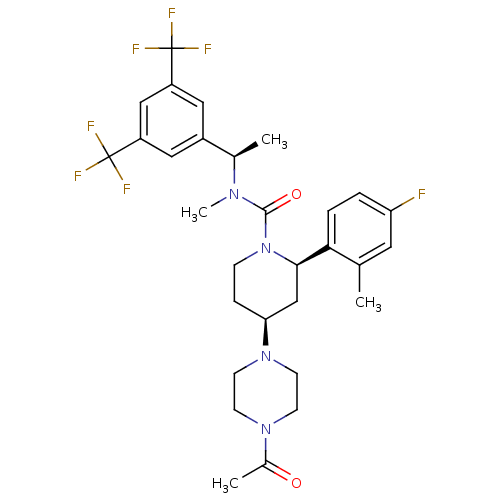
(CHEMBL1672054 | cis-(1'-Acetyl-N-{(1R)-1-[3,5-bis(...)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCN(CC1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C30H35F7N4O2/c1-18-13-24(31)5-6-26(18)27-17-25(40-11-9-39(10-12-40)20(3)42)7-8-41(27)28(43)38(4)19(2)21-14-22(29(32,33)34)16-23(15-21)30(35,36)37/h5-6,13-16,19,25,27H,7-12,17H2,1-4H3/t19-,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR205171 from human NK1 receptor in cortex homogenate by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384820
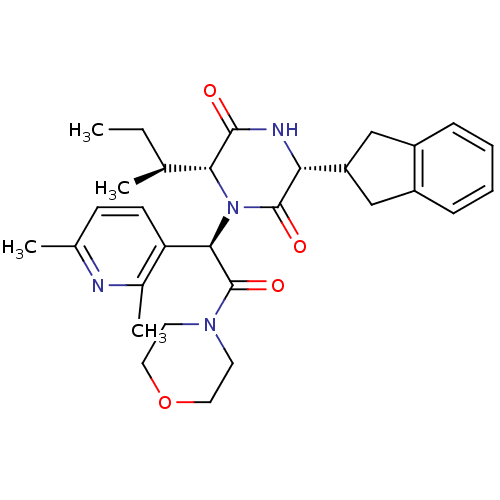
(EPELSIBAN | GSK557296B)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-16-21-8-6-7-9-22(21)17-23)29(36)34(26)27(24-11-10-19(3)31-20(24)4)30(37)33-12-14-38-15-13-33/h6-11,18,23,25-27H,5,12-17H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417972
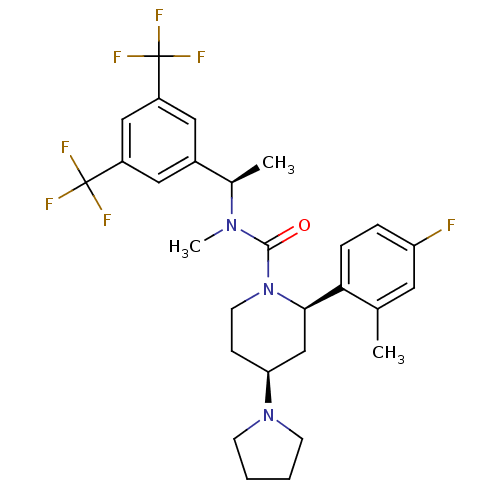
(CHEMBL1672051)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-17-12-22(29)6-7-24(17)25-16-23(37-9-4-5-10-37)8-11-38(25)26(39)36(3)18(2)19-13-20(27(30,31)32)15-21(14-19)28(33,34)35/h6-7,12-15,18,23,25H,4-5,8-11,16H2,1-3H3/t18-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384803
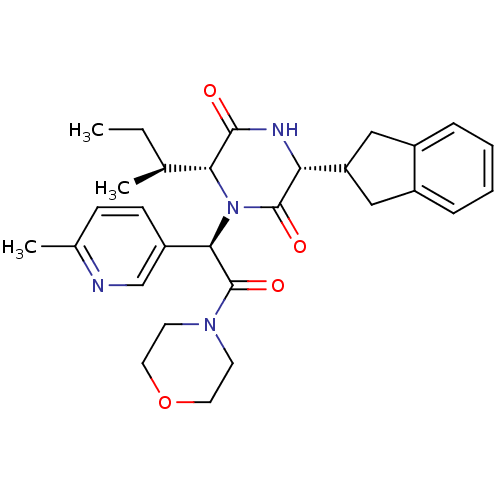
(CHEMBL2037501)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50212148
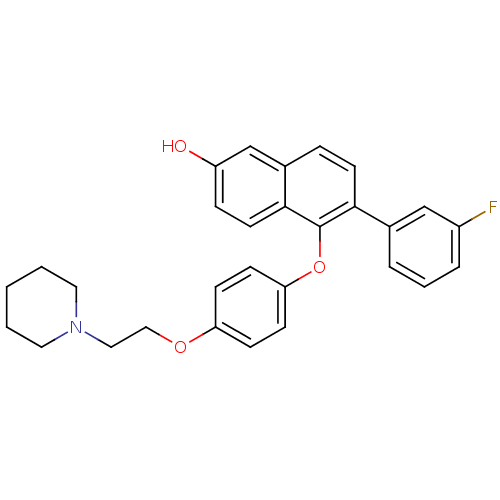
(6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C29H28FNO3/c30-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-31-15-2-1-3-16-31/h4-14,19-20,32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417982
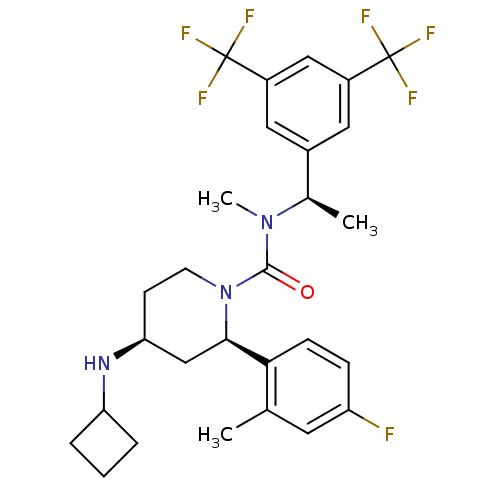
(CHEMBL1672058)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)NC1CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-16-11-21(29)7-8-24(16)25-15-23(36-22-5-4-6-22)9-10-38(25)26(39)37(3)17(2)18-12-19(27(30,31)32)14-20(13-18)28(33,34)35/h7-8,11-14,17,22-23,25,36H,4-6,9-10,15H2,1-3H3/t17-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417970
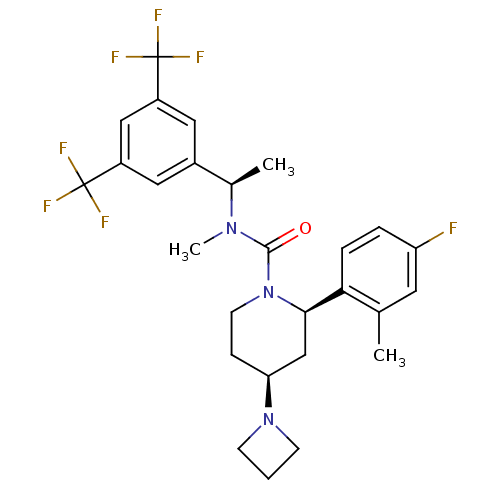
(CHEMBL1672048)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N1CCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H30F7N3O/c1-16-11-21(28)5-6-23(16)24-15-22(36-8-4-9-36)7-10-37(24)25(38)35(3)17(2)18-12-19(26(29,30)31)14-20(13-18)27(32,33)34/h5-6,11-14,17,22,24H,4,7-10,15H2,1-3H3/t17-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212148

(6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C29H28FNO3/c30-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-31-15-2-1-3-16-31/h4-14,19-20,32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM30704

((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50279697
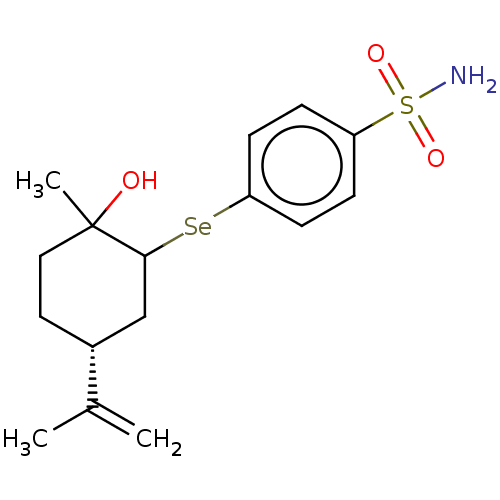
(CHEMBL4164295)Show SMILES [#6]-[#6](=[#6])-[#6@@H]-1-[#6]-[#6]C([#6])([#8])[#6](-[#6]-1)[Se;v2]c1ccc(cc1)S([#7])(=O)=O |r| Show InChI InChI=1S/C16H23NO3SSe/c1-11(2)12-8-9-16(3,18)15(10-12)22-14-6-4-13(5-7-14)21(17,19)20/h4-7,12,15,18H,1,8-10H2,2-3H3,(H2,17,19,20)/t12-,15?,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 8: 1213-1217 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00387
BindingDB Entry DOI: 10.7270/Q22Z182G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM30704

((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430877
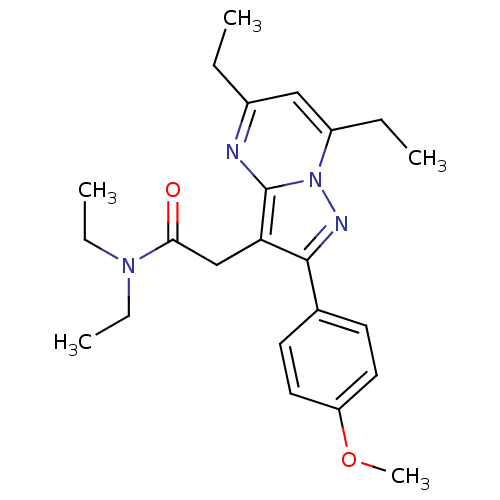
(CHEMBL2336480)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(CC)cc(CC)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C23H30N4O2/c1-6-17-14-18(7-2)27-23(24-17)20(15-21(28)26(8-3)9-4)22(25-27)16-10-12-19(29-5)13-11-16/h10-14H,6-9,15H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417980
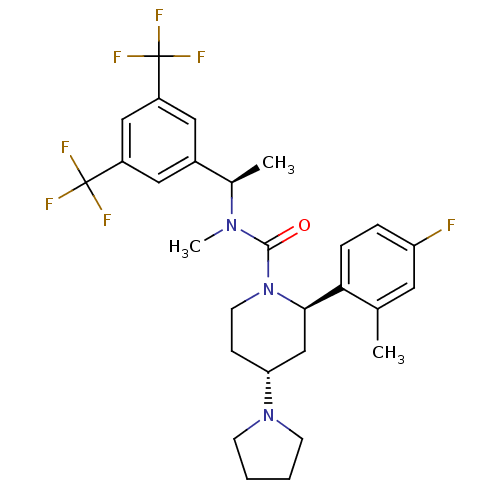
(CHEMBL1672052)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@H](C[C@@H]1c1ccc(F)cc1C)N1CCCC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C28H32F7N3O/c1-17-12-22(29)6-7-24(17)25-16-23(37-9-4-5-10-37)8-11-38(25)26(39)36(3)18(2)19-13-20(27(30,31)32)15-21(14-19)28(33,34)35/h6-7,12-15,18,23,25H,4-5,8-11,16H2,1-3H3/t18-,23-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417974
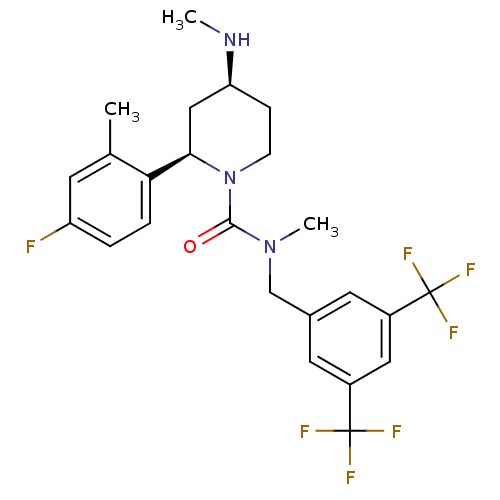
(CHEMBL1672056)Show SMILES CN[C@H]1CCN([C@H](C1)c1ccc(F)cc1C)C(=O)N(C)Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H26F7N3O/c1-14-8-18(25)4-5-20(14)21-12-19(32-2)6-7-34(21)22(35)33(3)13-15-9-16(23(26,27)28)11-17(10-15)24(29,30)31/h4-5,8-11,19,21,32H,6-7,12-13H2,1-3H3/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212157

(6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)c(F)c1 Show InChI InChI=1S/C29H27F2NO3/c30-27-13-5-21(19-28(27)31)25-11-4-20-18-22(33)6-12-26(20)29(25)35-24-9-7-23(8-10-24)34-17-16-32-14-2-1-3-15-32/h4-13,18-19,33H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384805
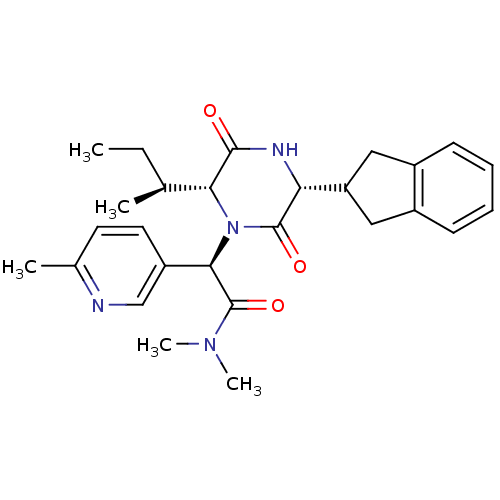
(CHEMBL2037499)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417976
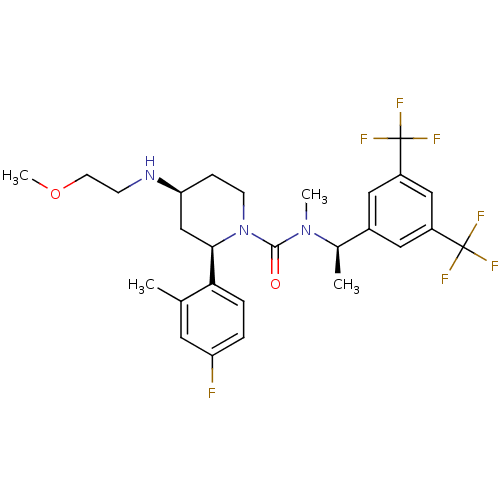
(CHEMBL1672059)Show SMILES COCCN[C@H]1CCN([C@H](C1)c1ccc(F)cc1C)C(=O)N(C)[C@H](C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H32F7N3O2/c1-16-11-21(28)5-6-23(16)24-15-22(35-8-10-39-4)7-9-37(24)25(38)36(3)17(2)18-12-19(26(29,30)31)14-20(13-18)27(32,33)34/h5-6,11-14,17,22,24,35H,7-10,15H2,1-4H3/t17-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212149
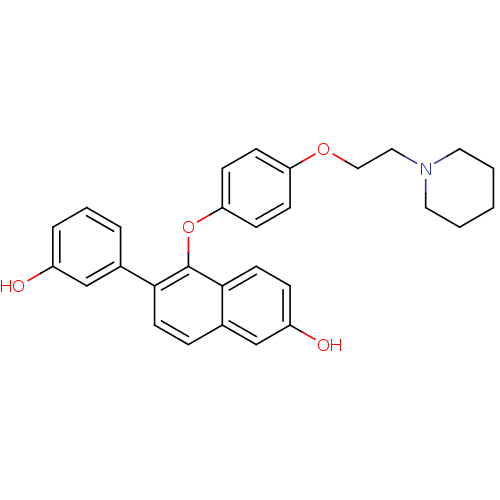
(6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...)Show SMILES Oc1cccc(c1)-c1ccc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C29H29NO4/c31-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-30-15-2-1-3-16-30/h4-14,19-20,31-32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413891

(VESTIPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CCNC[C@@H]1c1ccc(F)cc1C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F7N3O/c1-13-8-18(24)4-5-19(13)20-12-31-6-7-33(20)21(34)32(3)14(2)15-9-16(22(25,26)27)11-17(10-15)23(28,29)30/h4-5,8-11,14,20,31H,6-7,12H2,1-3H3/t14-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413891

(VESTIPITANT)Show SMILES C[C@@H](N(C)C(=O)N1CCNC[C@@H]1c1ccc(F)cc1C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F7N3O/c1-13-8-18(24)4-5-19(13)20-12-31-6-7-33(20)21(34)32(3)14(2)15-9-16(22(25,26)27)11-17(10-15)23(28,29)30/h4-5,8-11,14,20,31H,6-7,12H2,1-3H3/t14-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human recombinant NK1 receptor expressed in CHO cells |
J Med Chem 52: 3238-47 (2009)
Article DOI: 10.1021/jm900023b
BindingDB Entry DOI: 10.7270/Q2BP0425 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50417968
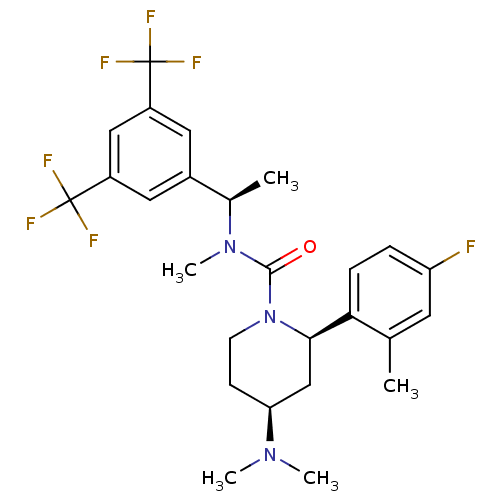
(CHEMBL1672045)Show SMILES C[C@@H](N(C)C(=O)N1CC[C@@H](C[C@@H]1c1ccc(F)cc1C)N(C)C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H30F7N3O/c1-15-10-20(27)6-7-22(15)23-14-21(34(3)4)8-9-36(23)24(37)35(5)16(2)17-11-18(25(28,29)30)13-19(12-17)26(31,32)33/h6-7,10-13,16,21,23H,8-9,14H2,1-5H3/t16-,21+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 54: 1071-9 (2011)
Article DOI: 10.1021/jm1013264
BindingDB Entry DOI: 10.7270/Q2W66M1X |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19967
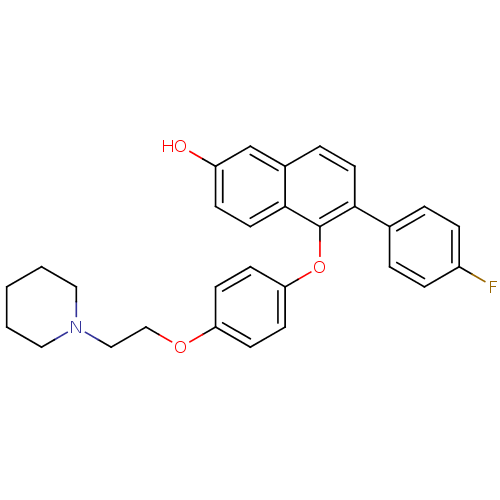
(6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C29H28FNO3/c30-23-7-4-21(5-8-23)27-14-6-22-20-24(32)9-15-28(22)29(27)34-26-12-10-25(11-13-26)33-19-18-31-16-2-1-3-17-31/h4-15,20,32H,1-3,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212160
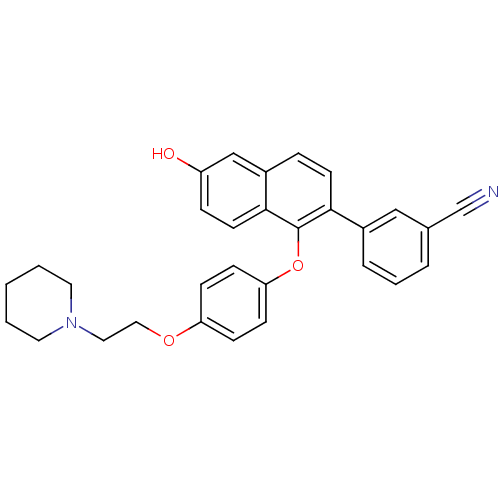
(3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(c1)C#N Show InChI InChI=1S/C30H28N2O3/c31-21-22-5-4-6-23(19-22)28-13-7-24-20-25(33)8-14-29(24)30(28)35-27-11-9-26(10-12-27)34-18-17-32-15-2-1-3-16-32/h4-14,19-20,33H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384812
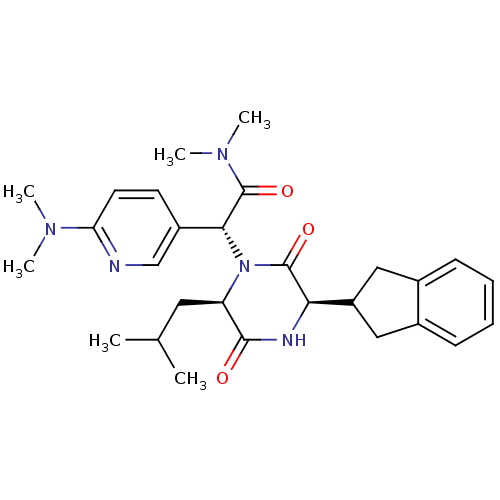
(CHEMBL2037489)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H37N5O3/c1-17(2)13-22-26(34)30-24(21-14-18-9-7-8-10-19(18)15-21)27(35)33(22)25(28(36)32(5)6)20-11-12-23(29-16-20)31(3)4/h7-12,16-17,21-22,24-25H,13-15H2,1-6H3,(H,30,34)/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data