

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394718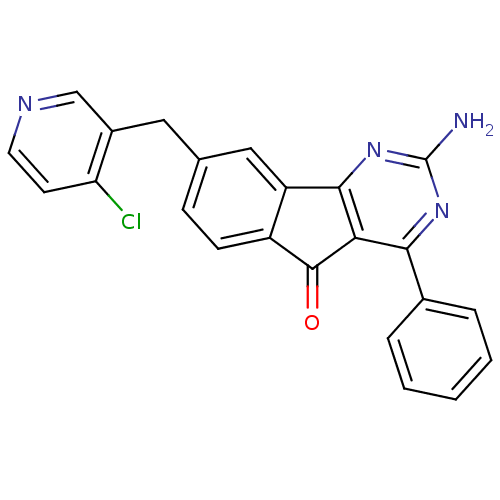 (CHEMBL2165801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394722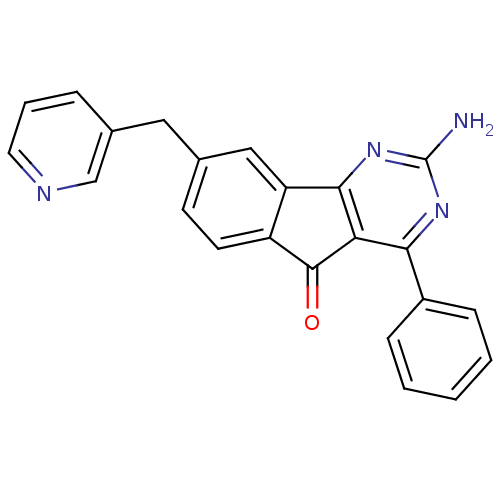 (CHEMBL2165807) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394717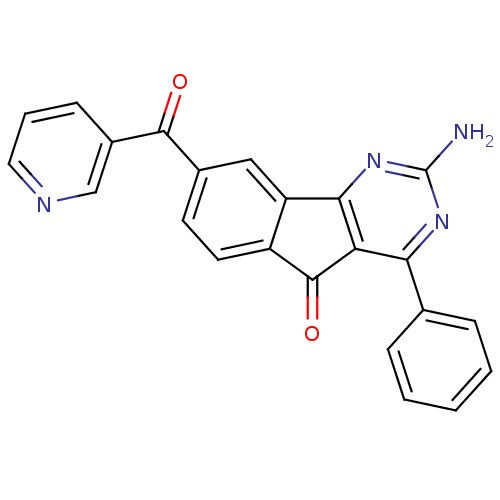 (CHEMBL2165802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394718 (CHEMBL2165801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394719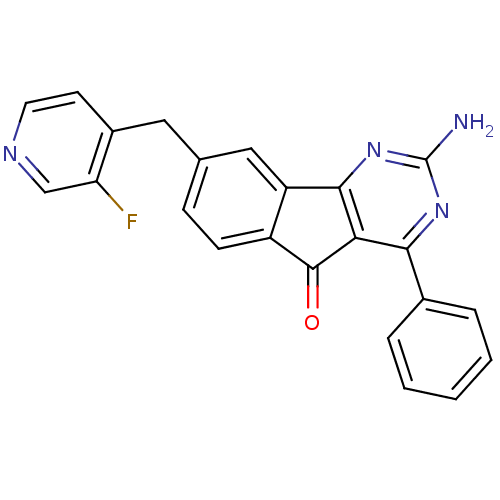 (CHEMBL2165800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394720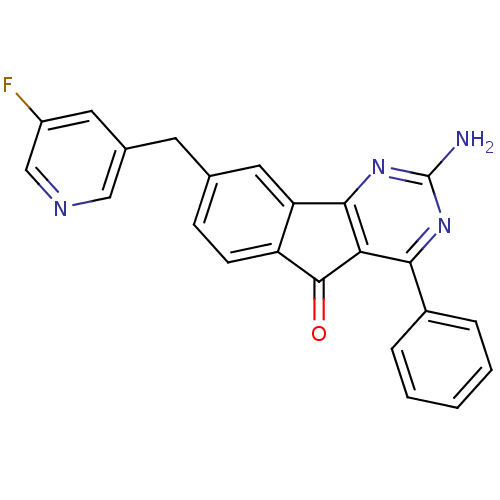 (CHEMBL2165799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394721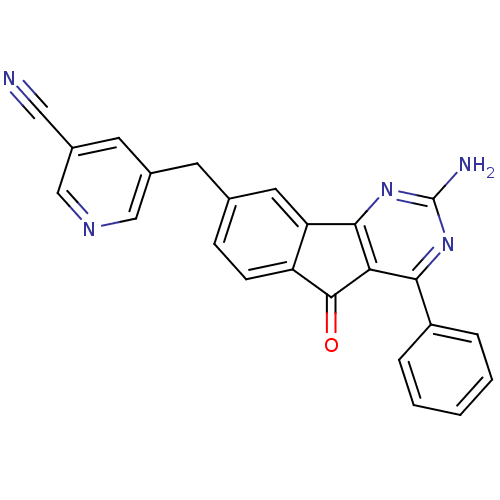 (CHEMBL2165808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394716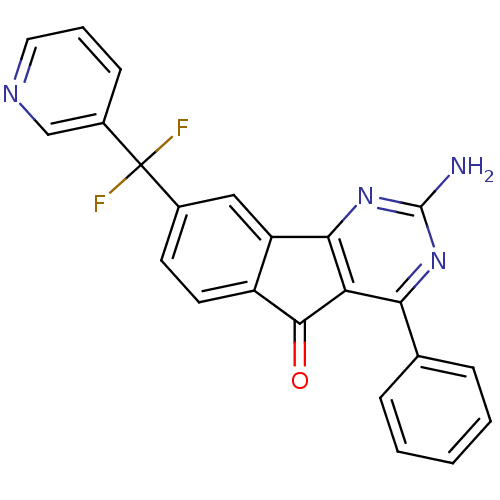 (CHEMBL2165803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394715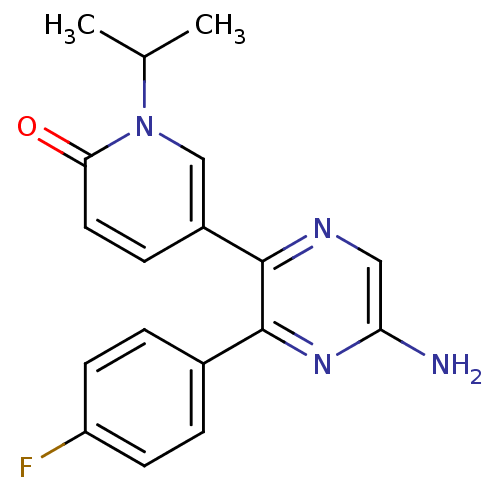 (CHEMBL2165804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394717 (CHEMBL2165802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 30 ... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394722 (CHEMBL2165807) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394719 (CHEMBL2165800) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394723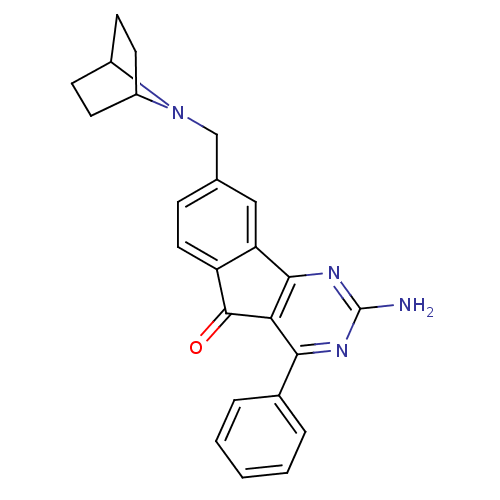 (CHEMBL2165806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50330987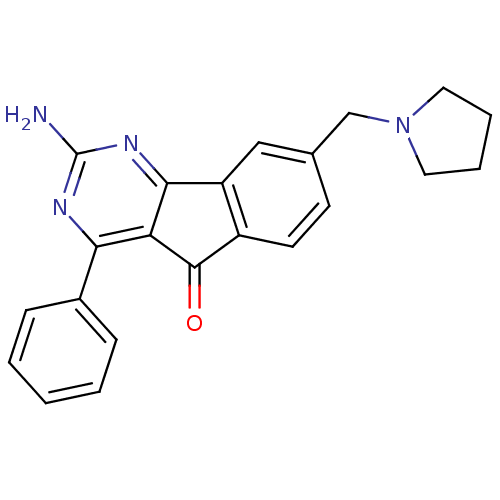 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50330987 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | J Med Chem 53: 8104-15 (2010) Checked by Author Article DOI: 10.1021/jm100971t BindingDB Entry DOI: 10.7270/Q2VT1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254266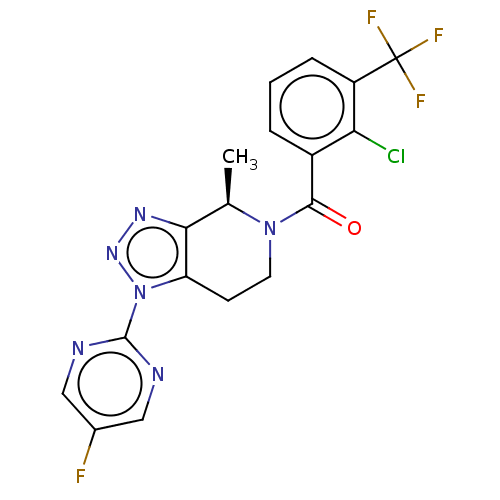 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-A-804598 from recombinant human P2X7 expressed in human 1321N1 cells after 1 hr | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254266 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-A-804598 from recombinant rat P2X7 expressed in human 1321N1 cells after 1 hr | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394721 (CHEMBL2165808) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394720 (CHEMBL2165799) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316887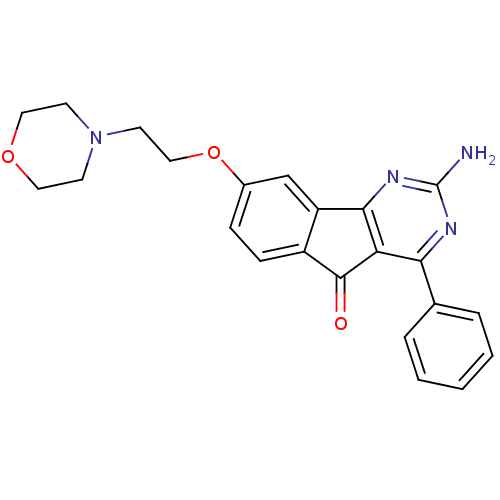 (2-amino-8-(2-morpholinoethoxy)-4-phenyl-5H-indeno[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316876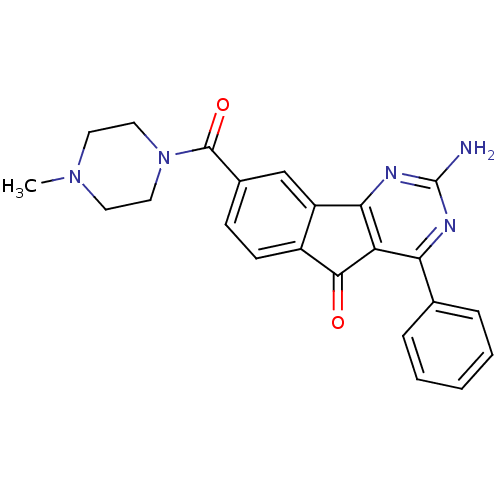 (2-amino-8-(4-methylpiperazine-1-carbonyl)-4-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394715 (CHEMBL2165804) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 3... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394724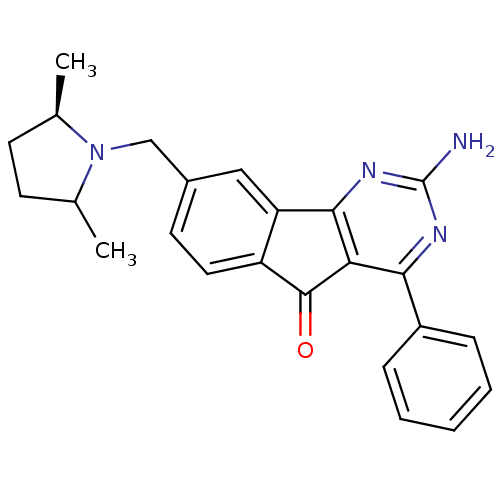 (CHEMBL2165805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50330987 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor | J Med Chem 53: 8104-15 (2010) Checked by Author Article DOI: 10.1021/jm100971t BindingDB Entry DOI: 10.7270/Q2VT1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50330987 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394716 (CHEMBL2165803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394724 (CHEMBL2165805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50316887 (2-amino-8-(2-morpholinoethoxy)-4-phenyl-5H-indeno[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 48.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50316876 (2-amino-8-(4-methylpiperazine-1-carbonyl)-4-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 58.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394723 (CHEMBL2165806) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254266 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 30 ... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 30 ... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 3... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254266 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 3... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254189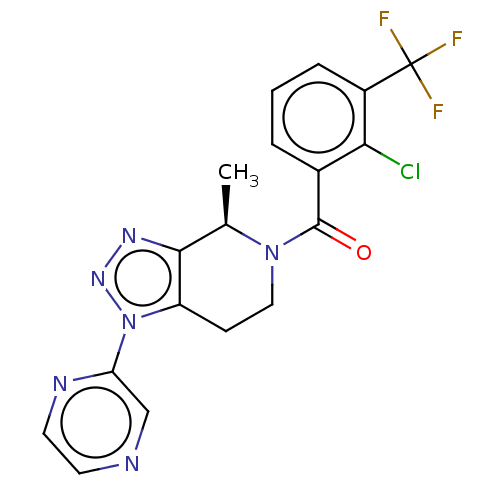 (US10112937, Example 74 | US10150765, Example 74 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 3... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276796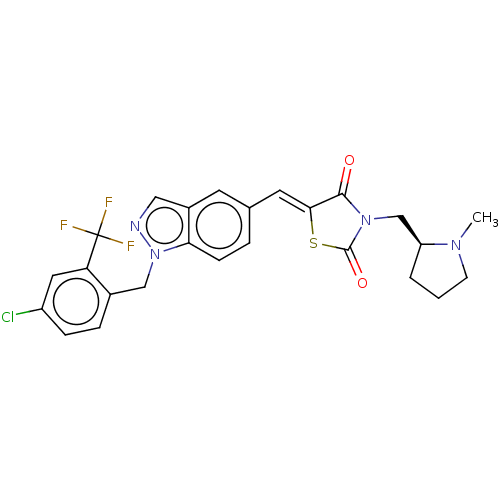 (CHEMBL4170306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254332 (US10112937, Example 235 | US10150765, Example 235 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 3... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50276802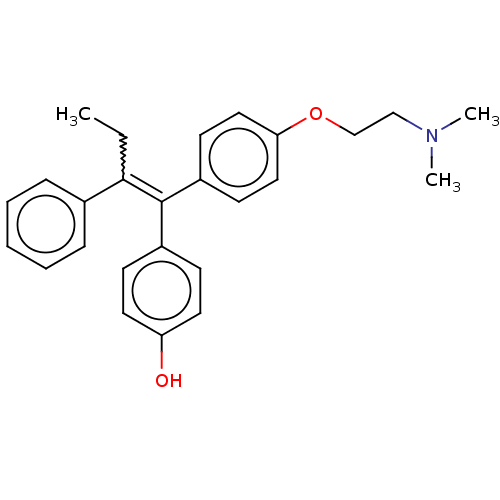 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 green binding to recombinant full length human ERalpha expressed in insect cells by fluorescence polarization assay | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254332 (US10112937, Example 235 | US10150765, Example 235 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 30 ... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 binding to recombinant full length human ERbeta expressed in insect cells by fluorescence polarization assay | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254266 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at P2X7 in human whole blood assessed as inhibition of BzATP-induced IL-1beta release preincubated for 30 mins followed by BzATP ... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor (Macaca mulatta) | BDBM254266 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at macaque P2X7 assessed as inhibition of BzATP-induced calcium flux preincubated for 30 mins followed by BzATP addition measured... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276805 (CHEMBL4166492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276803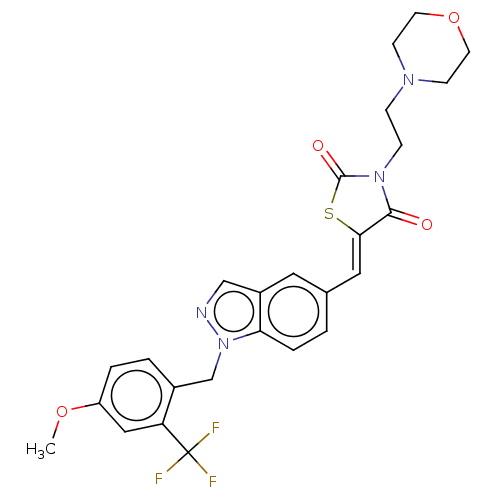 (CHEMBL4169272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276818 (CHEMBL4161346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254258 (US10112937, Example 148 | US10150765, Example 148 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 3... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276817 (CHEMBL4159613) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50276834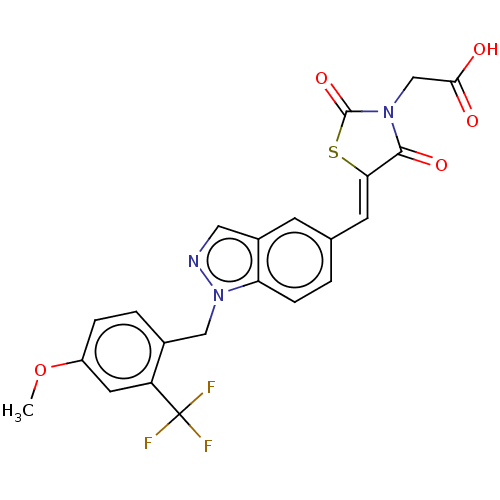 (CHEMBL4163909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18... | Eur J Med Chem 138: 830-853 (2017) Article DOI: 10.1016/j.ejmech.2017.07.015 BindingDB Entry DOI: 10.7270/Q2W098F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 138 total ) | Next | Last >> |