 Found 424 hits with Last Name = 'guthy' and Initial = 'da'
Found 424 hits with Last Name = 'guthy' and Initial = 'da' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862
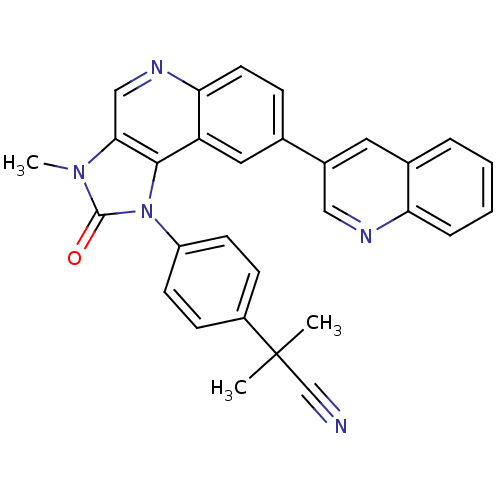
(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM608937
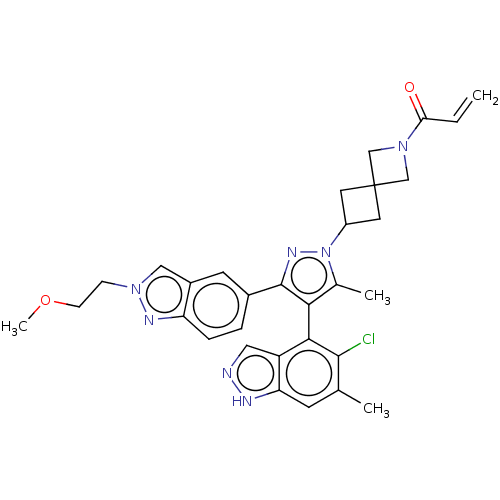
(1-(6-{(4M)-4-(5-Chloro-6- methyl-1H-indazol-4-yl)-...)Show SMILES COCCn1cc2cc(ccc2n1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(9.82,-.8,;9.42,-2.28,;7.94,-2.68,;6.85,-1.59,;5.36,-1.99,;4.21,-.96,;2.88,-1.73,;1.42,-1.26,;.27,-2.29,;.59,-3.79,;2.06,-4.27,;3.2,-3.24,;4.73,-3.4,;-1.19,-1.81,;-1.67,-.35,;-3.21,-.35,;-3.98,.99,;-5.47,1.39,;-5.07,2.87,;-3.58,2.48,;-6.55,3.27,;-6.16,4.76,;-4.67,4.36,;-7.25,5.85,;-6.85,7.34,;-8.73,5.45,;-9.82,6.54,;-3.68,-1.81,;-5.15,-2.29,;-2.44,-2.72,;-2.44,-4.26,;-3.77,-5.03,;-5.11,-4.26,;-3.77,-6.57,;-5.11,-7.34,;-2.44,-7.34,;-1.1,-6.57,;.36,-7.04,;1.27,-5.8,;.36,-4.55,;-1.1,-5.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112819

(CHEMBL3609522)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C19H24N6O2S/c1-19(2,3)16-21-9-10-6-7-11-14(13(10)23-16)28-17(22-11)24-18(27)25-8-4-5-12(25)15(20)26/h9,12H,4-8H2,1-3H3,(H2,20,26)(H,22,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112822
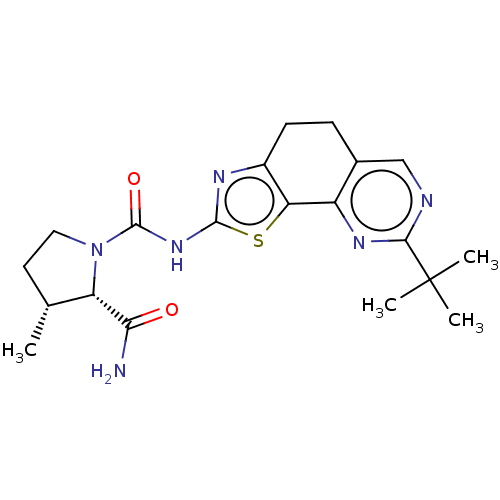
(CHEMBL3609525)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C20H26N6O2S/c1-10-7-8-26(14(10)16(21)27)19(28)25-18-23-12-6-5-11-9-22-17(20(2,3)4)24-13(11)15(12)29-18/h9-10,14H,5-8H2,1-4H3,(H2,21,27)(H,23,25,28)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112823

(CHEMBL3609526)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O2S/c1-9-6-7-29(13(9)15(24)30)18(31)28-17-26-11-5-4-10-8-25-16(19(2,3)20(21,22)23)27-12(10)14(11)32-17/h8-9,13H,4-7H2,1-3H3,(H2,24,30)(H,26,28,31)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112820
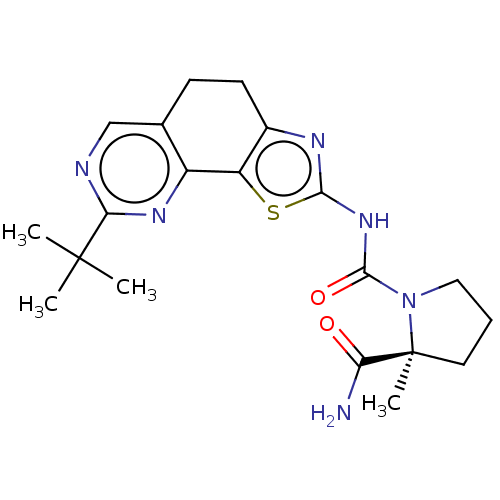
(CHEMBL3609523)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C20H26N6O2S/c1-19(2,3)16-22-10-11-6-7-12-14(13(11)24-16)29-17(23-12)25-18(28)26-9-5-8-20(26,4)15(21)27/h10H,5-9H2,1-4H3,(H2,21,27)(H,23,25,28)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112826
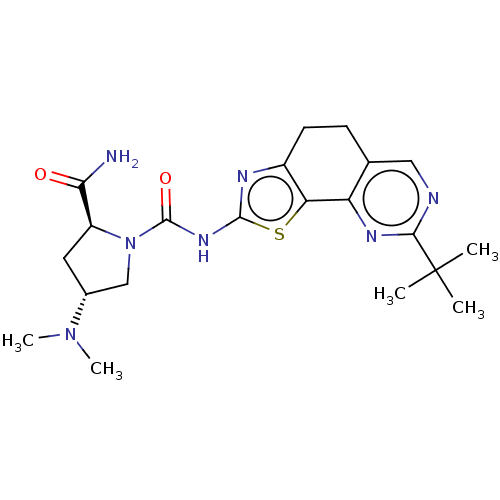
(CHEMBL3609529)Show SMILES CN(C)[C@@H]1C[C@H](N(C1)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C21H29N7O2S/c1-21(2,3)18-23-9-11-6-7-13-16(15(11)25-18)31-19(24-13)26-20(30)28-10-12(27(4)5)8-14(28)17(22)29/h9,12,14H,6-8,10H2,1-5H3,(H2,22,29)(H,24,26,30)/t12-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459
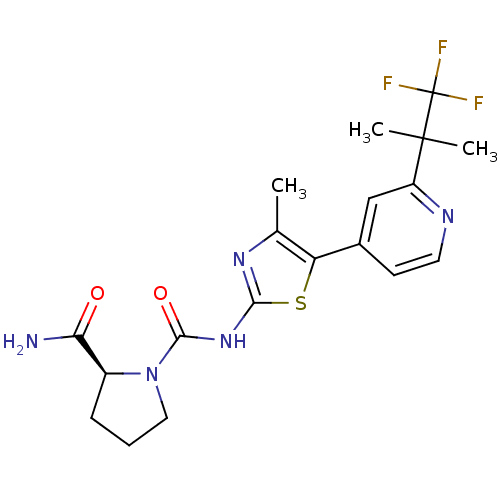
(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 3569-74 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.078
BindingDB Entry DOI: 10.7270/Q2W098XH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112817
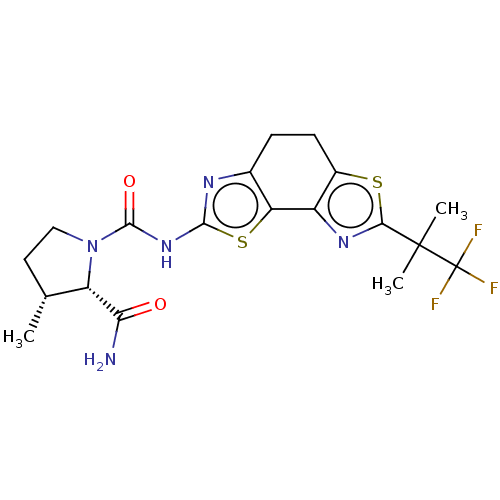
(CHEMBL3609520)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S2/c1-8-6-7-27(12(8)14(23)28)17(29)26-16-24-9-4-5-10-11(13(9)31-16)25-15(30-10)18(2,3)19(20,21)22/h8,12H,4-7H2,1-3H3,(H2,23,28)(H,24,26,29)/t8-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112820

(CHEMBL3609523)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C20H26N6O2S/c1-19(2,3)16-22-10-11-6-7-12-14(13(11)24-16)29-17(23-12)25-18(28)26-9-5-8-20(26,4)15(21)27/h10H,5-9H2,1-4H3,(H2,21,27)(H,23,25,28)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112823

(CHEMBL3609526)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O2S/c1-9-6-7-29(13(9)15(24)30)18(31)28-17-26-11-5-4-10-8-25-16(19(2,3)20(21,22)23)27-12(10)14(11)32-17/h8-9,13H,4-7H2,1-3H3,(H2,24,30)(H,26,28,31)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50112822

(CHEMBL3609525)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C20H26N6O2S/c1-10-7-8-26(14(10)16(21)27)19(28)25-18-23-12-6-5-11-9-22-17(20(2,3)4)24-13(11)15(12)29-18/h9-10,14H,5-8H2,1-4H3,(H2,21,27)(H,23,25,28)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PI or PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50113952
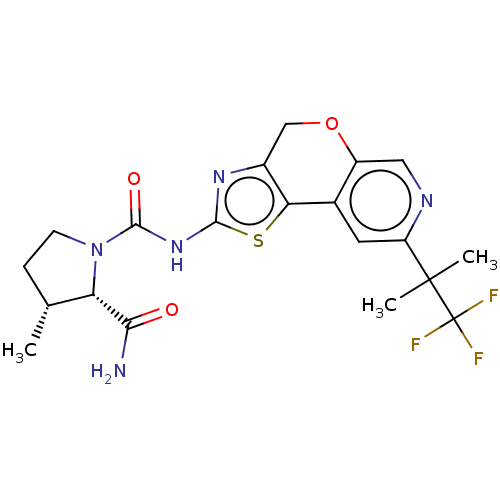
(CHEMBL3605177)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2COc3cnc(cc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H22F3N5O3S/c1-9-4-5-28(14(9)16(24)29)18(30)27-17-26-11-8-31-12-7-25-13(6-10(12)15(11)32-17)19(2,3)20(21,22)23/h6-7,9,14H,4-5,8H2,1-3H3,(H2,24,29)(H,26,27,30)/t9-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) by Kinase-Glo assay |
Bioorg Med Chem Lett 25: 3582-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.077
BindingDB Entry DOI: 10.7270/Q2G73GJG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112824
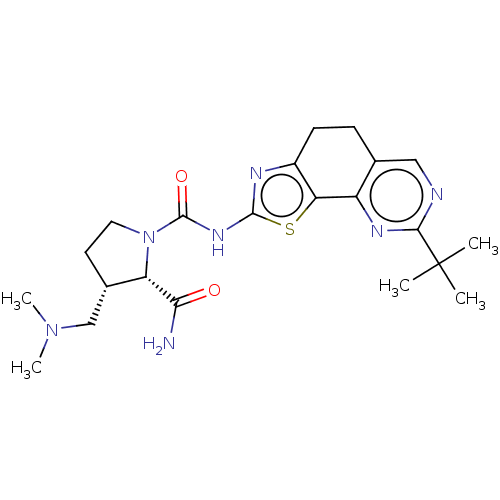
(CHEMBL3609527)Show SMILES CN(C)C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C22H31N7O2S/c1-22(2,3)19-24-10-12-6-7-14-17(15(12)26-19)32-20(25-14)27-21(31)29-9-8-13(11-28(4)5)16(29)18(23)30/h10,13,16H,6-9,11H2,1-5H3,(H2,23,30)(H,25,27,31)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) by Kinase-Glo assay |
Bioorg Med Chem Lett 25: 3582-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.077
BindingDB Entry DOI: 10.7270/Q2G73GJG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Rattus norvegicus (Rat)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112821

(CHEMBL3609524)Show SMILES [H][C@@]12C[C@@]1(N(CC2)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C20H24N6O2S/c1-19(2,3)16-22-9-10-4-5-12-14(13(10)24-16)29-17(23-12)25-18(28)26-7-6-11-8-20(11,26)15(21)27/h9,11H,4-8H2,1-3H3,(H2,21,27)(H,23,25,28)/t11-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112819

(CHEMBL3609522)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C19H24N6O2S/c1-19(2,3)16-21-9-10-6-7-11-14(13(10)23-16)28-17(22-11)24-18(27)25-8-4-5-12(25)15(20)26/h9,12H,4-8H2,1-3H3,(H2,20,26)(H,22,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112822

(CHEMBL3609525)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C20H26N6O2S/c1-10-7-8-26(14(10)16(21)27)19(28)25-18-23-12-6-5-11-9-22-17(20(2,3)4)24-13(11)15(12)29-18/h9-10,14H,5-8H2,1-4H3,(H2,21,27)(H,23,25,28)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448
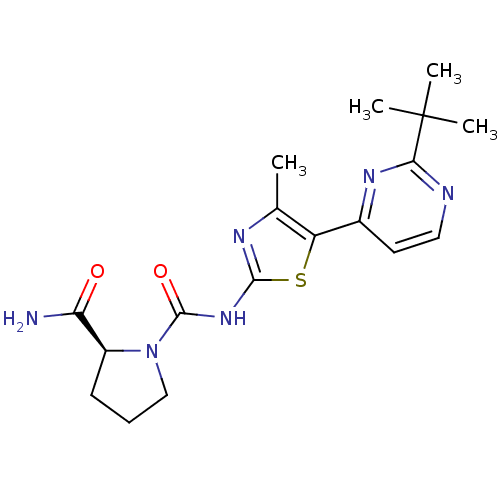
(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 3569-74 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.078
BindingDB Entry DOI: 10.7270/Q2W098XH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112829
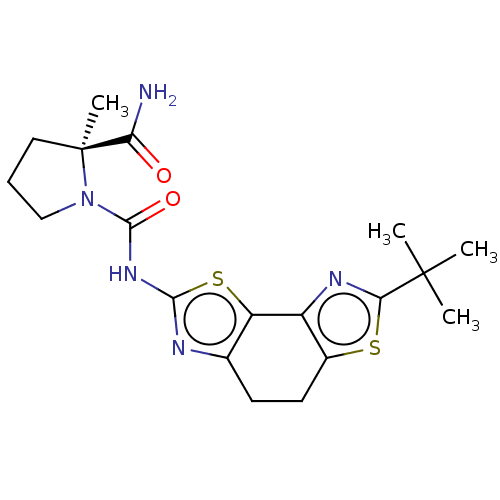
(CHEMBL3609516)Show SMILES CC(C)(C)c1nc-2c(CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc-23)s1 |r| Show InChI InChI=1S/C19H25N5O2S2/c1-18(2,3)15-22-12-11(27-15)7-6-10-13(12)28-16(21-10)23-17(26)24-9-5-8-19(24,4)14(20)25/h5-9H2,1-4H3,(H2,20,25)(H,21,23,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50609524
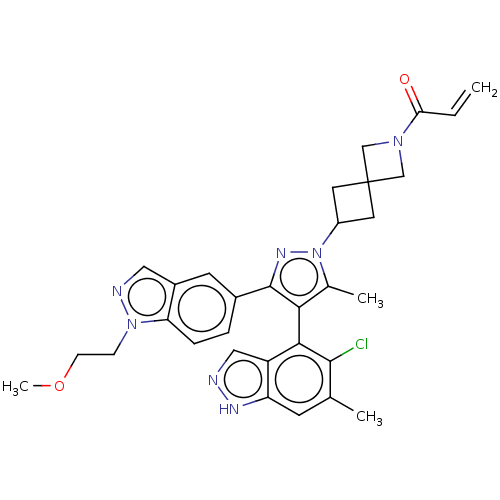
(CHEMBL5281254)Show SMILES COCCn1ncc2cc(ccc12)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(6.51,-8.61,;5.19,-9.32,;3.92,-8.53,;2.6,-9.24,;1.33,-8.44,;-.1,-9.02,;-1.09,-7.85,;-.28,-6.54,;-.71,-5.06,;.35,-3.95,;1.85,-4.31,;2.28,-5.79,;1.22,-6.91,;.03,-2.44,;1.07,-1.29,;.29,.05,;.92,1.46,;2.28,2.07,;1.62,3.49,;.22,2.79,;3.03,4.13,;2.4,5.53,;.99,4.9,;2.94,6.97,;4.17,7.17,;1.97,8.17,;2.4,9.32,;-1.21,-.28,;-2.14,.54,;-1.35,-1.8,;-2.7,-2.58,;-2.7,-4.12,;-1.62,-4.74,;-4.03,-4.89,;-4.03,-6.12,;-5.37,-4.12,;-5.37,-2.58,;-6.51,-1.54,;-5.88,-.13,;-4.35,-.29,;-4.03,-1.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50598037
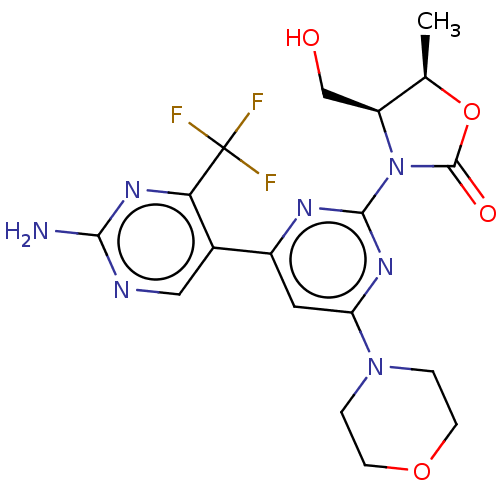
(CHEMBL5203121)Show SMILES C[C@H]1OC(=O)N([C@H]1CO)c1nc(cc(n1)-c1cnc(N)nc1C(F)(F)F)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50598037

(CHEMBL5203121)Show SMILES C[C@H]1OC(=O)N([C@H]1CO)c1nc(cc(n1)-c1cnc(N)nc1C(F)(F)F)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112830
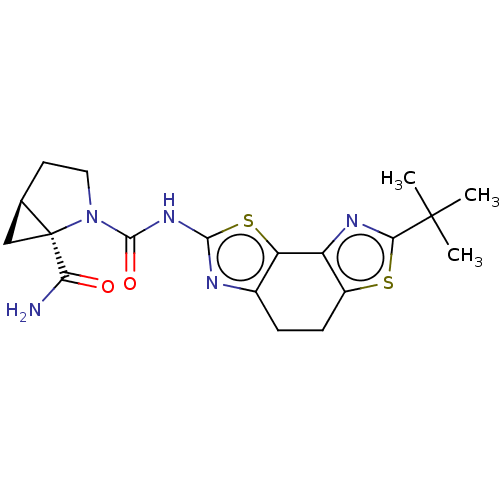
(CHEMBL3609517)Show SMILES [H][C@@]12C[C@@]1(N(CC2)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C19H23N5O2S2/c1-18(2,3)15-22-12-11(27-15)5-4-10-13(12)28-16(21-10)23-17(26)24-7-6-9-8-19(9,24)14(20)25/h9H,4-8H2,1-3H3,(H2,20,25)(H,21,23,26)/t9-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112817

(CHEMBL3609520)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S2/c1-8-6-7-27(12(8)14(23)28)17(29)26-16-24-9-4-5-10-11(13(9)31-16)25-15(30-10)18(2,3)19(20,21)22/h8,12H,4-7H2,1-3H3,(H2,23,28)(H,24,26,29)/t8-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Rattus norvegicus (Rat)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50609523
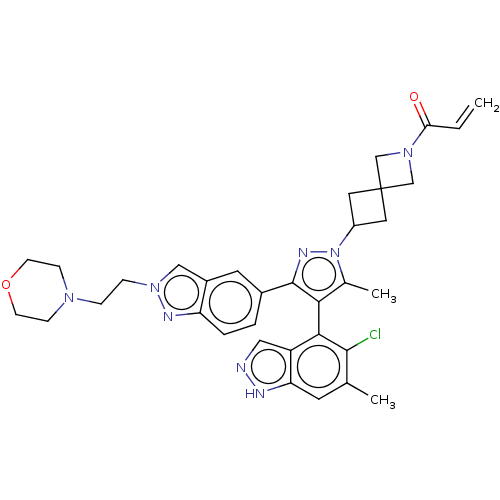
(CHEMBL5271997)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2nn(CCN3CCOCC3)cc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-1.82,5.46,;-1.41,4.3,;-2.27,3.04,;-1.37,1.81,;.09,2.31,;.06,3.85,;1.29,4.77,;2.79,4.65,;2.9,6.21,;1.34,6.28,;4.43,6.08,;4.56,7.61,;3.03,7.74,;5.73,8.61,;6.89,8.19,;5.46,10.12,;6.4,10.92,;-1.83,.34,;-3.29,-.12,;-3.64,-1.62,;-2.51,-2.66,;-2.53,-4.2,;-1.07,-4.7,;-.61,-6.17,;.89,-6.51,;1.35,-7.98,;2.85,-8.32,;3.31,-9.79,;2.26,-10.92,;.76,-10.58,;.3,-9.11,;-.15,-3.47,;-1.04,-2.21,;-.69,-.71,;-3.81,3.01,;-4.56,1.67,;-3.93,.61,;-6.1,1.65,;-6.7,.57,;-6.89,2.97,;-6.14,4.31,;-6.64,5.77,;-5.41,6.69,;-4.15,5.81,;-4.6,4.34,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112831
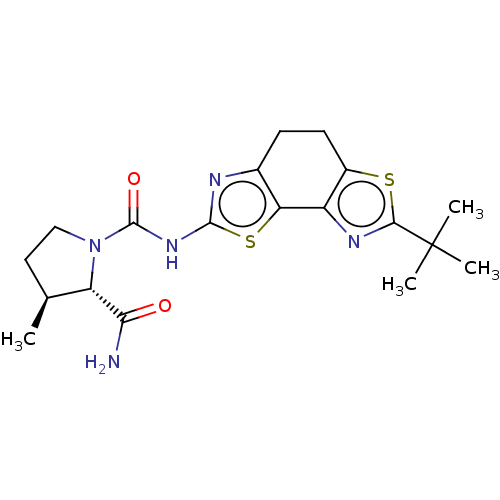
(CHEMBL3609518)Show SMILES C[C@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S2/c1-9-7-8-24(13(9)15(20)25)18(26)23-17-21-10-5-6-11-12(14(10)28-17)22-16(27-11)19(2,3)4/h9,13H,5-8H2,1-4H3,(H2,20,25)(H,21,23,26)/t9-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112816

(CHEMBL3609519)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S2/c1-9-7-8-24(13(9)15(20)25)18(26)23-17-21-10-5-6-11-12(14(10)28-17)22-16(27-11)19(2,3)4/h9,13H,5-8H2,1-4H3,(H2,20,25)(H,21,23,26)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112818
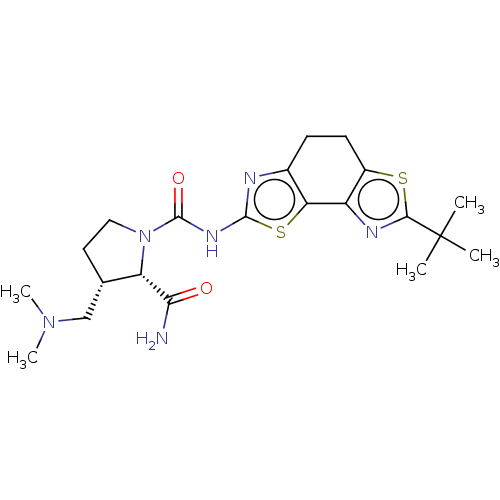
(CHEMBL3609521)Show SMILES CN(C)C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C21H30N6O2S2/c1-21(2,3)18-24-14-13(30-18)7-6-12-16(14)31-19(23-12)25-20(29)27-9-8-11(10-26(4)5)15(27)17(22)28/h11,15H,6-10H2,1-5H3,(H2,22,28)(H,23,25,29)/t11-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112816

(CHEMBL3609519)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S2/c1-9-7-8-24(13(9)15(20)25)18(26)23-17-21-10-5-6-11-12(14(10)28-17)22-16(27-11)19(2,3)4/h9,13H,5-8H2,1-4H3,(H2,20,25)(H,21,23,26)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50113952

(CHEMBL3605177)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2COc3cnc(cc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H22F3N5O3S/c1-9-4-5-28(14(9)16(24)29)18(30)27-17-26-11-8-31-12-7-25-13(6-10(12)15(11)32-17)19(2,3)20(21,22)23/h6-7,9,14H,4-5,8H2,1-3H3,(H2,24,29)(H,26,27,30)/t9-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of myristoylated human P110alpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylation at Serine 473 by Western blot analy... |
Bioorg Med Chem Lett 25: 3582-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.077
BindingDB Entry DOI: 10.7270/Q2G73GJG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112830

(CHEMBL3609517)Show SMILES [H][C@@]12C[C@@]1(N(CC2)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C19H23N5O2S2/c1-18(2,3)15-22-12-11(27-15)5-4-10-13(12)28-16(21-10)23-17(26)24-7-6-9-8-19(9,24)14(20)25/h9H,4-8H2,1-3H3,(H2,20,25)(H,21,23,26)/t9-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50598037

(CHEMBL5203121)Show SMILES C[C@H]1OC(=O)N([C@H]1CO)c1nc(cc(n1)-c1cnc(N)nc1C(F)(F)F)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112821

(CHEMBL3609524)Show SMILES [H][C@@]12C[C@@]1(N(CC2)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C20H24N6O2S/c1-19(2,3)16-22-9-10-4-5-12-14(13(10)24-16)29-17(23-12)25-18(28)26-7-6-11-8-20(11,26)15(21)27/h9,11H,4-8H2,1-3H3,(H2,21,27)(H,23,25,28)/t11-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112827

(CHEMBL3609514)Show SMILES CC(C)(C)c1nc-2c(CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc-23)s1 |r| Show InChI InChI=1S/C18H23N5O2S2/c1-18(2,3)15-21-12-11(26-15)7-6-9-13(12)27-16(20-9)22-17(25)23-8-4-5-10(23)14(19)24/h10H,4-8H2,1-3H3,(H2,19,24)(H,20,22,25)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112831

(CHEMBL3609518)Show SMILES C[C@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S2/c1-9-7-8-24(13(9)15(20)25)18(26)23-17-21-10-5-6-11-12(14(10)28-17)22-16(27-11)19(2,3)4/h9,13H,5-8H2,1-4H3,(H2,20,25)(H,21,23,26)/t9-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112825

(CHEMBL3609528)Show SMILES CN(C)C[C@]1(CCCN1C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C22H31N7O2S/c1-21(2,3)18-24-11-13-7-8-14-16(15(13)26-18)32-19(25-14)27-20(31)29-10-6-9-22(29,17(23)30)12-28(4)5/h11H,6-10,12H2,1-5H3,(H2,23,30)(H,25,27,31)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50498563
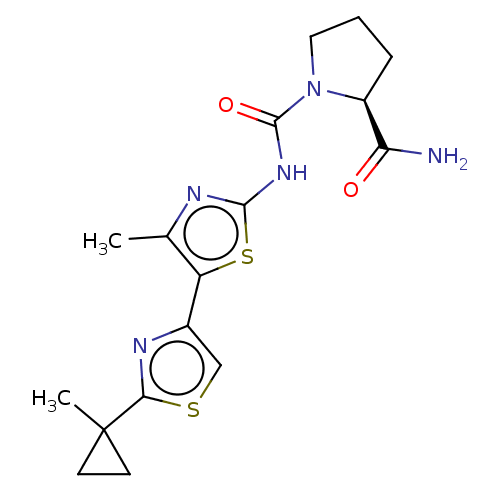
(CHEMBL3609540)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1csc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C17H21N5O2S2/c1-9-12(10-8-25-14(20-10)17(2)5-6-17)26-15(19-9)21-16(24)22-7-3-4-11(22)13(18)23/h8,11H,3-7H2,1-2H3,(H2,18,23)(H,19,21,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 3569-74 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.078
BindingDB Entry DOI: 10.7270/Q2W098XH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50498545

(CHEMBL3608927)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc(C)c(s1)-c1csc(n1)C1(CC1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S2/c1-8-3-6-26(11(8)13(22)27)16(28)25-15-23-9(2)12(30-15)10-7-29-14(24-10)17(4-5-17)18(19,20)21/h7-8,11H,3-6H2,1-2H3,(H2,22,27)(H,23,25,28)/t8-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 3569-74 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.078
BindingDB Entry DOI: 10.7270/Q2W098XH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50042922

(CHEMBL3218581)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1csc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H23N5O2S2/c1-9-12(10-8-25-14(20-10)17(2,3)4)26-15(19-9)21-16(24)22-7-5-6-11(22)13(18)23/h8,11H,5-7H2,1-4H3,(H2,18,23)(H,19,21,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50042922

(CHEMBL3218581)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1csc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H23N5O2S2/c1-9-12(10-8-25-14(20-10)17(2,3)4)26-15(19-9)21-16(24)22-7-5-6-11(22)13(18)23/h8,11H,5-7H2,1-4H3,(H2,18,23)(H,19,21,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 3569-74 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.078
BindingDB Entry DOI: 10.7270/Q2W098XH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112824

(CHEMBL3609527)Show SMILES CN(C)C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C22H31N7O2S/c1-22(2,3)19-24-10-12-6-7-14-17(15(12)26-19)32-20(25-14)27-21(31)29-9-8-13(11-28(4)5)16(29)18(23)30/h10,13,16H,6-9,11H2,1-5H3,(H2,23,30)(H,25,27,31)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50112823

(CHEMBL3609526)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O2S/c1-9-6-7-29(13(9)15(24)30)18(31)28-17-26-11-5-4-10-8-25-16(19(2,3)20(21,22)23)27-12(10)14(11)32-17/h8-9,13H,4-7H2,1-3H3,(H2,24,30)(H,26,28,31)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PI or PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50112819

(CHEMBL3609522)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C19H24N6O2S/c1-19(2,3)16-21-9-10-6-7-11-14(13(10)23-16)28-17(22-11)24-18(27)25-8-4-5-12(25)15(20)26/h9,12H,4-8H2,1-3H3,(H2,20,26)(H,22,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PI or PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112827

(CHEMBL3609514)Show SMILES CC(C)(C)c1nc-2c(CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc-23)s1 |r| Show InChI InChI=1S/C18H23N5O2S2/c1-18(2,3)15-21-12-11(26-15)7-6-9-13(12)27-16(20-9)22-17(25)23-8-4-5-10(23)14(19)24/h10H,4-8H2,1-3H3,(H2,19,24)(H,20,22,25)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50113951
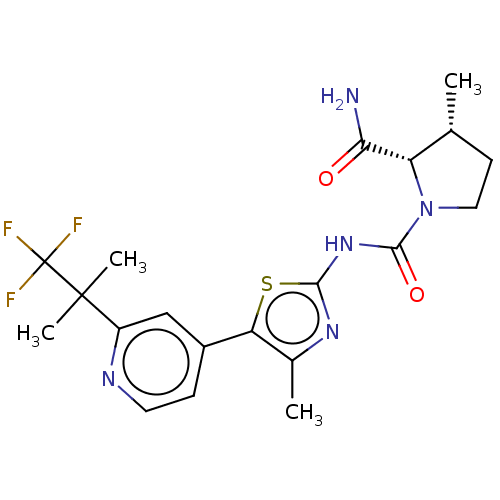
(CHEMBL3605178)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc(C)c(s1)-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H24F3N5O2S/c1-10-6-8-28(14(10)16(24)29)18(30)27-17-26-11(2)15(31-17)12-5-7-25-13(9-12)19(3,4)20(21,22)23/h5,7,9-10,14H,6,8H2,1-4H3,(H2,24,29)(H,26,27,30)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of myristoylated human P110alpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylation at Serine 473 by Western blot analy... |
Bioorg Med Chem Lett 25: 3582-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.077
BindingDB Entry DOI: 10.7270/Q2G73GJG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data