

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134262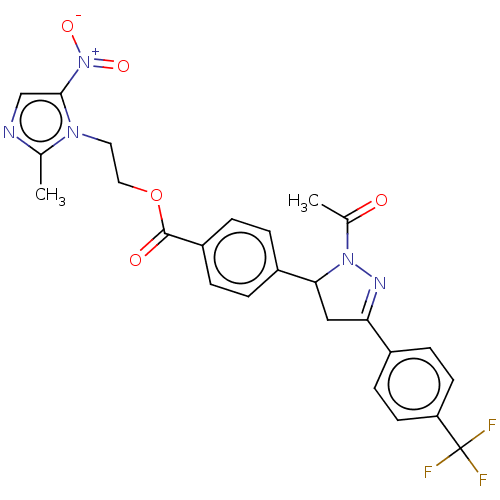 (CHEMBL3746557) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134262 (CHEMBL3746557) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016095 (CHEMBL3261192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134262 (CHEMBL3746557) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134236 (CHEMBL3746473) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134236 (CHEMBL3746473) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 601 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase (Staphylococcus aureus (strain MSSA476)) | BDBM199134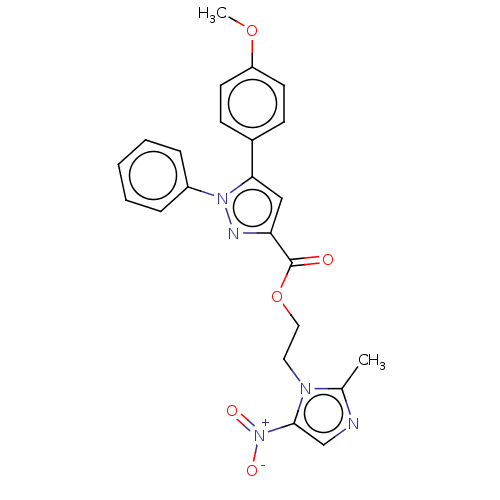 (2-(2-Methyl-5-nitro-1H-imidazol-1-yl)-ethyl-5-(4-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Nanjing University | Assay Description The assays were conducted at 37 °C in a mixture containing 100 mM Tris/Cl pH 7.9, 50 mM KCl, 16 mM MgCl2, 5 mM ATP, 3 mM DTT, 4 mg/ml E. coli MRE600 ... | Chem Biol Drug Des 88: 592-8 (2016) Article DOI: 10.1111/cbdd.12793 BindingDB Entry DOI: 10.7270/Q2V69HDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016096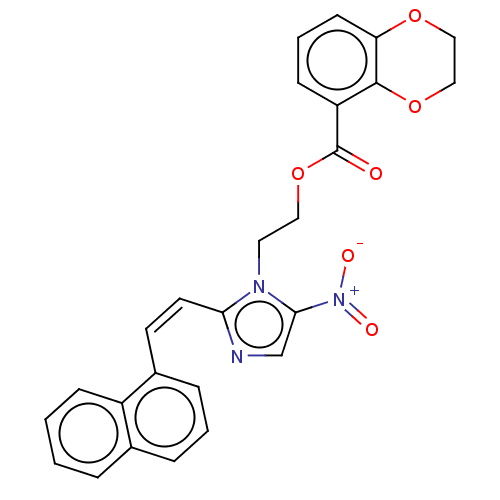 (CHEMBL3261193) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134258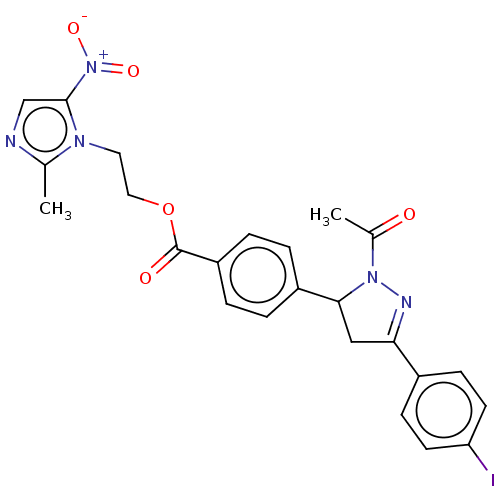 (CHEMBL3746711) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134258 (CHEMBL3746711) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134241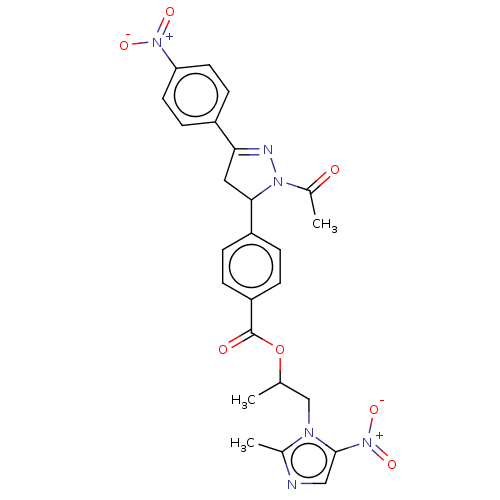 (CHEMBL3746642) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134241 (CHEMBL3746642) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase (Staphylococcus aureus (strain MSSA476)) | BDBM199139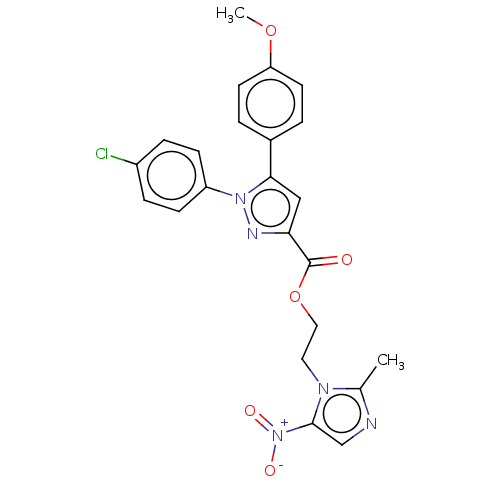 (2-(2-Methyl-5-nitro-1H-imidazol-1-yl)-ethyl-1-(4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Nanjing University | Assay Description The assays were conducted at 37 °C in a mixture containing 100 mM Tris/Cl pH 7.9, 50 mM KCl, 16 mM MgCl2, 5 mM ATP, 3 mM DTT, 4 mg/ml E. coli MRE600 ... | Chem Biol Drug Des 88: 592-8 (2016) Article DOI: 10.1111/cbdd.12793 BindingDB Entry DOI: 10.7270/Q2V69HDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016084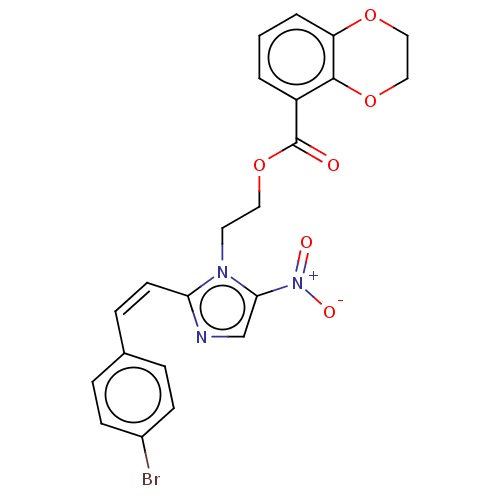 (CHEMBL3261182) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of bovine brain tubulin preincubated for 20 mins by turbidimetry | Eur J Med Chem 85: 341-51 (2014) Article DOI: 10.1016/j.ejmech.2014.07.082 BindingDB Entry DOI: 10.7270/Q29C7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134236 (CHEMBL3746473) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50056757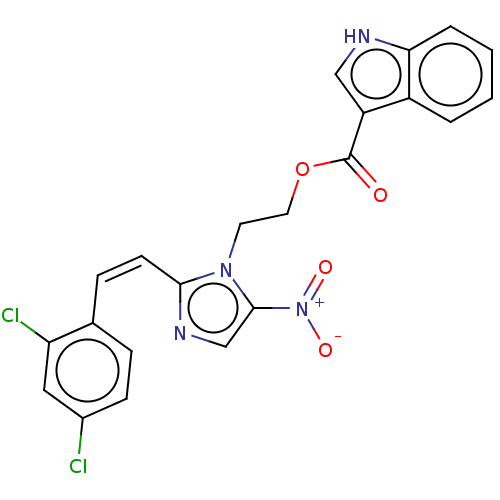 (CHEMBL3331011) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of bovine brain tubulin preincubated for 20 mins by turbidimetry | Eur J Med Chem 85: 341-51 (2014) Article DOI: 10.1016/j.ejmech.2014.07.082 BindingDB Entry DOI: 10.7270/Q29C7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134258 (CHEMBL3746711) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016080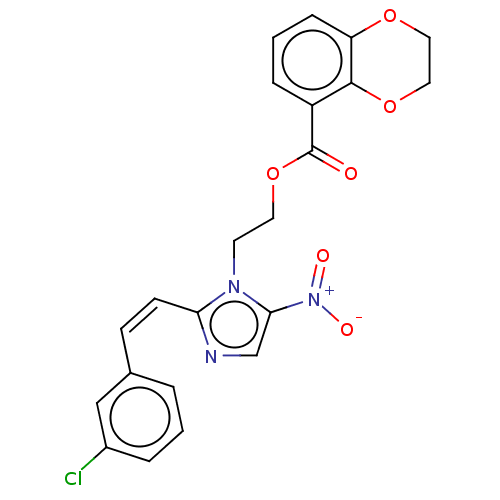 (CHEMBL3261178) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134264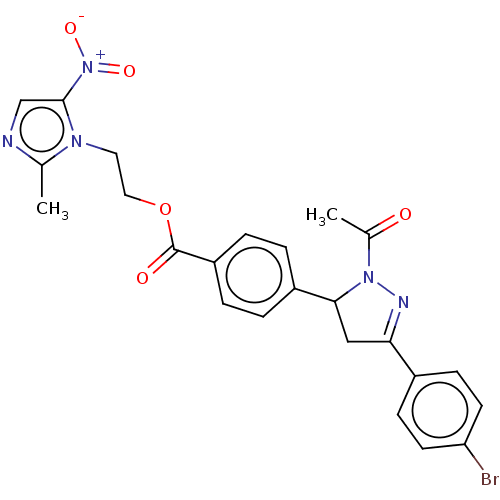 (CHEMBL3746212) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134241 (CHEMBL3746642) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134256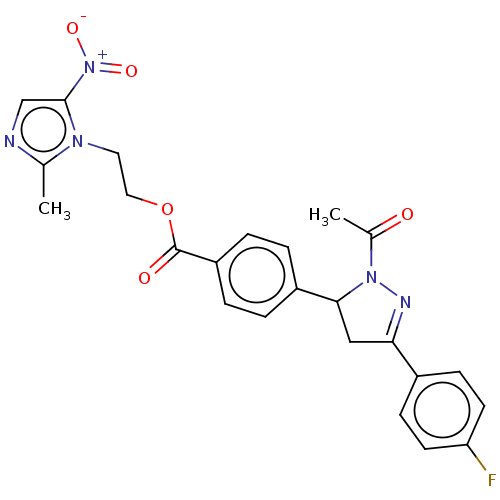 (CHEMBL3747281) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134254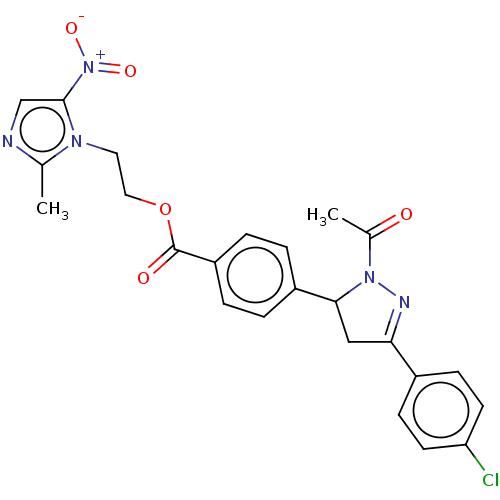 (CHEMBL3747277) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134264 (CHEMBL3746212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134264 (CHEMBL3746212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016085 (CHEMBL3261183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase (Staphylococcus aureus (strain MSSA476)) | BDBM199144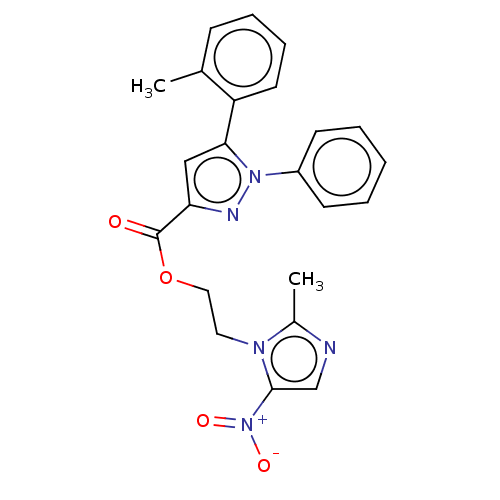 (2-(2-Methyl-5-nitro-1H-imidazol-1-yl)-ethyl-1-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.55E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Nanjing University | Assay Description The assays were conducted at 37 °C in a mixture containing 100 mM Tris/Cl pH 7.9, 50 mM KCl, 16 mM MgCl2, 5 mM ATP, 3 mM DTT, 4 mg/ml E. coli MRE600 ... | Chem Biol Drug Des 88: 592-8 (2016) Article DOI: 10.1111/cbdd.12793 BindingDB Entry DOI: 10.7270/Q2V69HDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134256 (CHEMBL3747281) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134256 (CHEMBL3747281) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase (Staphylococcus aureus (strain MSSA476)) | BDBM199145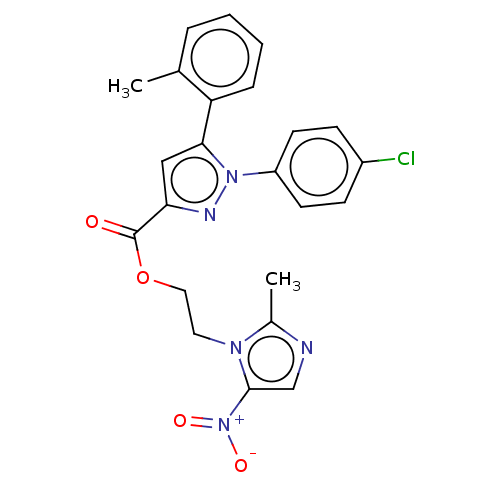 (2-(2-Methyl-5-nitro-1H-imidazol-1-yl)-ethyl-1-(4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Nanjing University | Assay Description The assays were conducted at 37 °C in a mixture containing 100 mM Tris/Cl pH 7.9, 50 mM KCl, 16 mM MgCl2, 5 mM ATP, 3 mM DTT, 4 mg/ml E. coli MRE600 ... | Chem Biol Drug Des 88: 592-8 (2016) Article DOI: 10.1111/cbdd.12793 BindingDB Entry DOI: 10.7270/Q2V69HDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016087 (CHEMBL3261184) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134263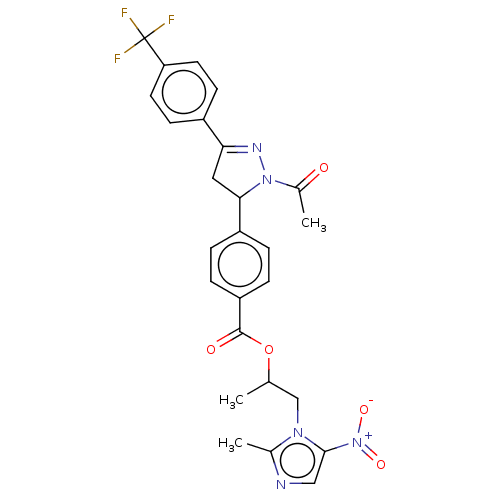 (CHEMBL3746421) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134267 (CHEMBL3747776) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134267 (CHEMBL3747776) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50056748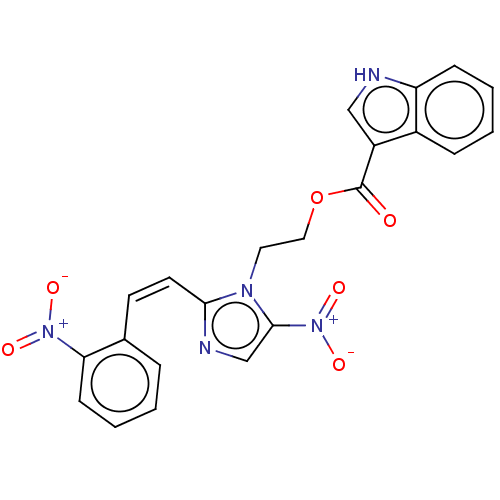 (CHEMBL3331002) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of bovine brain tubulin preincubated for 20 mins by turbidimetry | Eur J Med Chem 85: 341-51 (2014) Article DOI: 10.1016/j.ejmech.2014.07.082 BindingDB Entry DOI: 10.7270/Q29C7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016079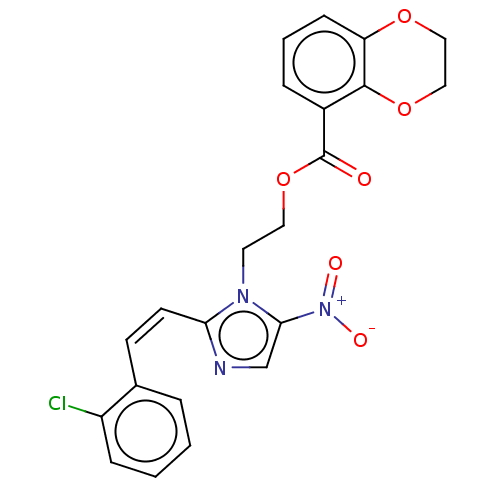 (CHEMBL3260970) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134257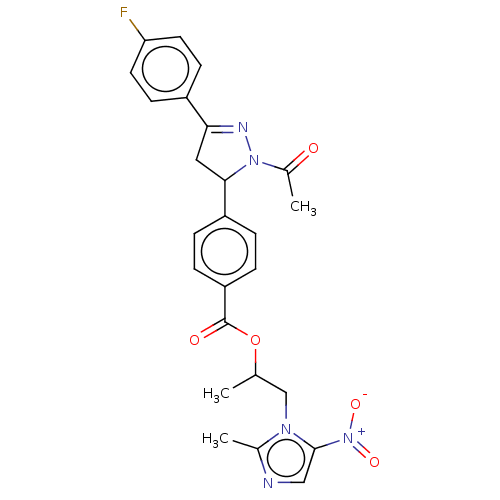 (CHEMBL3747152) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134257 (CHEMBL3747152) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134263 (CHEMBL3746421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134263 (CHEMBL3746421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50056750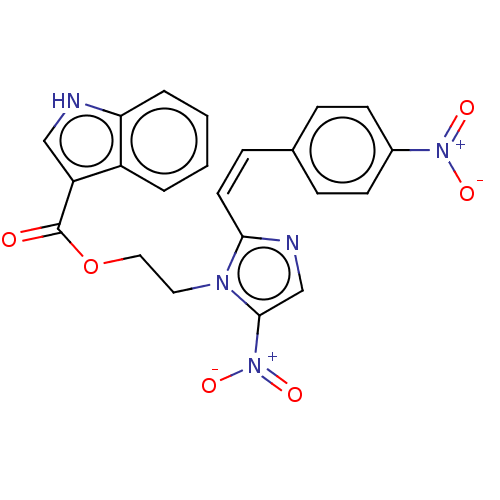 (CHEMBL3331004) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of bovine brain tubulin preincubated for 20 mins by turbidimetry | Eur J Med Chem 85: 341-51 (2014) Article DOI: 10.1016/j.ejmech.2014.07.082 BindingDB Entry DOI: 10.7270/Q29C7036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134254 (CHEMBL3747277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50134254 (CHEMBL3747277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50016088 (CHEMBL3261185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly after 20 mins by turbidimetric method | Bioorg Med Chem 22: 2947-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.005 BindingDB Entry DOI: 10.7270/Q25B041Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50134267 (CHEMBL3747776) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged human HER-2 cytoplasmic domain (676-1245 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometr... | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 127 total ) | Next | Last >> |