

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (RAT) | BDBM60917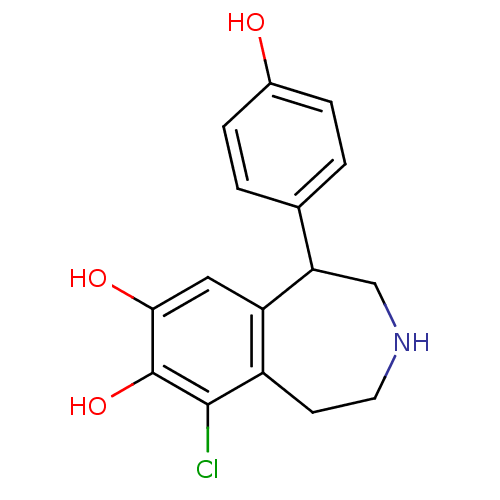 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025202 (9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025206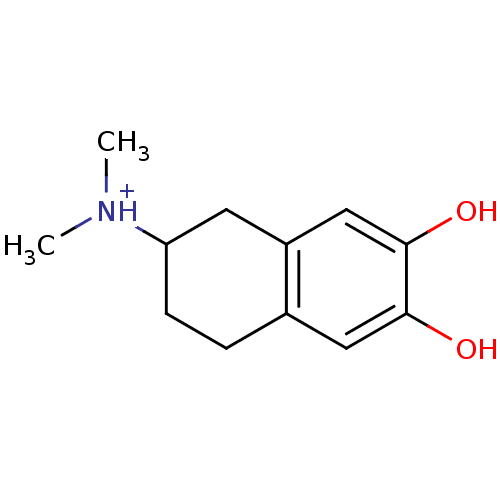 ((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50403606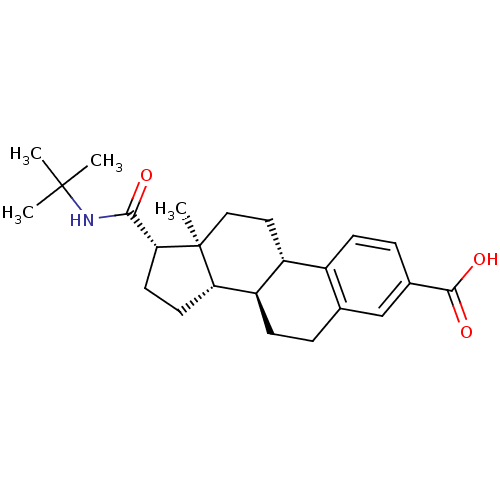 (CHEMBL1627951) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50022533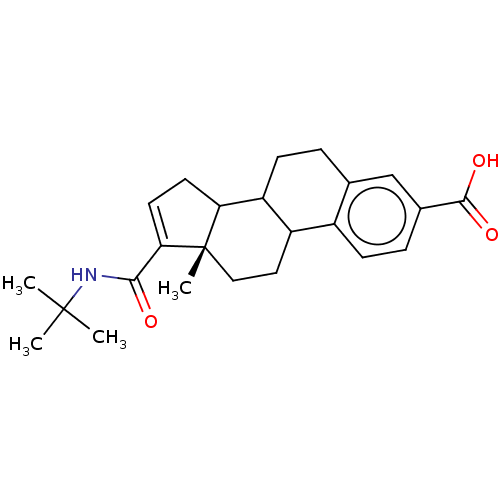 (CHEMBL368288) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50017807 (CHEMBL1628008) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50025206 ((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50022535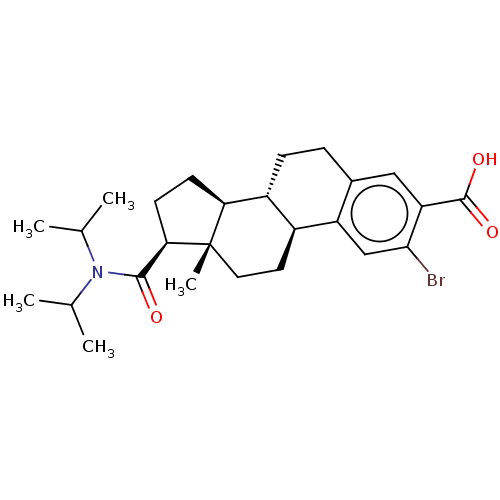 (CHEMBL1628036) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50021747 (CHEMBL1628035) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50017798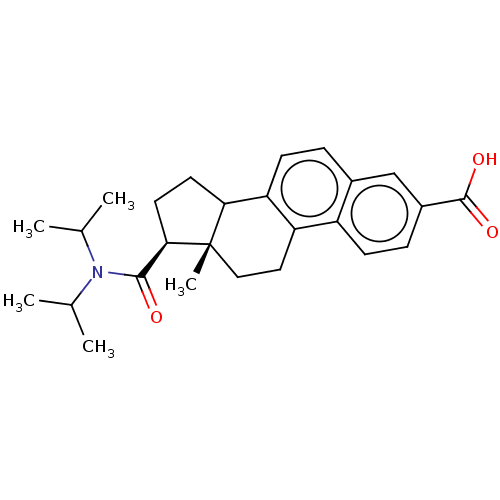 (CHEMBL353414) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50366682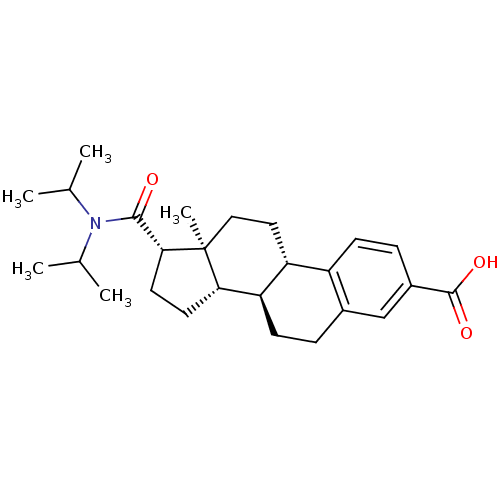 (CHEMBL1627395) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025201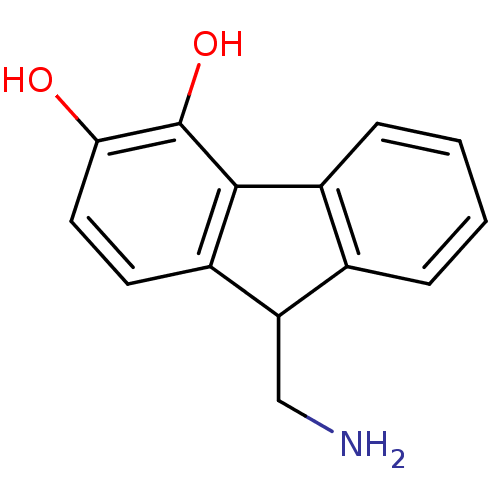 (9-Aminomethyl-9H-fluorene-3,4-diol | CHEMBL55693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50016781 (CHEMBL176429) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50016782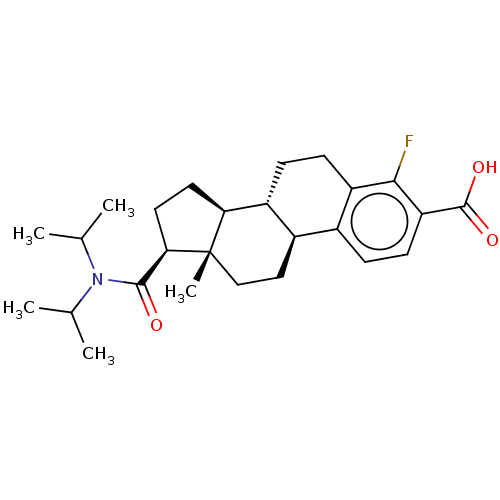 (CHEMBL1627708) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025208 (6-Chloro-9-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM60917 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50017219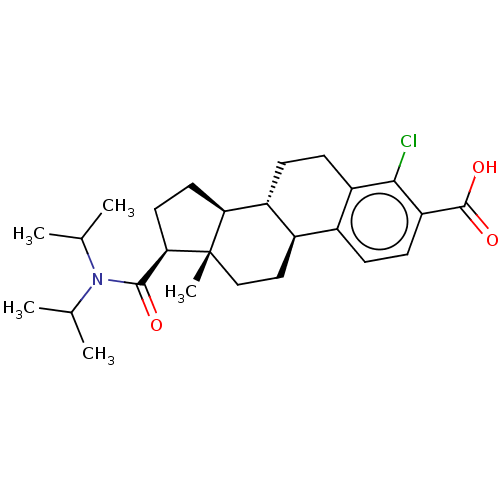 (CHEMBL1627982) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50022534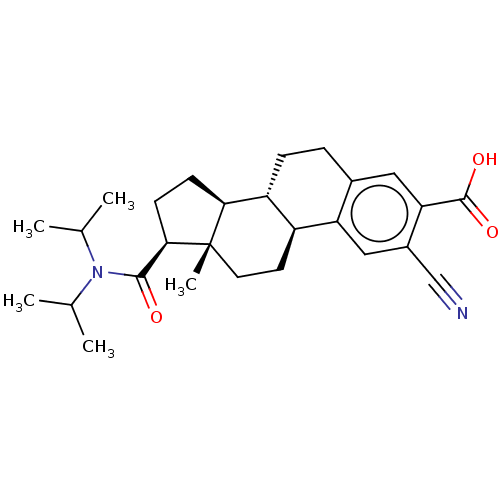 (CHEMBL1628011) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50004821 (2,3,4,5-Tetrahydro-1H-benzo[d]azepine-7,8-diol | C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against dDopamine receptor D1 using [3H]fenoldopam as a radioligand | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025204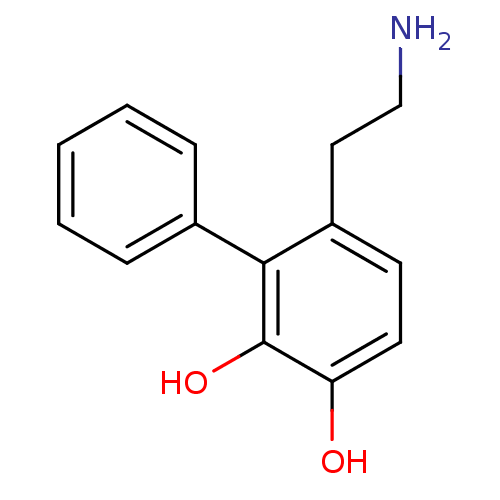 (6-(2-Amino-ethyl)-biphenyl-2,3-diol | CHEMBL299511) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025209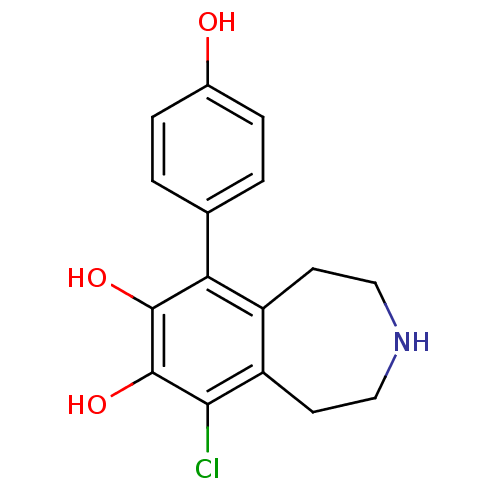 (6-Chloro-9-(4-hydroxy-phenyl)-2,3,4,5-tetrahydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025207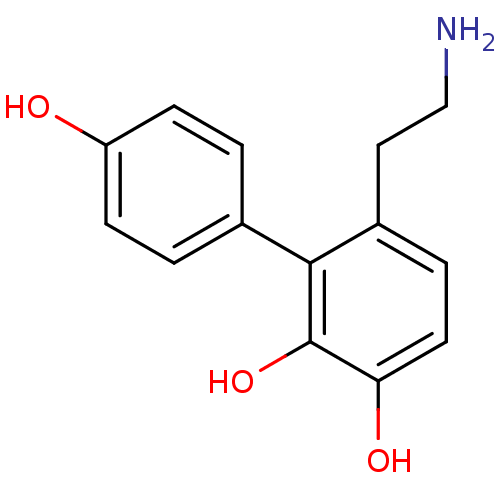 (6-(2-Amino-ethyl)-biphenyl-2,3,4'-triol | CHEMBL57...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025203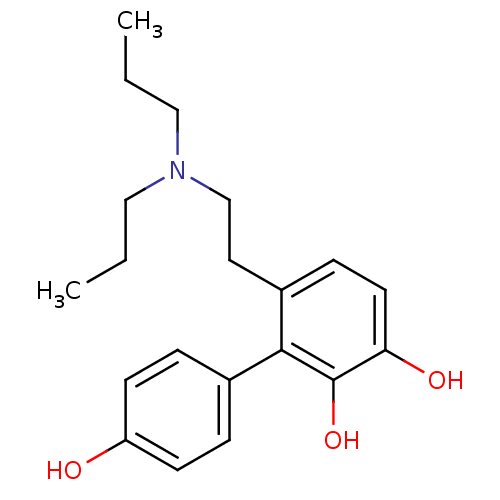 (6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025210 (6-Phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50025203 (6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50017222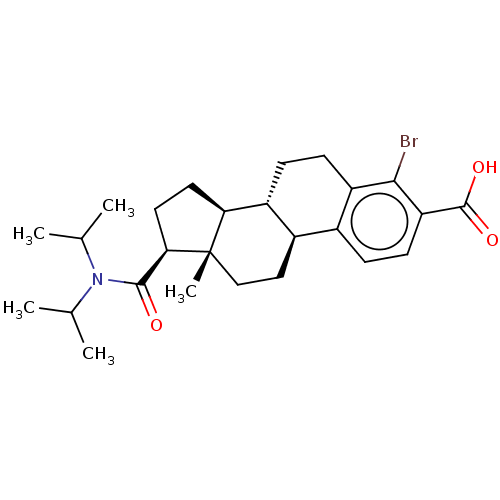 (CHEMBL1627706) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025205 (6-(2-Dipropylamino-ethyl)-biphenyl-2,3-diol | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025211 (6-(4-Hydroxy-phenyl)-2,3,4,5-tetrahydro-1H-benzo[d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50406344 (CHEMBL1628191) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description In vitro inhibition against Steroid 5-alpha-reductase of benign hyperplastic human prostatic tissue expressed as apparent inhibition constant | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50025202 (9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50020394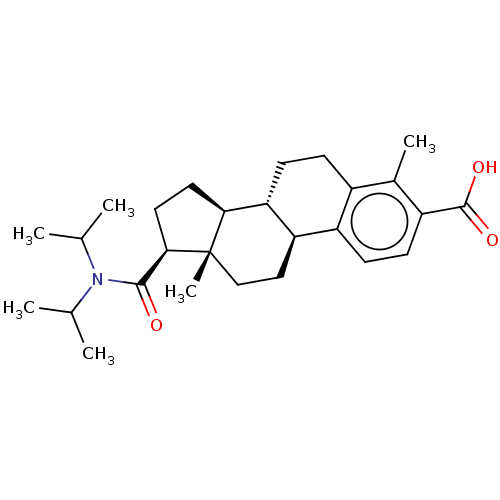 (CHEMBL1627710) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50406344 (CHEMBL1628191) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description In vitro inhibition against Steroid 5-alpha-reductase of whole rat ventral prostate tissue expressed as apparent inhibition constant | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50017763 (CHEMBL1628002) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020475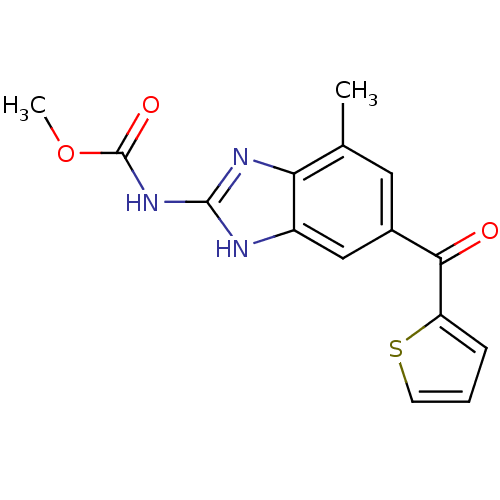 (CHEMBL341192 | [7-Methyl-5-(thiophene-2-carbonyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020464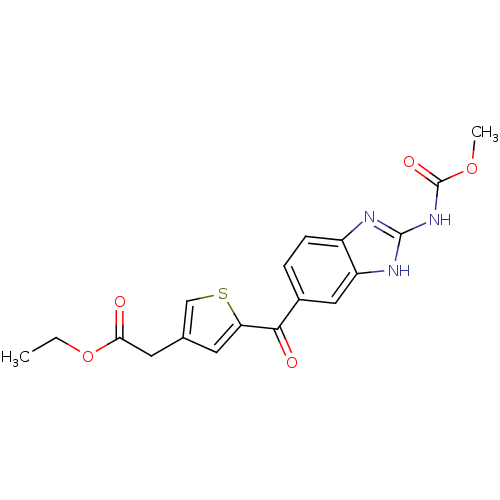 (CHEMBL135161 | [5-(2-Methoxycarbonylamino-1H-benzo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020463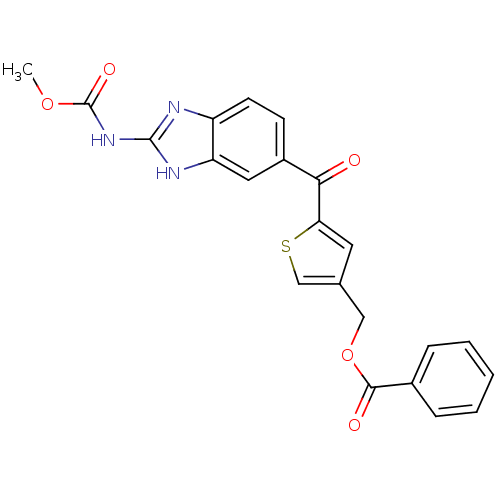 (Benzoic acid 5-(2-methoxycarbonylamino-1H-benzoimi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020478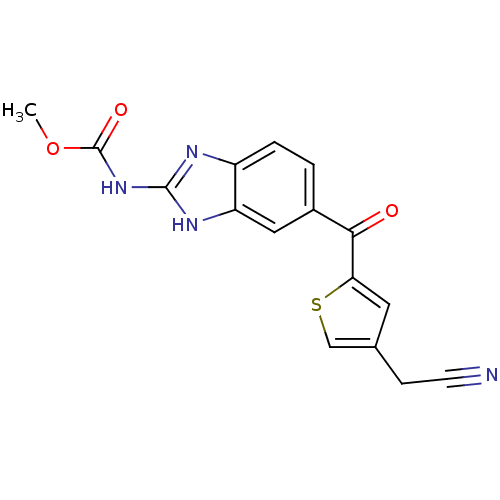 (CHEMBL133799 | [5-(4-Cyanomethyl-thiophene-2-carbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020459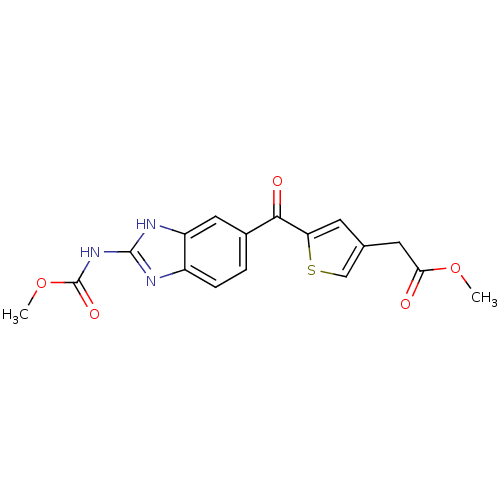 (CHEMBL336862 | [5-(2-Methoxycarbonylamino-1H-benzo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020480 (CHEMBL422615 | [5-(2-Methoxycarbonylamino-1H-benzo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM97233 (CHEMBL9514 | MLS001164242 | N-[6-(2-thenoyl)-1H-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020479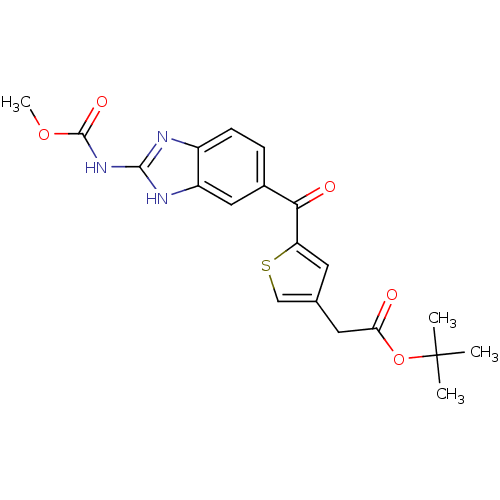 (CHEMBL133900 | [5-(2-Methoxycarbonylamino-1H-benzo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020460 (2,2,2-Trifluoro-1-[5-(2-methoxycarbonylamino-1H-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020461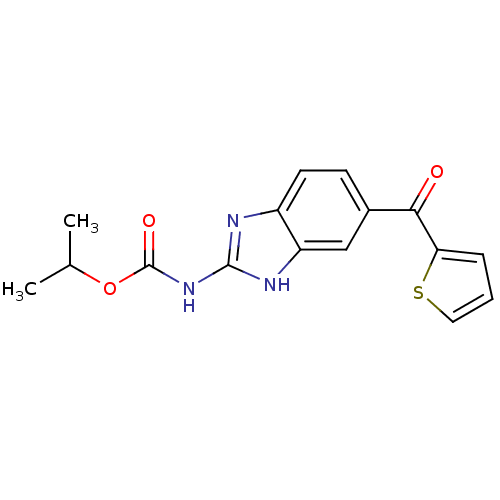 (CHEMBL422269 | [5-(Thiophene-2-carbonyl)-1H-benzoi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020469 (CHEMBL134616 | {5-[4-(2,2,2-Trifluoro-acetyl)-thio...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020474 (CHEMBL133155 | [5-(4-Methyl-thiophene-2-carbonyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020455 (CHEMBL130090 | [5-(4-Hydroxymethyl-thiophene-2-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |