 Found 517 hits with Last Name = 'le' and Initial = 'dt'
Found 517 hits with Last Name = 'le' and Initial = 'dt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin A receptor in cultured rabbit renal artery vascular smooth muscle cells |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50420996
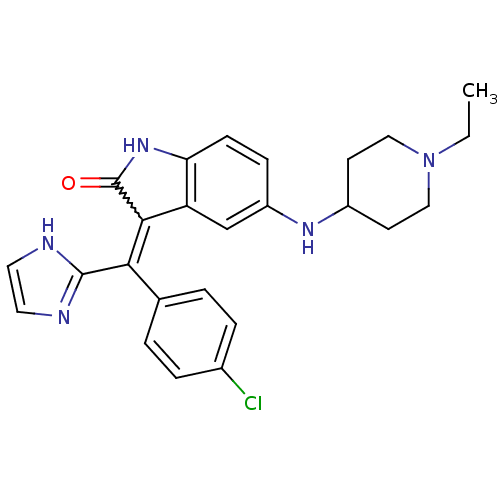
(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380930
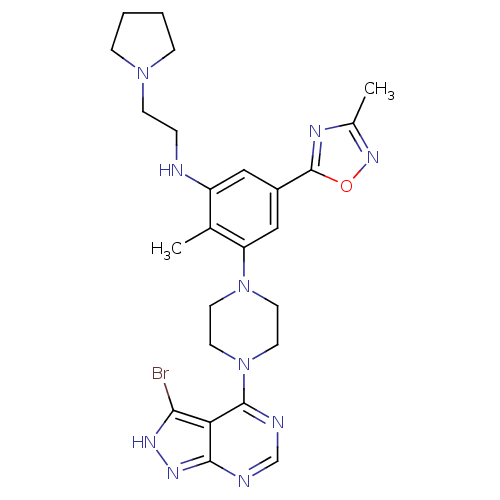
(CHEMBL2016890)Show SMILES Cc1noc(n1)-c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H31BrN10O/c1-16-19(27-5-8-34-6-3-4-7-34)13-18(25-30-17(2)33-37-25)14-20(16)35-9-11-36(12-10-35)24-21-22(26)31-32-23(21)28-15-29-24/h13-15,27H,3-12H2,1-2H3,(H,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380929
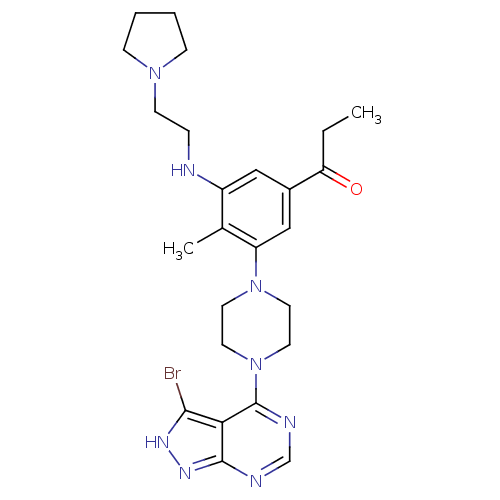
(CHEMBL2016892)Show SMILES CCC(=O)c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H33BrN8O/c1-3-21(35)18-14-19(27-6-9-32-7-4-5-8-32)17(2)20(15-18)33-10-12-34(13-11-33)25-22-23(26)30-31-24(22)28-16-29-25/h14-16,27H,3-13H2,1-2H3,(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380935
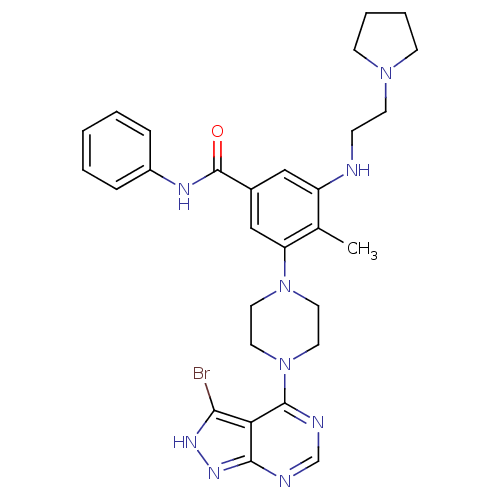
(CHEMBL2016887)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H34BrN9O/c1-20-23(31-9-12-37-10-5-6-11-37)17-21(29(40)34-22-7-3-2-4-8-22)18-24(20)38-13-15-39(16-14-38)28-25-26(30)35-36-27(25)32-19-33-28/h2-4,7-8,17-19,31H,5-6,9-16H2,1H3,(H,34,40)(H,32,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged Flt1 using poly(Glu,Tyr) as substrate after 60 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50380939
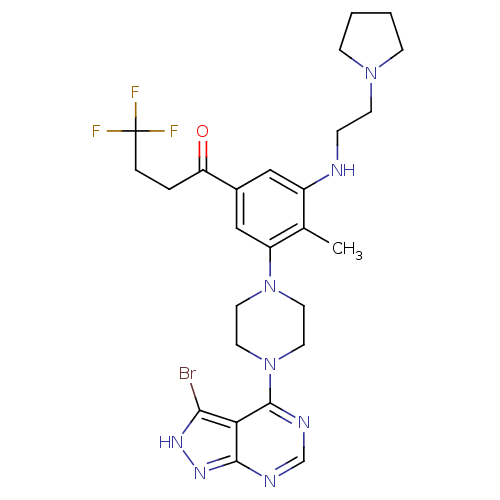
(CHEMBL2016893)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)CCC(F)(F)F Show InChI InChI=1S/C26H32BrF3N8O/c1-17-19(31-6-9-36-7-2-3-8-36)14-18(21(39)4-5-26(28,29)30)15-20(17)37-10-12-38(13-11-37)25-22-23(27)34-35-24(22)32-16-33-25/h14-16,31H,2-13H2,1H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380931
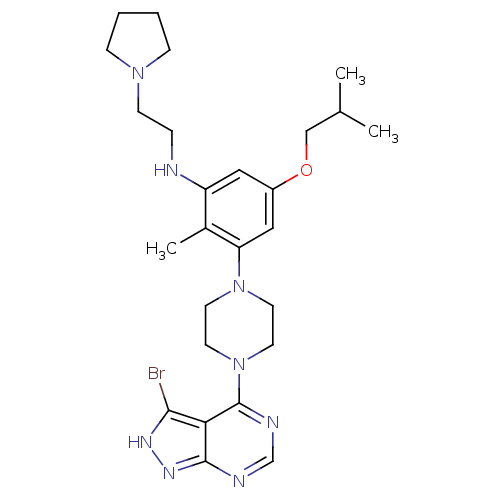
(CHEMBL2016886)Show SMILES CC(C)COc1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C26H37BrN8O/c1-18(2)16-36-20-14-21(28-6-9-33-7-4-5-8-33)19(3)22(15-20)34-10-12-35(13-11-34)26-23-24(27)31-32-25(23)29-17-30-26/h14-15,17-18,28H,4-13,16H2,1-3H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of ET-1 binding to Endothelin B receptor in cultured rat cerebellar membranes |
J Med Chem 35: 3301-3 (1992)
BindingDB Entry DOI: 10.7270/Q21C1XGJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25077
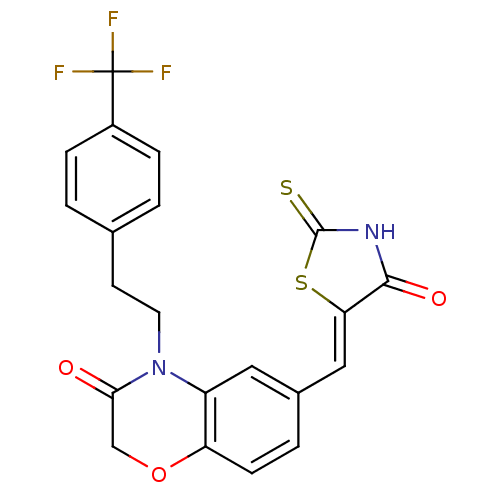
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES FC(F)(F)c1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H15F3N2O3S2/c22-21(23,24)14-4-1-12(2-5-14)7-8-26-15-9-13(3-6-16(15)29-11-18(26)27)10-17-19(28)25-20(30)31-17/h1-6,9-10H,7-8,11H2,(H,25,28,30)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421033
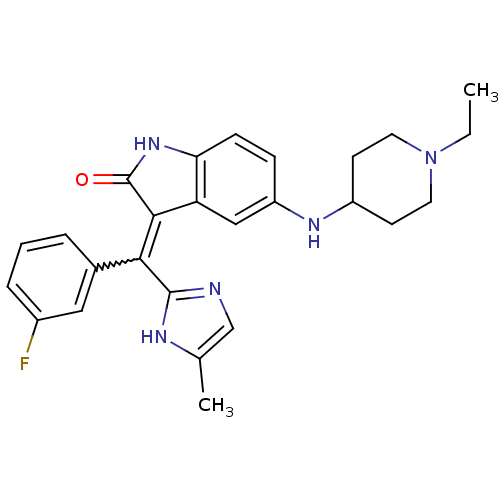
(CHEMBL2087167)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cccc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-11-9-19(10-12-32)30-20-7-8-22-21(14-20)24(26(33)31-22)23(25-28-15-16(2)29-25)17-5-4-6-18(27)13-17/h4-8,13-15,19,30H,3,9-12H2,1-2H3,(H,28,29)(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380939

(CHEMBL2016893)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)CCC(F)(F)F Show InChI InChI=1S/C26H32BrF3N8O/c1-17-19(31-6-9-36-7-2-3-8-36)14-18(21(39)4-5-26(28,29)30)15-20(17)37-10-12-38(13-11-37)25-22-23(27)34-35-24(22)32-16-33-25/h14-16,31H,2-13H2,1H3,(H,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380938
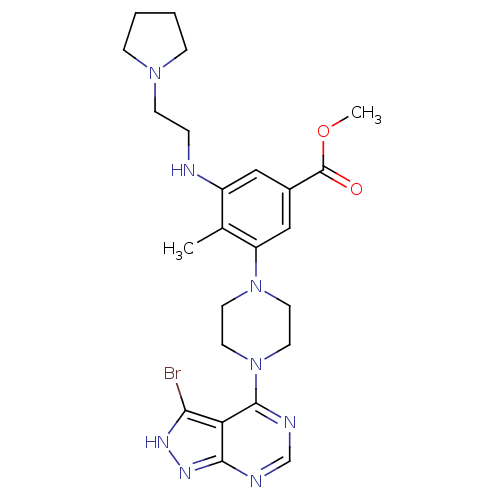
(CHEMBL2016891)Show SMILES COC(=O)c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C24H31BrN8O2/c1-16-18(26-5-8-31-6-3-4-7-31)13-17(24(34)35-2)14-19(16)32-9-11-33(12-10-32)23-20-21(25)29-30-22(20)27-15-28-23/h13-15,26H,3-12H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380937
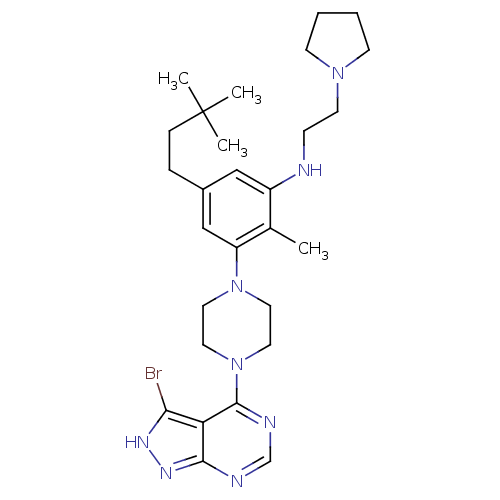
(CHEMBL2016889)Show SMILES Cc1c(NCCN2CCCC2)cc(CCC(C)(C)C)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C28H41BrN8/c1-20-22(30-9-12-35-10-5-6-11-35)17-21(7-8-28(2,3)4)18-23(20)36-13-15-37(16-14-36)27-24-25(29)33-34-26(24)31-19-32-27/h17-19,30H,5-16H2,1-4H3,(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50379530
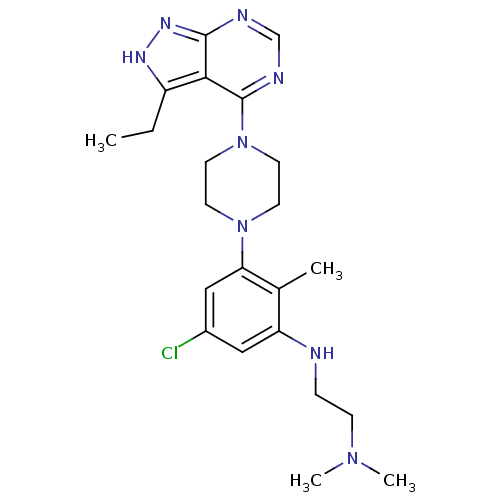
(CHEMBL2012701)Show SMILES CCc1[nH]nc2ncnc(N3CCN(CC3)c3cc(Cl)cc(NCCN(C)C)c3C)c12 Show InChI InChI=1S/C22H31ClN8/c1-5-17-20-21(28-27-17)25-14-26-22(20)31-10-8-30(9-11-31)19-13-16(23)12-18(15(19)2)24-6-7-29(3)4/h12-14,24H,5-11H2,1-4H3,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380934
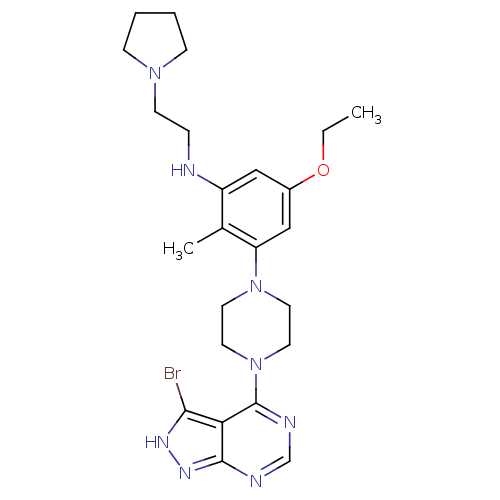
(CHEMBL2016885)Show SMILES CCOc1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C24H33BrN8O/c1-3-34-18-14-19(26-6-9-31-7-4-5-8-31)17(2)20(15-18)32-10-12-33(13-11-32)24-21-22(25)29-30-23(21)27-16-28-24/h14-16,26H,3-13H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50379531
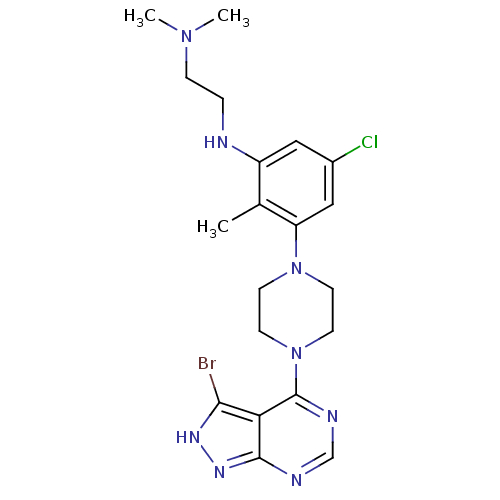
(CHEMBL2012702)Show SMILES CN(C)CCNc1cc(Cl)cc(N2CCN(CC2)c2ncnc3n[nH]c(Br)c23)c1C Show InChI InChI=1S/C20H26BrClN8/c1-13-15(23-4-5-28(2)3)10-14(22)11-16(13)29-6-8-30(9-7-29)20-17-18(21)26-27-19(17)24-12-25-20/h10-12,23H,4-9H2,1-3H3,(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380932
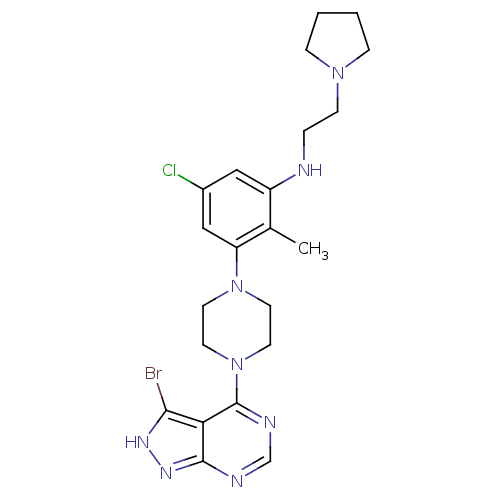
(CHEMBL2016771)Show SMILES Cc1c(NCCN2CCCC2)cc(Cl)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C22H28BrClN8/c1-15-17(25-4-7-30-5-2-3-6-30)12-16(24)13-18(15)31-8-10-32(11-9-31)22-19-20(23)28-29-21(19)26-14-27-22/h12-14,25H,2-11H2,1H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380936
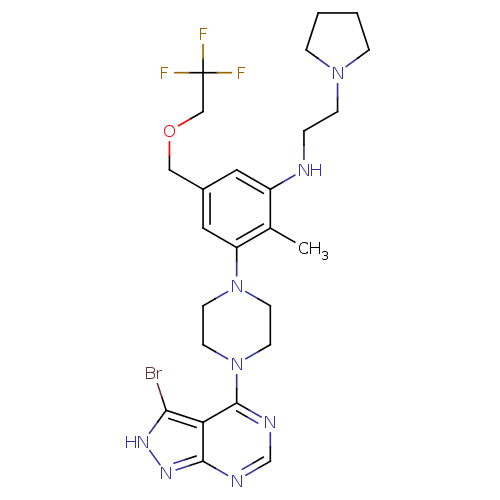
(CHEMBL2016888)Show SMILES Cc1c(NCCN2CCCC2)cc(COCC(F)(F)F)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H32BrF3N8O/c1-17-19(30-4-7-35-5-2-3-6-35)12-18(14-38-15-25(27,28)29)13-20(17)36-8-10-37(11-9-36)24-21-22(26)33-34-23(21)31-16-32-24/h12-13,16,30H,2-11,14-15H2,1H3,(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25073
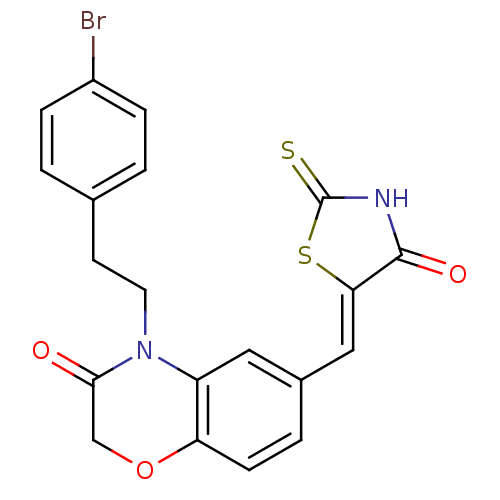
(4-[2-(4-bromophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Brc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15BrN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25061
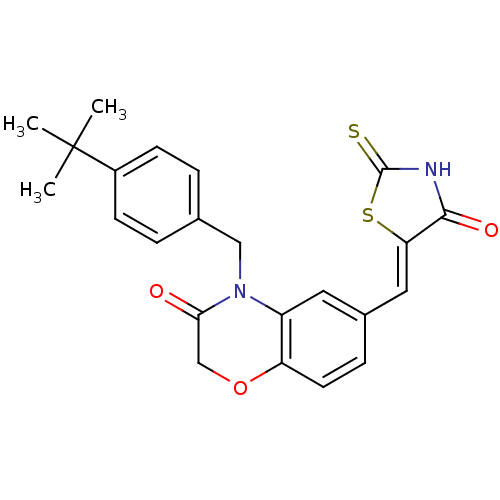
(4-[(4-tert-butylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES CC(C)(C)c1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C23H22N2O3S2/c1-23(2,3)16-7-4-14(5-8-16)12-25-17-10-15(6-9-18(17)28-13-20(25)26)11-19-21(27)24-22(29)30-19/h4-11H,12-13H2,1-3H3,(H,24,27,29)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25066
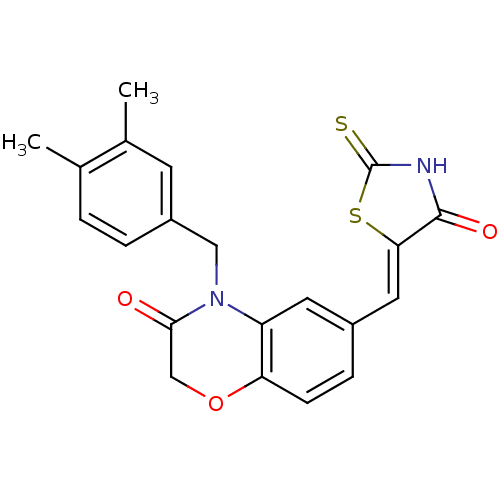
(4-[(3,4-dimethylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES Cc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1C Show InChI InChI=1S/C21H18N2O3S2/c1-12-3-4-15(7-13(12)2)10-23-16-8-14(5-6-17(16)26-11-19(23)24)9-18-20(25)22-21(27)28-18/h3-9H,10-11H2,1-2H3,(H,22,25,27)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25068
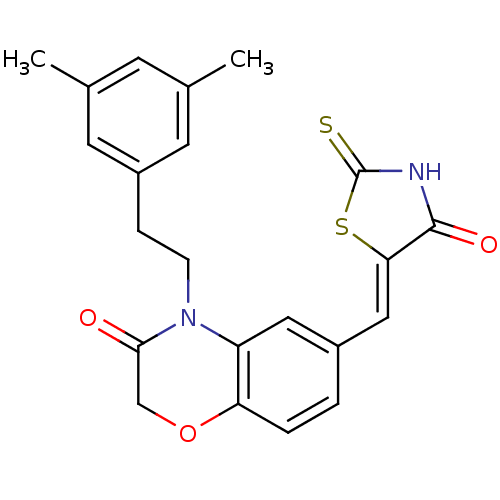
(4-[2-(3,5-dimethylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Cc1cc(C)cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C22H20N2O3S2/c1-13-7-14(2)9-16(8-13)5-6-24-17-10-15(3-4-18(17)27-12-20(24)25)11-19-21(26)23-22(28)29-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,28)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25080
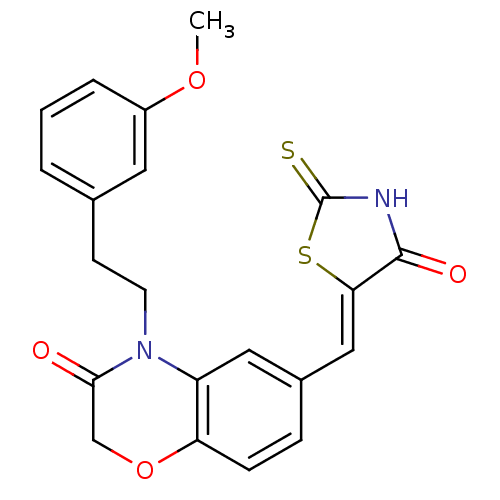
(4-[2-(3-methoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulf...)Show SMILES COc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O4S2/c1-26-15-4-2-3-13(9-15)7-8-23-16-10-14(5-6-17(16)27-12-19(23)24)11-18-20(25)22-21(28)29-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,28)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25069
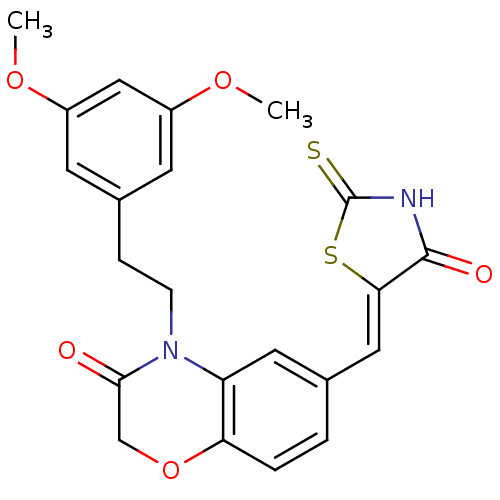
(4-[2-(3,5-dimethoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-...)Show SMILES COc1cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc(OC)c1 Show InChI InChI=1S/C22H20N2O5S2/c1-27-15-7-14(8-16(11-15)28-2)5-6-24-17-9-13(3-4-18(17)29-12-20(24)25)10-19-21(26)23-22(30)31-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,30)/b19-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421026
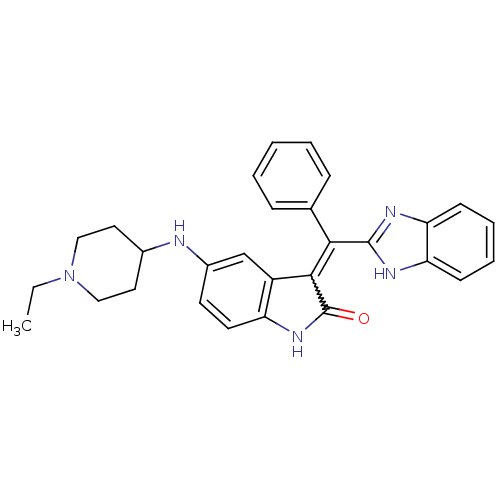
(CHEMBL2086753)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccccc3)c2c1 Show InChI InChI=1S/C29H29N5O/c1-2-34-16-14-20(15-17-34)30-21-12-13-23-22(18-21)27(29(35)33-23)26(19-8-4-3-5-9-19)28-31-24-10-6-7-11-25(24)32-28/h3-13,18,20,30H,2,14-17H2,1H3,(H,31,32)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421034
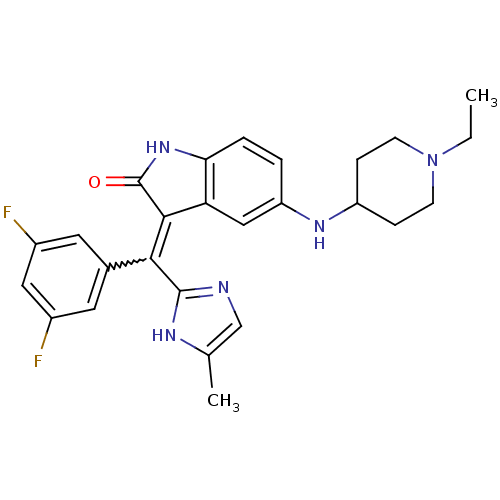
(CHEMBL2087168)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cc(F)cc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H27F2N5O/c1-3-33-8-6-19(7-9-33)31-20-4-5-22-21(13-20)24(26(34)32-22)23(25-29-14-15(2)30-25)16-10-17(27)12-18(28)11-16/h4-5,10-14,19,31H,3,6-9H2,1-2H3,(H,29,30)(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380933

(CHEMBL2012699)Show SMILES CCc1[nH]nc2ncnc(N3CCN(CC3)c3cc(Cl)cc(NCCN4CCCC4)c3C)c12 Show InChI InChI=1S/C24H33ClN8/c1-3-19-22-23(30-29-19)27-16-28-24(22)33-12-10-32(11-13-33)21-15-18(25)14-20(17(21)2)26-6-9-31-7-4-5-8-31/h14-16,26H,3-13H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421034

(CHEMBL2087168)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cc(F)cc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H27F2N5O/c1-3-33-8-6-19(7-9-33)31-20-4-5-22-21(13-20)24(26(34)32-22)23(25-29-14-15(2)30-25)16-10-17(27)12-18(28)11-16/h4-5,10-14,19,31H,3,6-9H2,1-2H3,(H,29,30)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421016
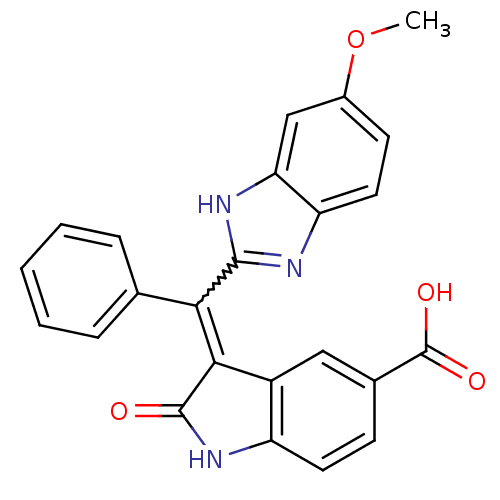
(CHEMBL2086742)Show SMILES COc1ccc2nc([nH]c2c1)C(=C1C(=O)Nc2ccc(cc12)C(O)=O)c1ccccc1 |w:11.12| Show InChI InChI=1S/C24H17N3O4/c1-31-15-8-10-18-19(12-15)26-22(25-18)20(13-5-3-2-4-6-13)21-16-11-14(24(29)30)7-9-17(16)27-23(21)28/h2-12H,1H3,(H,25,26)(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421028
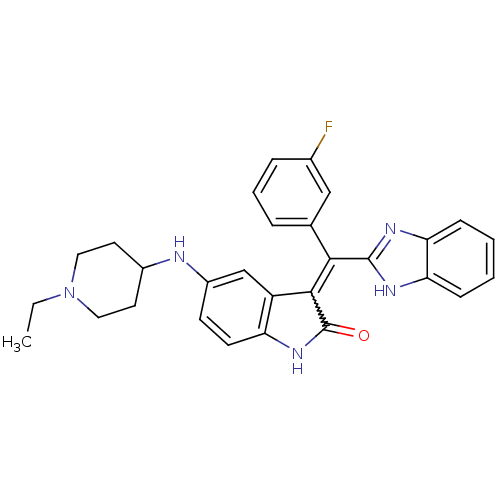
(CHEMBL2086756)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3cccc(F)c3)c2c1 Show InChI InChI=1S/C29H28FN5O/c1-2-35-14-12-20(13-15-35)31-21-10-11-23-22(17-21)27(29(36)34-23)26(18-6-5-7-19(30)16-18)28-32-24-8-3-4-9-25(24)33-28/h3-11,16-17,20,31H,2,12-15H2,1H3,(H,32,33)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421035
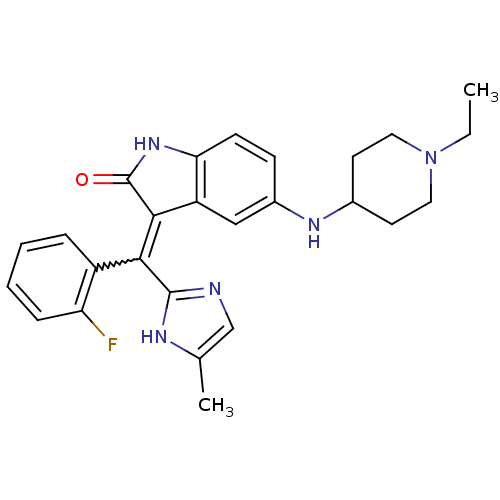
(CHEMBL2087169)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3ccccc3F)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-12-10-17(11-13-32)30-18-8-9-22-20(14-18)24(26(33)31-22)23(25-28-15-16(2)29-25)19-6-4-5-7-21(19)27/h4-9,14-15,17,30H,3,10-13H2,1-2H3,(H,28,29)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of Flt4 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50421033

(CHEMBL2087167)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cccc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-11-9-19(10-12-32)30-20-7-8-22-21(14-20)24(26(33)31-22)23(25-28-15-16(2)29-25)17-5-4-6-18(27)13-17/h4-8,13-15,19,30H,3,9-12H2,1-2H3,(H,28,29)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged FGFR1 using poly(Glu,Tyr) as substrate after 30 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421036
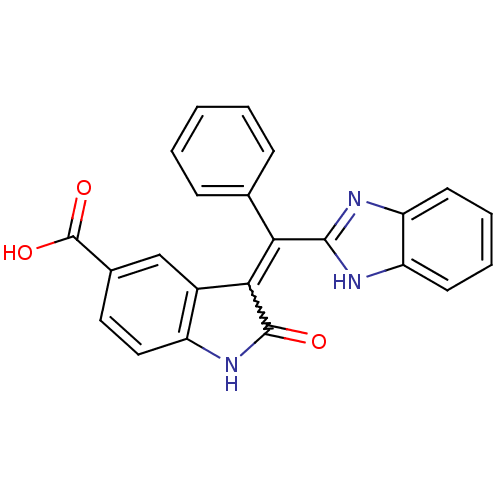
(CHEMBL2086736)Show SMILES OC(=O)c1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccccc3)c2c1 Show InChI InChI=1S/C23H15N3O3/c27-22-20(15-12-14(23(28)29)10-11-16(15)26-22)19(13-6-2-1-3-7-13)21-24-17-8-4-5-9-18(17)25-21/h1-12H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421029
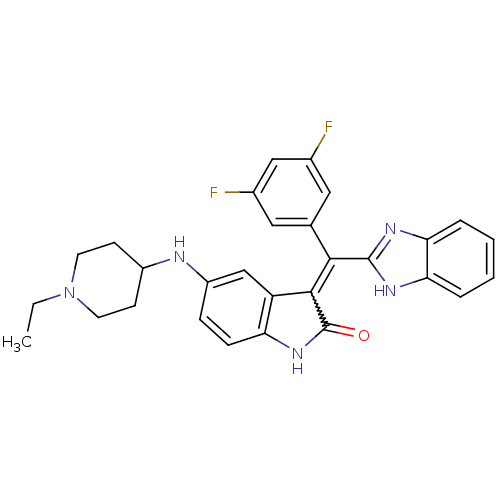
(CHEMBL2086757)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3cc(F)cc(F)c3)c2c1 Show InChI InChI=1S/C29H27F2N5O/c1-2-36-11-9-20(10-12-36)32-21-7-8-23-22(16-21)27(29(37)35-23)26(17-13-18(30)15-19(31)14-17)28-33-24-5-3-4-6-25(24)34-28/h3-8,13-16,20,32H,2,9-12H2,1H3,(H,33,34)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421033

(CHEMBL2087167)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cccc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-11-9-19(10-12-32)30-20-7-8-22-21(14-20)24(26(33)31-22)23(25-28-15-16(2)29-25)17-5-4-6-18(27)13-17/h4-8,13-15,19,30H,3,9-12H2,1-2H3,(H,28,29)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25074
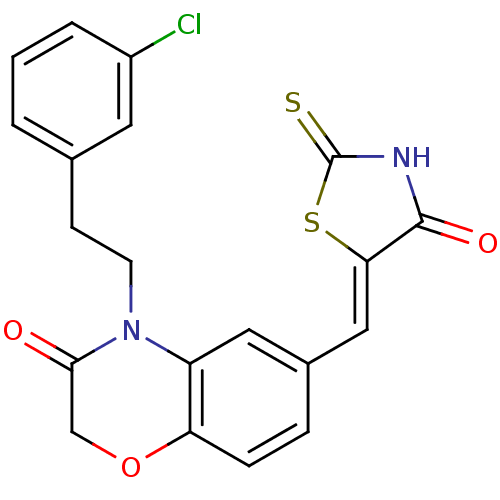
(4-[2-(3-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-3-1-2-12(8-14)6-7-23-15-9-13(4-5-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-5,8-10H,6-7,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.27 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25078
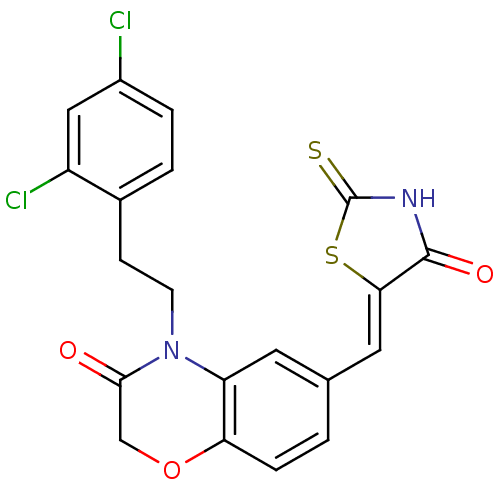
(4-[2-(2,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c(Cl)c1 Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-2-12(14(22)9-13)5-6-24-15-7-11(1-4-16(15)27-10-18(24)25)8-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.28 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
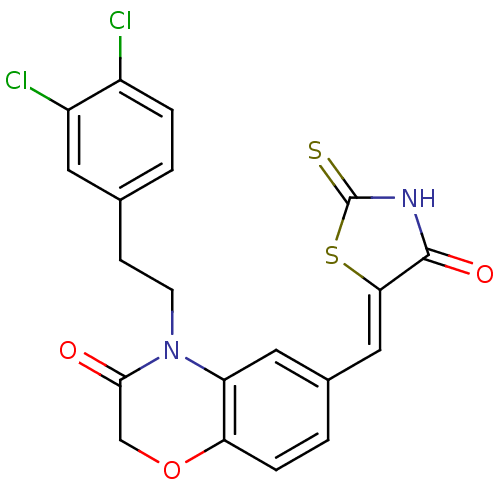
(Homo sapiens (Human)) | BDBM25075
(4-[2-(3,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1Cl Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.36 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25070
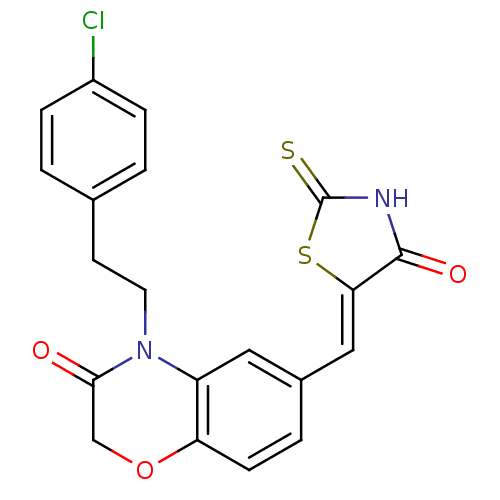
(4-[2-(4-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.76 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25081
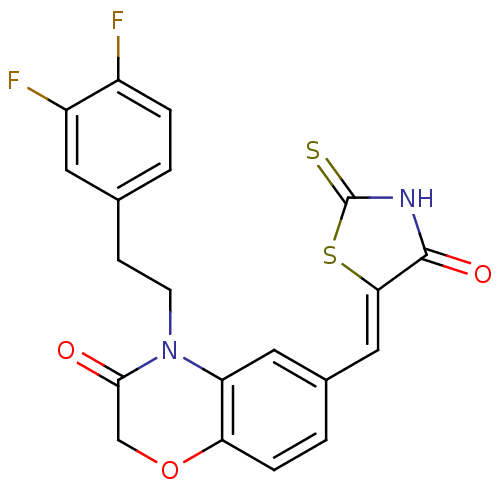
(4-[2-(3,4-difluorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Fc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1F Show InChI InChI=1S/C20H14F2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.84 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25079
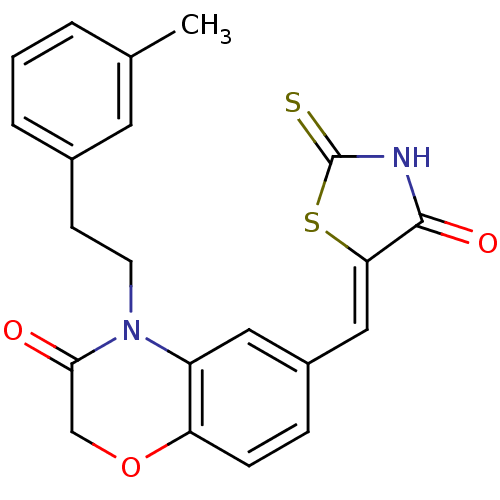
(4-[2-(3-methylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Cc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O3S2/c1-13-3-2-4-14(9-13)7-8-23-16-10-15(5-6-17(16)26-12-19(23)24)11-18-20(25)22-21(27)28-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,27)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.96 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50421026

(CHEMBL2086753)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccccc3)c2c1 Show InChI InChI=1S/C29H29N5O/c1-2-34-16-14-20(15-17-34)30-21-12-13-23-22(18-21)27(29(35)33-23)26(19-8-4-3-5-9-19)28-31-24-10-6-7-11-25(24)32-28/h3-13,18,20,30H,2,14-17H2,1H3,(H,31,32)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged FGFR1 using poly(Glu,Tyr) as substrate after 30 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50421028

(CHEMBL2086756)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3cccc(F)c3)c2c1 Show InChI InChI=1S/C29H28FN5O/c1-2-35-14-12-20(13-15-35)31-21-10-11-23-22(17-21)27(29(36)34-23)26(18-6-5-7-19(30)16-18)28-32-24-8-3-4-9-25(24)33-28/h3-11,16-17,20,31H,2,12-15H2,1H3,(H,32,33)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged FGFR1 using poly(Glu,Tyr) as substrate after 30 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421032
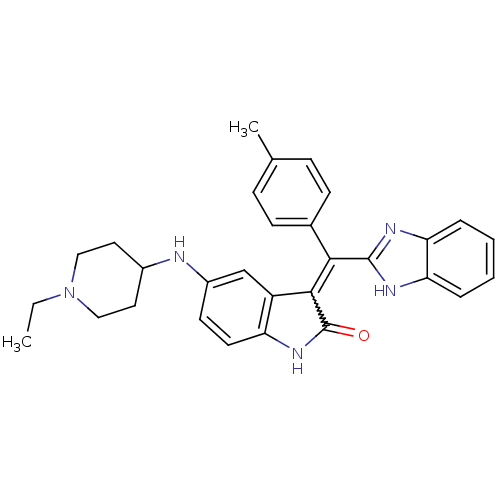
(CHEMBL2086754)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccc(C)cc3)c2c1 Show InChI InChI=1S/C30H31N5O/c1-3-35-16-14-21(15-17-35)31-22-12-13-24-23(18-22)28(30(36)34-24)27(20-10-8-19(2)9-11-20)29-32-25-6-4-5-7-26(25)33-29/h4-13,18,21,31H,3,14-17H2,1-2H3,(H,32,33)(H,34,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data