

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50322367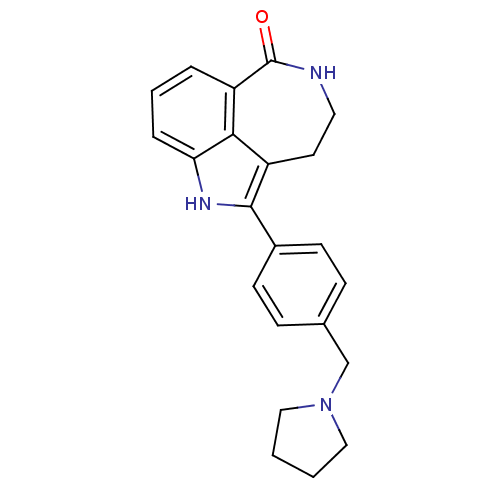 (5-(4-(pyrrolidin-1-ylmethyl)phenyl)-2,3,4,6-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50154730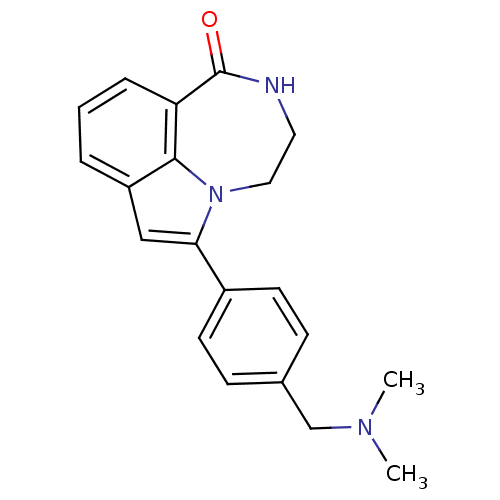 (6-(4-((dimethylamino)methyl)phenyl)-3,4-dihydro-[1...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM108460 (CHEMBL2178393 | US11191732, Example 1 | US8604016,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysis | J Med Chem 55: 10551-63 (2012) Article DOI: 10.1021/jm301191p BindingDB Entry DOI: 10.7270/Q2VD70M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Sus scrofa (pig)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Competitive inhibition of pig kidney DAAO using D-Alanine as substrate by Michaelis-Menten plot analysis | Bioorg Med Chem Lett 23: 3910-3 (2013) Article DOI: 10.1016/j.bmcl.2013.04.062 BindingDB Entry DOI: 10.7270/Q2K35W2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50304738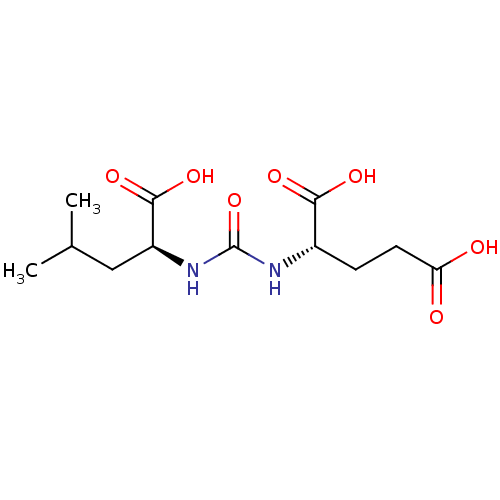 (2-(3-((S)-1-carboxy-3-methylbutyl)ureido)pentanedi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503760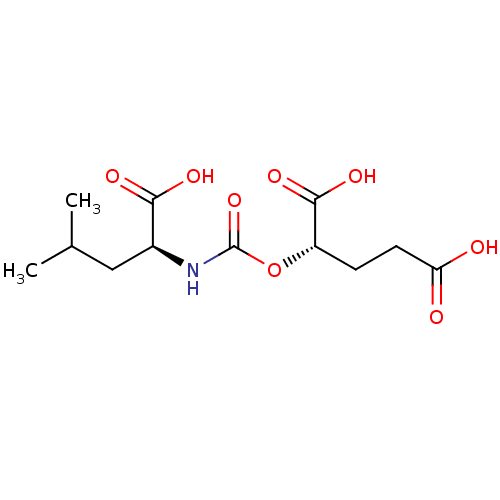 (CHEMBL4442450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27525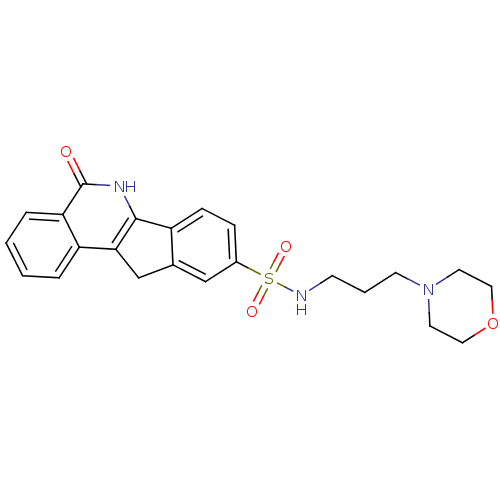 (N-[3-(morpholin-4-yl)propyl]-8-oxo-9-azatetracyclo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392045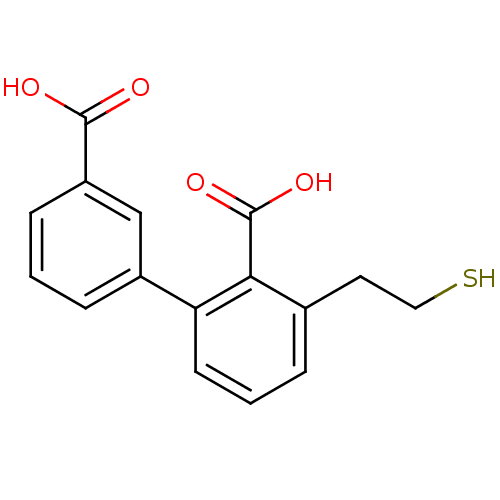 (CHEMBL2152561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392040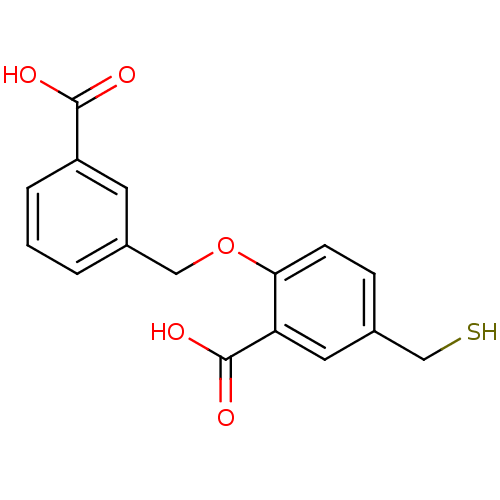 (CHEMBL2152556) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50322367 (5-(4-(pyrrolidin-1-ylmethyl)phenyl)-2,3,4,6-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392046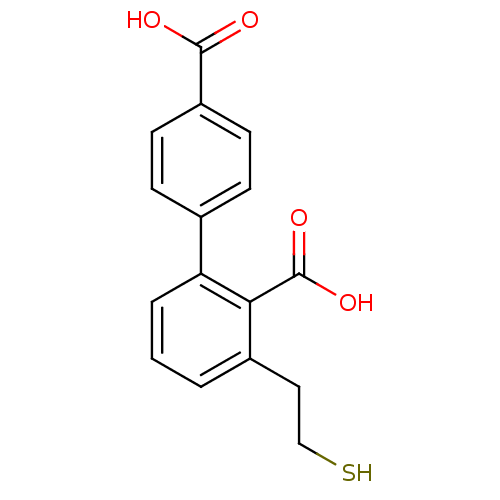 (CHEMBL2152562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM27721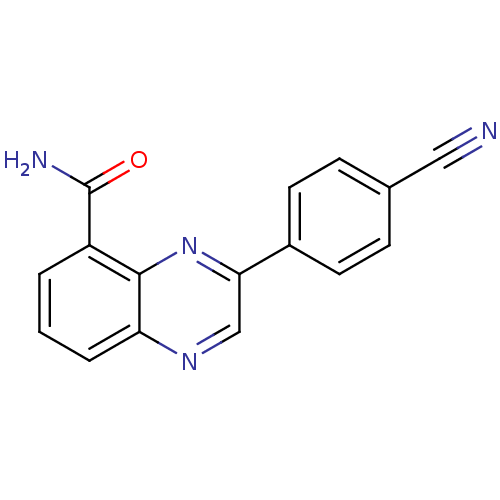 (3-(4-cyanophenyl)quinoxaline-5-carboxamide | CHEMB...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP2 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50220858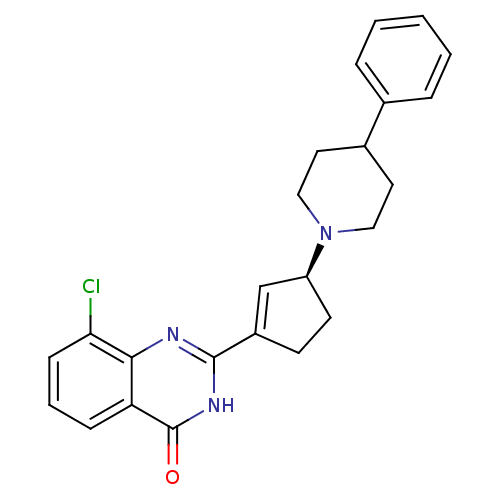 ((S)-8-chloro-2-(3-(4-phenylpiperidin-1-yl)cyclopen...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503756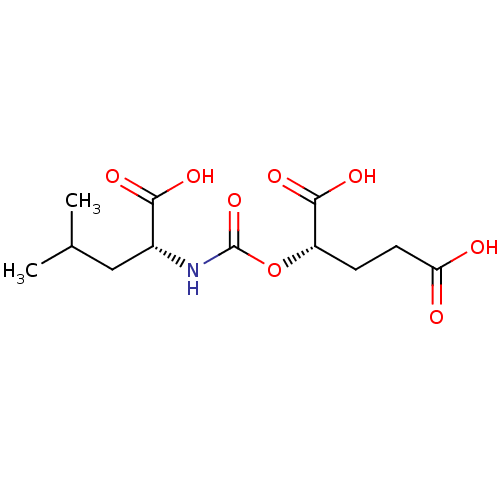 (CHEMBL4473741) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50068775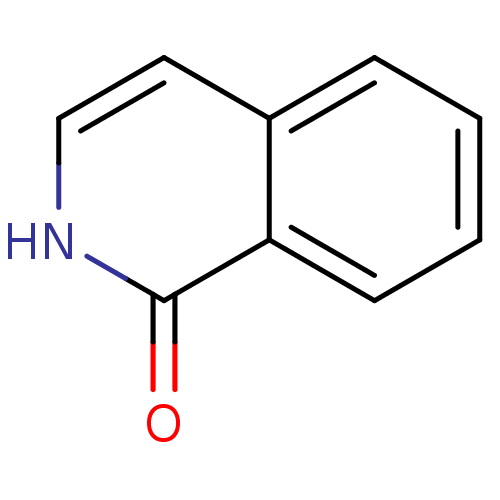 (2H-Isoquinolin-1-one | CHEMBL339695 | isoquinolin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50416360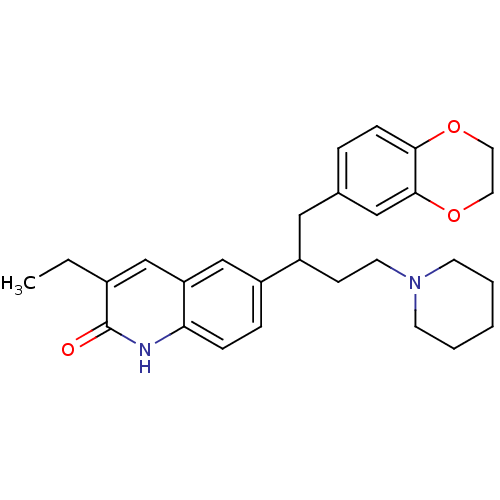 (CHEMBL1171298) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50416361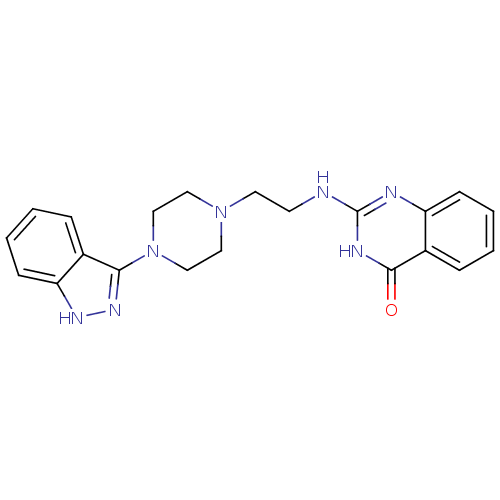 (CHEMBL1171304) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101114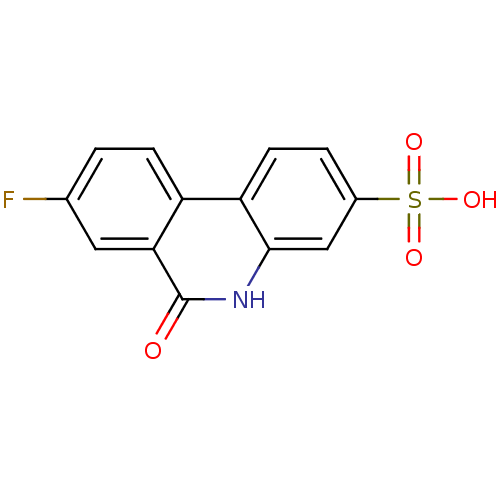 (8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27708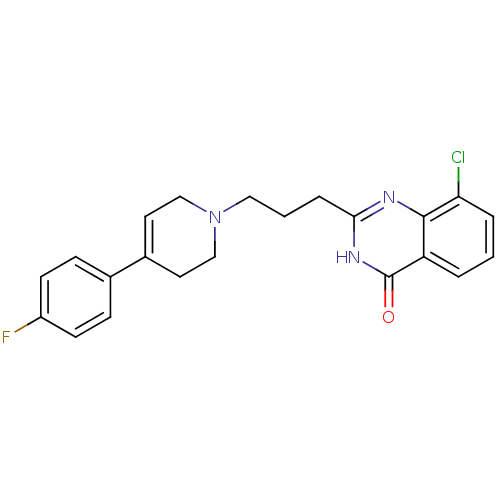 (8-chloro-2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101129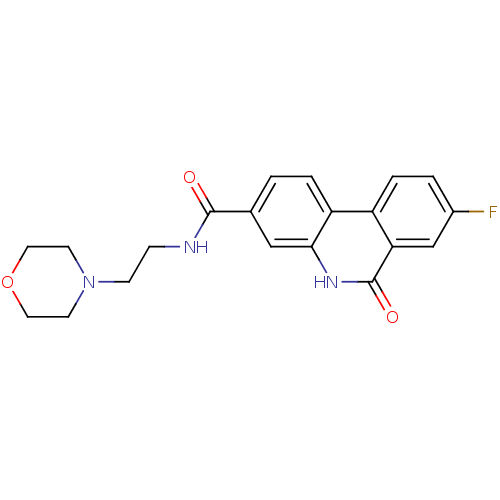 (8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-carbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101129 (8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-carbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17762 (3-[2-carboxy-2-(3-sulfanylpropyl)ethyl]benzoic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17762 (3-[2-carboxy-2-(3-sulfanylpropyl)ethyl]benzoic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503754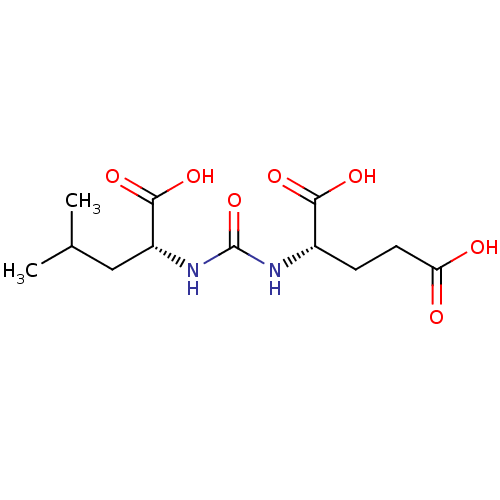 (CHEMBL4458733) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392041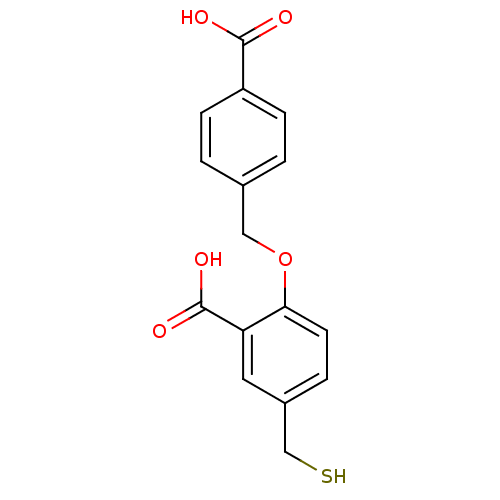 (CHEMBL2152557) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503757 (CHEMBL4541841) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120267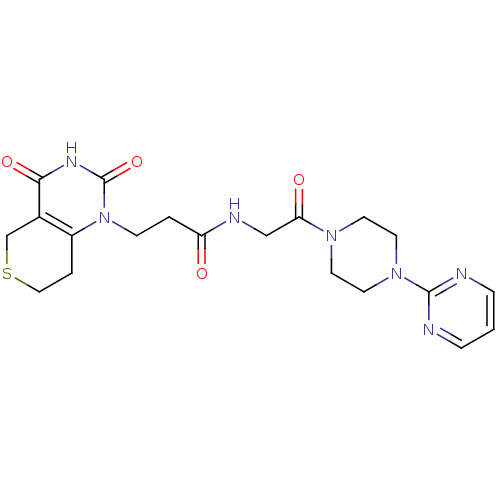 (3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27497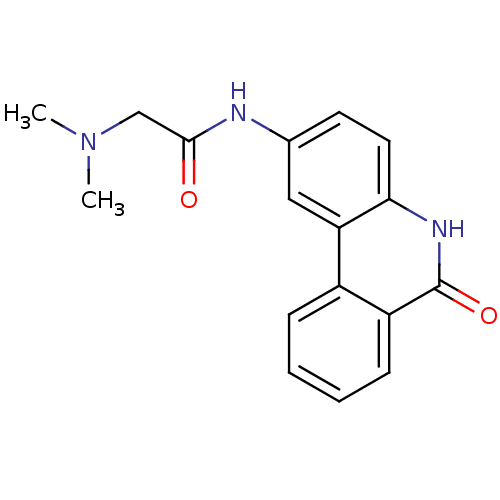 (2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridi...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101124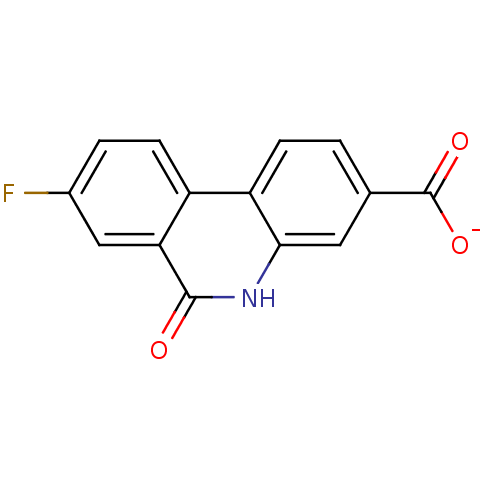 (CHEMBL298053 | sodium 8-fluoro-6-oxo-5,6-dihydroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50332217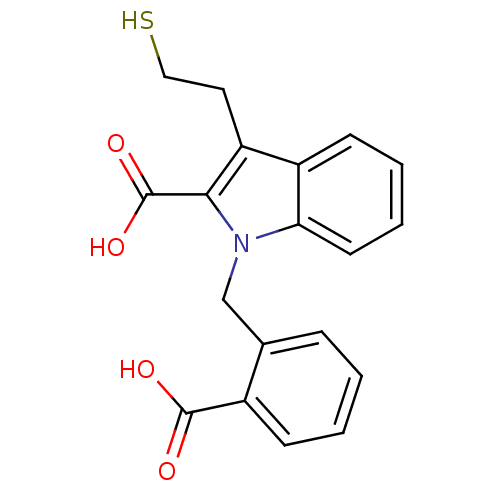 (1-[(2-carboxyphenyl)methyl]-3-(2-mercaptoethyl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50332218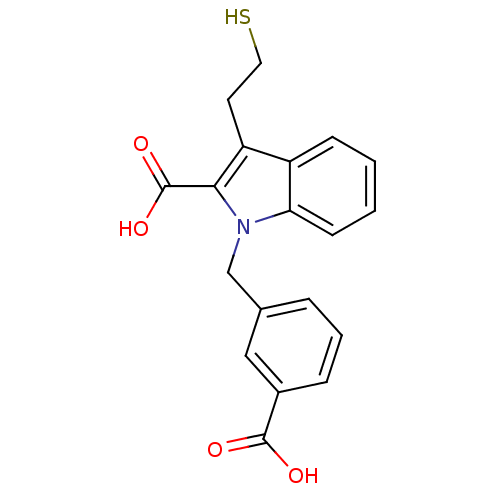 (1-[(3-carboxyphenyl)methyl]-3-(2-mercaptoethyl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50332228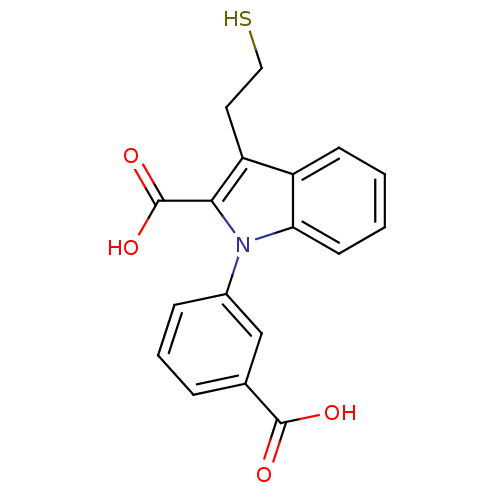 (1-(3-Carboxyphenyl)-3-(2-mercapto-ethyl)-1H-indole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50332228 (1-(3-Carboxyphenyl)-3-(2-mercapto-ethyl)-1H-indole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50131011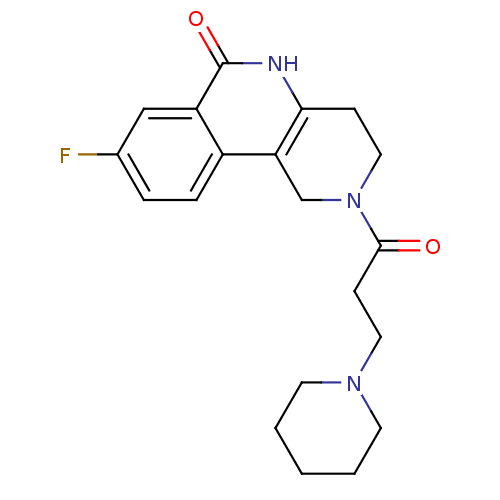 (8-Fluoro-2-(3-piperidin-1-yl-propionyl)-1,3,4,5-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50322366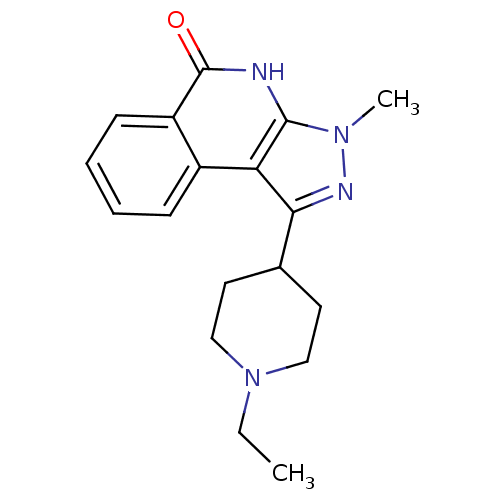 (1-(4-ethylcyclohexyl)-3-methyl-5-methylene-4,5-dih...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50322365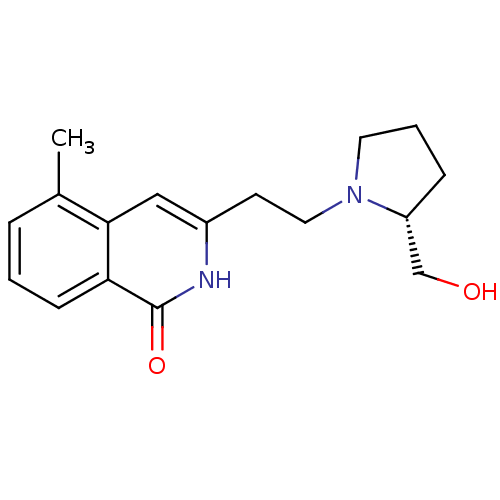 ((R)-3-(2-(2-(hydroxymethyl)pyrrolidin-1-yl)ethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Brain Science Institute Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 53: 4561-84 (2010) Article DOI: 10.1021/jm100012m BindingDB Entry DOI: 10.7270/Q2NV9JF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50160756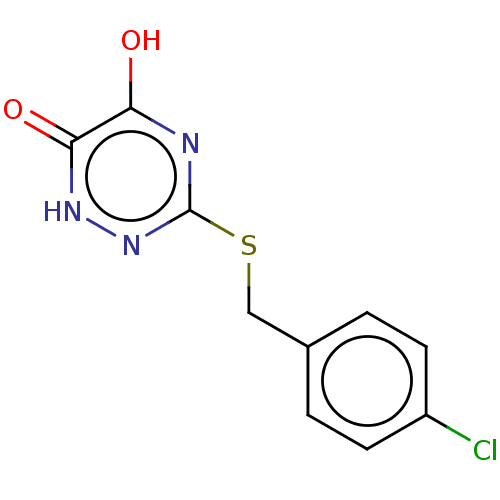 (CHEMBL3787162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of recombinant human DAAO assessed as oxidative deamination of D-serine in presence of molecular oxygen and FAD after 20 mins | Bioorg Med Chem Lett 26: 2088-91 (2016) Article DOI: 10.1016/j.bmcl.2016.02.068 BindingDB Entry DOI: 10.7270/Q2RB76HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50160750 (CHEMBL3786955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of recombinant human DAAO assessed as oxidative deamination of D-serine in presence of molecular oxygen and FAD after 20 mins | Bioorg Med Chem Lett 26: 2088-91 (2016) Article DOI: 10.1016/j.bmcl.2016.02.068 BindingDB Entry DOI: 10.7270/Q2RB76HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101105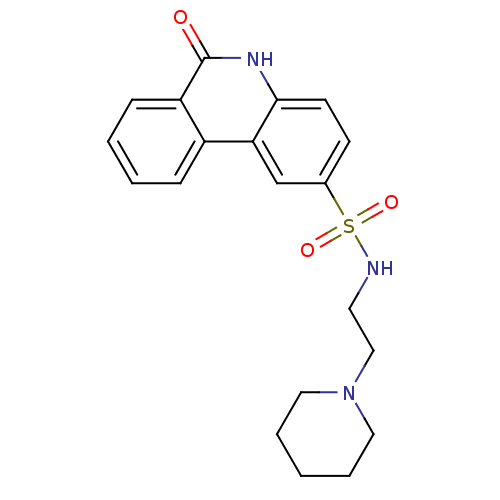 (6-Oxo-5,6-dihydro-phenanthridine-2-sulfonic acid (...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50117761 (CHEMBL3613920 | US9505753, 5aa) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.5 | 25 |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br... | US Patent US9505753 (2016) BindingDB Entry DOI: 10.7270/Q21J98QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM258332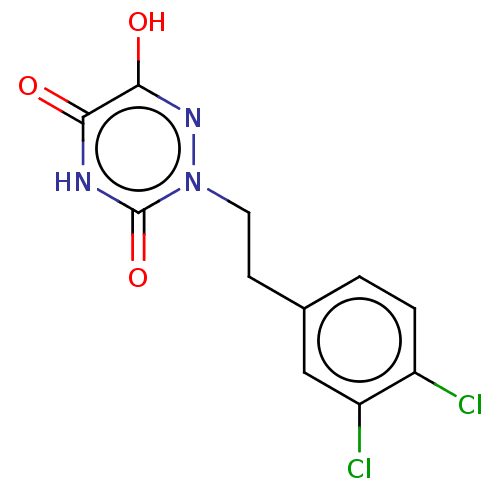 (US9505753, 5y) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.5 | 25 |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br... | US Patent US9505753 (2016) BindingDB Entry DOI: 10.7270/Q21J98QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50117818 (CHEMBL3613929 | US9505753, 5e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.5 | 25 |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br... | US Patent US9505753 (2016) BindingDB Entry DOI: 10.7270/Q21J98QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50117813 (CHEMBL3613946 | US9505753, 5o) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.5 | 25 |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br... | US Patent US9505753 (2016) BindingDB Entry DOI: 10.7270/Q21J98QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101108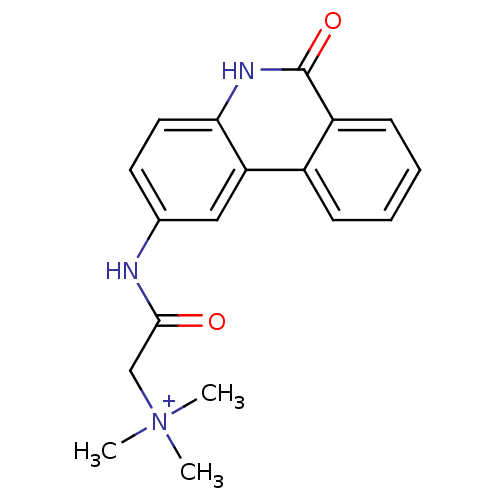 (CHEMBL289198 | Trimethyl-[(6-oxo-5,6-dihydro-phena...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human poly (ADP-ribose) polymerase-1 (PARP1) | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101104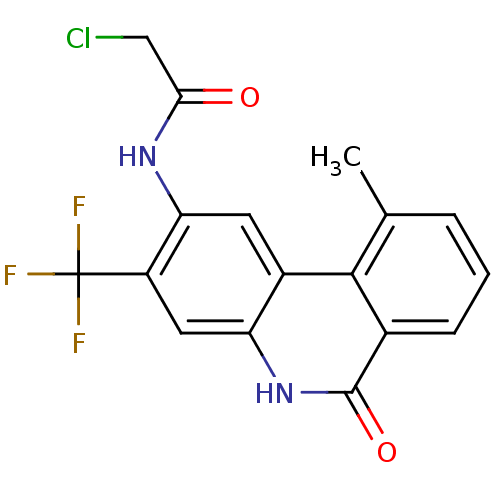 (2-Chloro-N-(10-methyl-6-oxo-3-trifluoromethyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50332221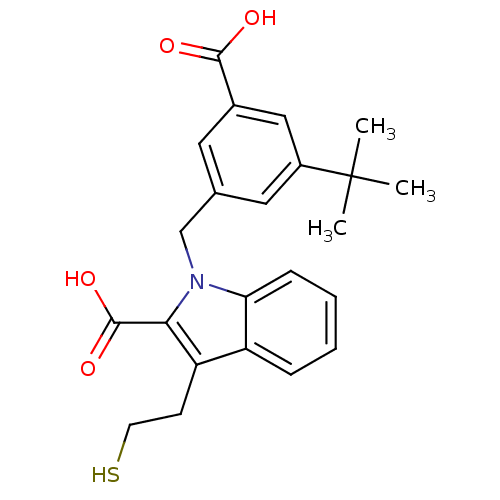 (1-[[3-carboxy-5-(1,1-dimethylethyl)phenyl]methyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101118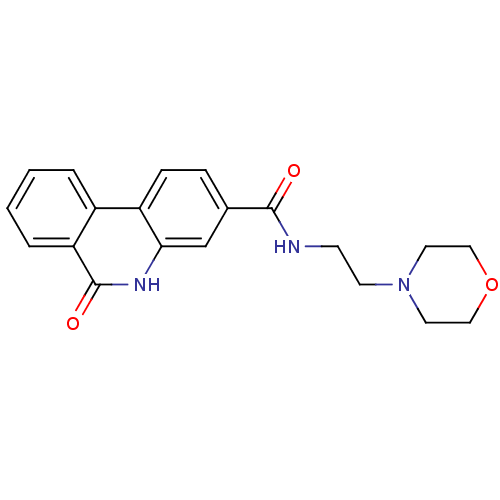 (6-Oxo-5,6-dihydro-phenanthridine-3-carboxylic acid...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101105 (6-Oxo-5,6-dihydro-phenanthridine-2-sulfonic acid (...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50101103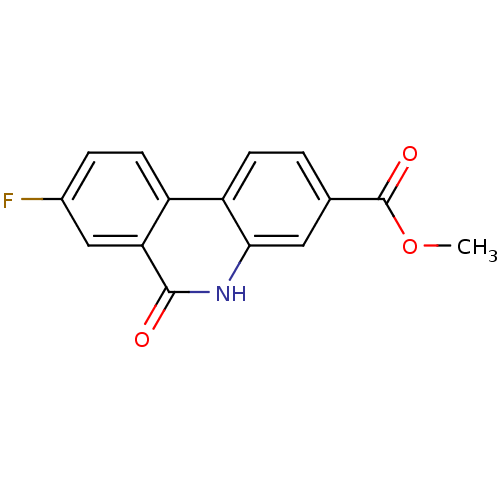 (8-Fluoro-6-oxo-5,6-dihydro-phenanthridine-3-carbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant human Poly (ADP-ribose) polymerase 1 | Bioorg Med Chem Lett 11: 1687-90 (2001) BindingDB Entry DOI: 10.7270/Q2348KWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 369 total ) | Next | Last >> |