 Found 14507 hits with Last Name = 'hu' and Initial = 'e'
Found 14507 hits with Last Name = 'hu' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50511450
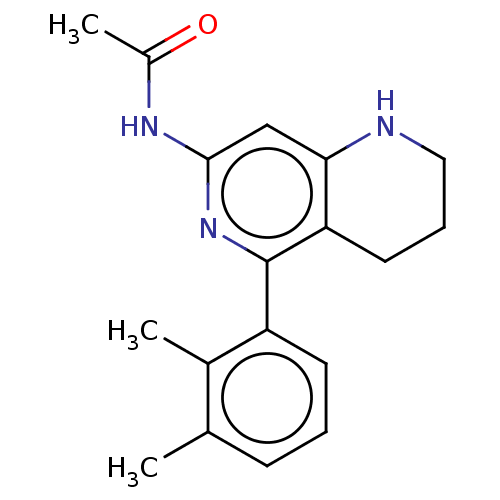
(CHEMBL4436749)Show InChI InChI=1S/C18H21N3O/c1-11-6-4-7-14(12(11)2)18-15-8-5-9-19-16(15)10-17(21-18)20-13(3)22/h4,6-7,10,19H,5,8-9H2,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... |
ACS Med Chem Lett 11: 358-364 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00420
BindingDB Entry DOI: 10.7270/Q2PV6PPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50511450

(CHEMBL4436749)Show InChI InChI=1S/C18H21N3O/c1-11-6-4-7-14(12(11)2)18-15-8-5-9-19-16(15)10-17(21-18)20-13(3)22/h4,6-7,10,19H,5,8-9H2,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... |
ACS Med Chem Lett 11: 358-364 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00420
BindingDB Entry DOI: 10.7270/Q2PV6PPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50229232
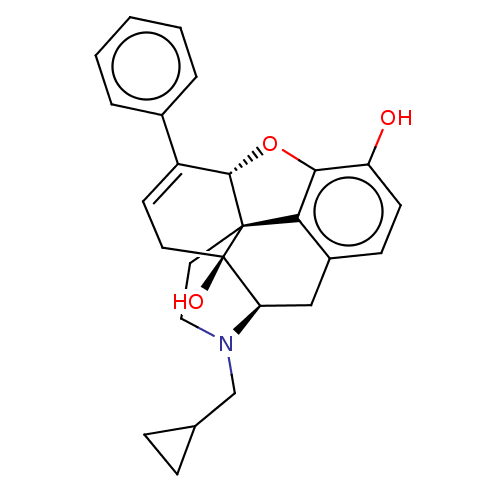
(CHEMBL610527)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC=C2c1ccccc1)ccc3O |r,c:24,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27NO3/c28-20-9-8-18-14-21-26(29)11-10-19(17-4-2-1-3-5-17)24-25(26,22(18)23(20)30-24)12-13-27(21)15-16-6-7-16/h1-5,8-10,16,21,24,28-29H,6-7,11-15H2/t21-,24+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Opioid receptor mu using [3H]DAMGO as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50229232

(CHEMBL610527)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC=C2c1ccccc1)ccc3O |r,c:24,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27NO3/c28-20-9-8-18-14-21-26(29)11-10-19(17-4-2-1-3-5-17)24-25(26,22(18)23(20)30-24)12-13-27(21)15-16-6-7-16/h1-5,8-10,16,21,24,28-29H,6-7,11-15H2/t21-,24+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to opioid receptor kappa using [3H]-EK as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM82552
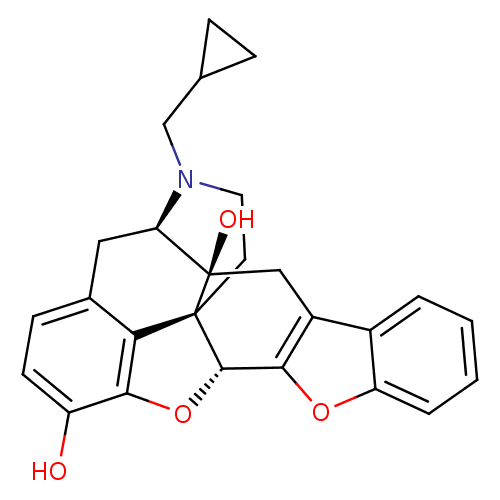
(CAS_111555-58-9 | NTB | naltrindolebenzofuran)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1oc2ccccc2c1C[C@@]35O |r| Show InChI InChI=1S/C26H25NO4/c28-18-8-7-15-11-20-26(29)12-17-16-3-1-2-4-19(16)30-22(17)24-25(26,21(15)23(18)31-24)9-10-27(20)13-14-5-6-14/h1-4,7-8,14,20,24,28-29H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to opioid receptor delta using [3H]DADLE as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50451343
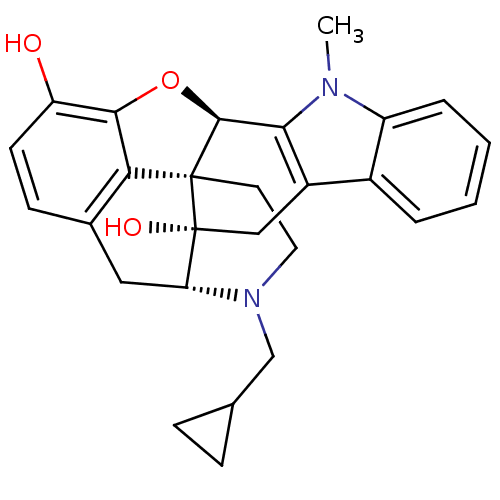
(CHEMBL477154)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)Cc1c2n(C)c2ccccc12)ccc3O |THB:10:9:17:5.6.4| Show InChI InChI=1S/C27H28N2O3/c1-28-19-5-3-2-4-17(19)18-13-27(31)21-12-16-8-9-20(30)24-22(16)26(27,25(32-24)23(18)28)10-11-29(21)14-15-6-7-15/h2-5,8-9,15,21,25,30-31H,6-7,10-14H2,1H3/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to opioid receptor delta using [3H]DADLE as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50370067
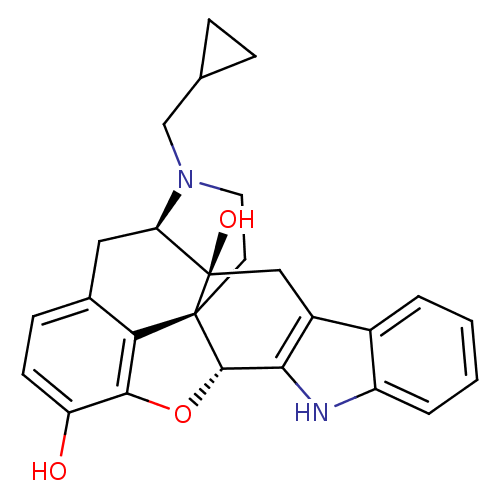
(CHEMBL1237164)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2ccccc2c1C[C@@]35O |r,TLB:28:29:7.12.13:4.5.18,30:29:7.12.13:4.5.18| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to opioid receptor delta using [3H]DADLE as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514220
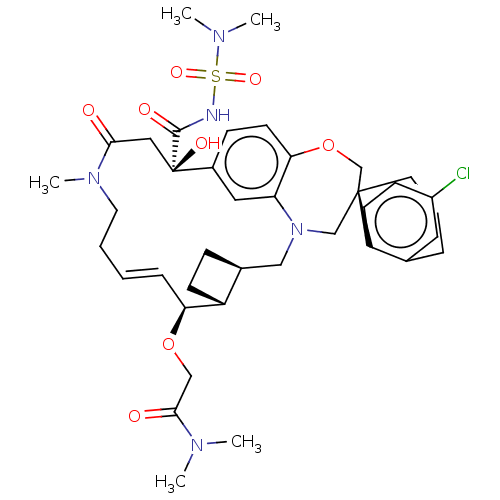
(CHEMBL4535151 | US11274105, Example 188)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCC(=O)N(C)C)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:15| Show InChI InChI=1S/C39H52ClN5O8S/c1-42(2)36(47)23-52-33-10-6-7-18-44(5)35(46)21-39(49,37(48)41-54(50,51)43(3)4)28-12-16-34-32(20-28)45(22-27-11-14-30(27)33)24-38(25-53-34)17-8-9-26-19-29(40)13-15-31(26)38/h6,10,12-13,15-16,19-20,27,30,33,49H,7-9,11,14,17-18,21-25H2,1-5H3,(H,41,48)/b10-6+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514222
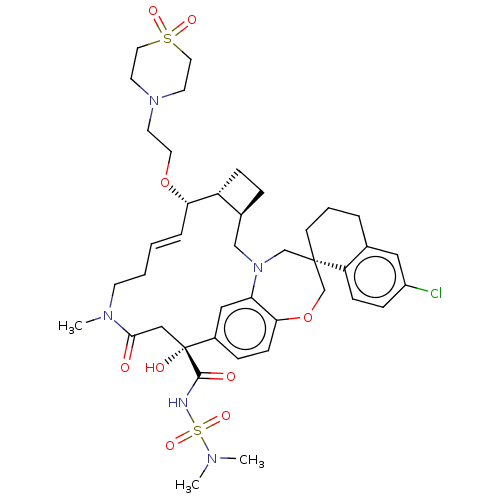
(CHEMBL4580244 | US11274105, Example 193)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCS(=O)(=O)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C41H56ClN5O9S2/c1-44(2)58(53,54)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(55-20-17-46-18-21-57(51,52)22-19-46)33-12-9-30(33)26-47-27-40(28-56-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514203
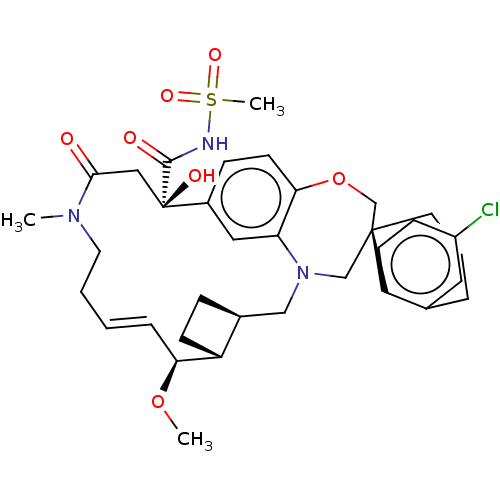
(CHEMBL4593361 | US11274105, Example 6)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(C)(=O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C35H44ClN3O7S/c1-38-16-5-4-8-30(45-2)27-12-9-24(27)20-39-21-34(15-6-7-23-17-26(36)11-13-28(23)34)22-46-31-14-10-25(18-29(31)39)35(42,19-32(38)40)33(41)37-47(3,43)44/h4,8,10-11,13-14,17-18,24,27,30,42H,5-7,9,12,15-16,19-22H2,1-3H3,(H,37,41)/b8-4+/t24-,27+,30-,34-,35+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514196
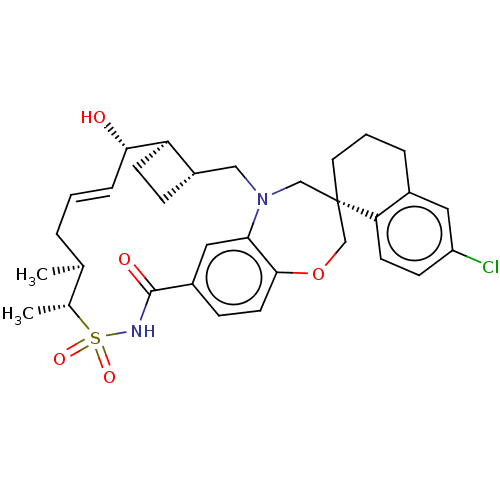
(CHEMBL4476472)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C32H39ClN2O5S/c1-20-5-3-7-29(36)26-11-8-24(26)17-35-18-32(14-4-6-22-15-25(33)10-12-27(22)32)19-40-30-13-9-23(16-28(30)35)31(37)34-41(38,39)21(20)2/h3,7,9-10,12-13,15-16,20-21,24,26,29,36H,4-6,8,11,14,17-19H2,1-2H3,(H,34,37)/b7-3+/t20-,21+,24-,26+,29-,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50229231
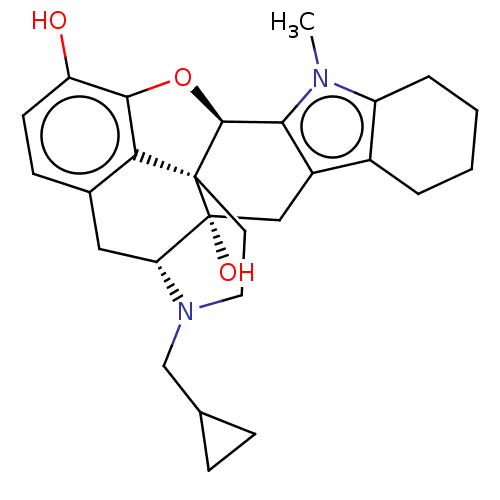
(CHEMBL610534)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)Cc1c4CCCCc4n(C)c21)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H32N2O3/c1-28-19-5-3-2-4-17(19)18-13-27(31)21-12-16-8-9-20(30)24-22(16)26(27,25(32-24)23(18)28)10-11-29(21)14-15-6-7-15/h8-9,15,21,25,30-31H,2-7,10-14H2,1H3/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to opioid receptor delta using [3H]DADLE as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931
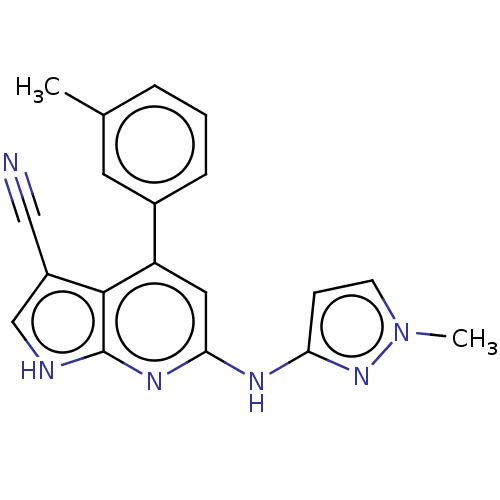
(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514202

(CHEMBL4446369 | US11274105, Example 179)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C40H52ClF2N5O7S/c1-45(2)56(52,53)44-37(50)40(51)21-36(49)46(3)16-5-4-8-34(54-18-17-47-24-39(42,43)25-47)31-12-9-28(31)22-48-23-38(26-55-35-14-10-29(40)20-33(35)48)15-6-7-27-19-30(41)11-13-32(27)38/h4,8,10-11,13-14,19-20,28,31,34,51H,5-7,9,12,15-18,21-26H2,1-3H3,(H,44,50)/b8-4+/t28-,31+,34-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514199
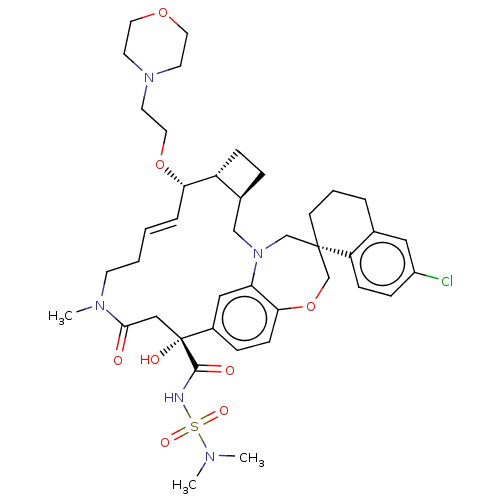
(CHEMBL4553660 | US11274105, Example 182)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCOCC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(54-22-19-46-17-20-53-21-18-46)33-12-9-30(33)26-47-27-40(28-55-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514200
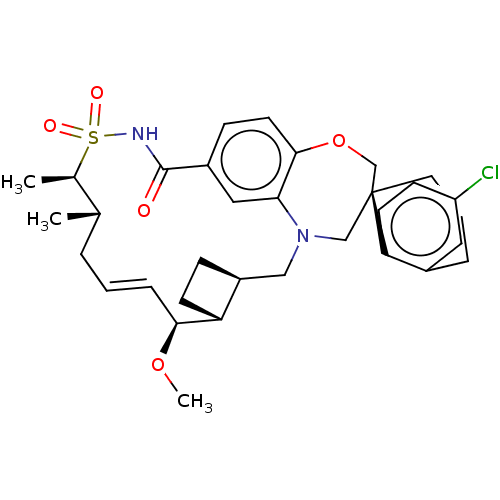
(CHEMBL4446378 | US10703733, Comparative Example 1)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C33H41ClN2O5S/c1-21-6-4-8-30(40-3)27-12-9-25(27)18-36-19-33(15-5-7-23-16-26(34)11-13-28(23)33)20-41-31-14-10-24(17-29(31)36)32(37)35-42(38,39)22(21)2/h4,8,10-11,13-14,16-17,21-22,25,27,30H,5-7,9,12,15,18-20H2,1-3H3,(H,35,37)/b8-4+/t21-,22+,25-,27+,30-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514215
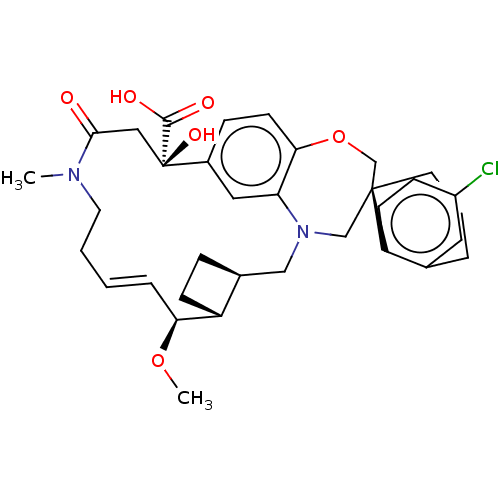
(CHEMBL4577379 | US11274105, Example 4)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(42-2)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(41,32(39)40)18-31(36)38/h3,7,9-10,12-13,16-17,23,26,29,41H,4-6,8,11,14-15,18-21H2,1-2H3,(H,39,40)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM82071
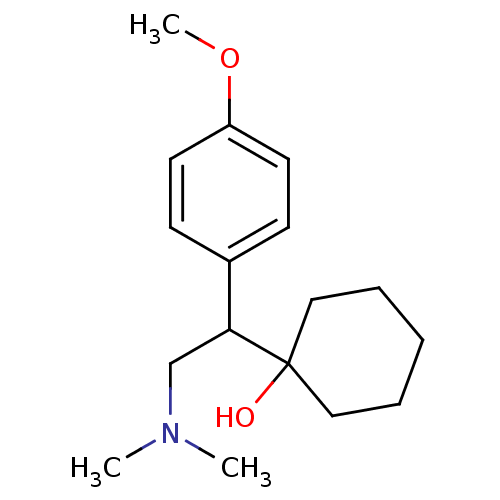
(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514206
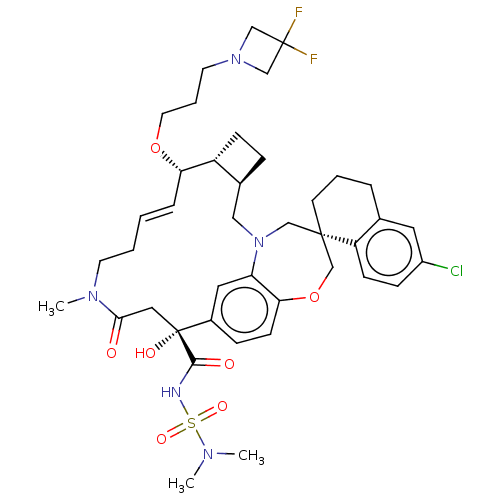
(CHEMBL4588330 | US11274105, Example 187)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:19| Show InChI InChI=1S/C41H54ClF2N5O7S/c1-46(2)57(53,54)45-38(51)41(52)22-37(50)47(3)17-5-4-9-35(55-19-7-18-48-25-40(43,44)26-48)32-13-10-29(32)23-49-24-39(27-56-36-15-11-30(41)21-34(36)49)16-6-8-28-20-31(42)12-14-33(28)39/h4,9,11-12,14-15,20-21,29,32,35,52H,5-8,10,13,16-19,22-27H2,1-3H3,(H,45,51)/b9-4+/t29-,32+,35-,39-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514218
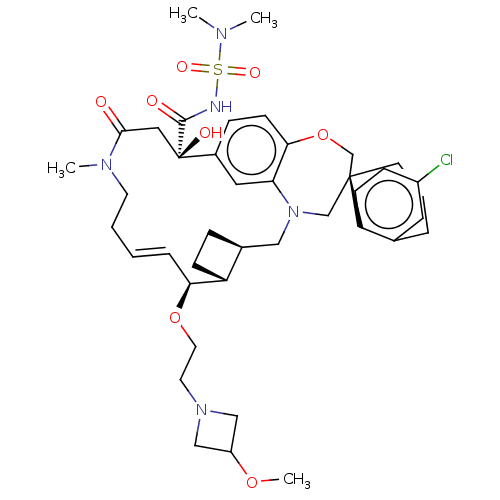
(CHEMBL4539543 | US11274105, Example 197)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(C1)OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)22-38(48)45(3)17-6-5-9-36(54-19-18-46-24-32(25-46)53-4)33-13-10-29(33)23-47-26-40(27-55-37-15-11-30(41)21-35(37)47)16-7-8-28-20-31(42)12-14-34(28)40/h5,9,11-12,14-15,20-21,29,32-33,36,50H,6-8,10,13,16-19,22-27H2,1-4H3,(H,43,49)/b9-5+/t29-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514219

(CHEMBL4438074 | US11274105, Example 181)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:17| Show InChI InChI=1S/C40H53ClFN5O7S/c1-44(2)55(51,52)43-38(49)40(50)21-37(48)45(3)16-5-4-8-35(53-18-17-46-23-31(42)24-46)32-12-9-28(32)22-47-25-39(26-54-36-14-10-29(40)20-34(36)47)15-6-7-27-19-30(41)11-13-33(27)39/h4,8,10-11,13-14,19-20,28,31-32,35,50H,5-7,9,12,15-18,21-26H2,1-3H3,(H,43,49)/b8-4+/t28-,32+,35-,39-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50381654
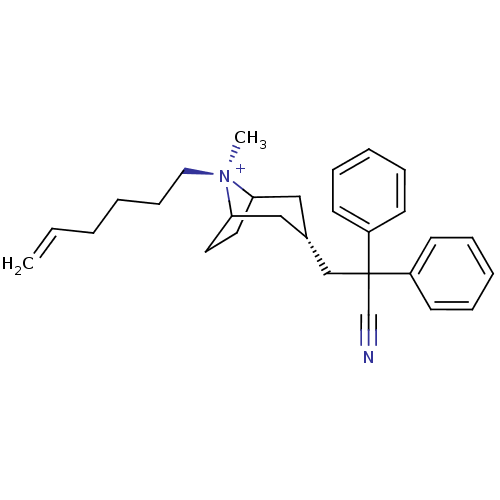
(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M2 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514214

(CHEMBL4542646 | US11274105, Example 41)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C33H39ClN2O6/c1-35-14-3-2-6-28(37)25-10-7-22(25)18-36-19-32(13-4-5-21-15-24(34)9-11-26(21)32)20-42-29-12-8-23(16-27(29)36)33(41,31(39)40)17-30(35)38/h2,6,8-9,11-12,15-16,22,25,28,37,41H,3-5,7,10,13-14,17-20H2,1H3,(H,39,40)/b6-2+/t22-,25+,28-,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514201

(CHEMBL4547370 | US11274105, Example 191)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCOCC(F)F)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:16| Show InChI InChI=1S/C39H51ClF2N4O8S/c1-44(2)55(50,51)43-37(48)39(49)21-36(47)45(3)16-5-4-8-33(53-18-17-52-23-35(41)42)30-12-9-27(30)22-46-24-38(25-54-34-14-10-28(39)20-32(34)46)15-6-7-26-19-29(40)11-13-31(26)38/h4,8,10-11,13-14,19-20,27,30,33,35,49H,5-7,9,12,15-18,21-25H2,1-3H3,(H,43,48)/b8-4+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931

(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514216
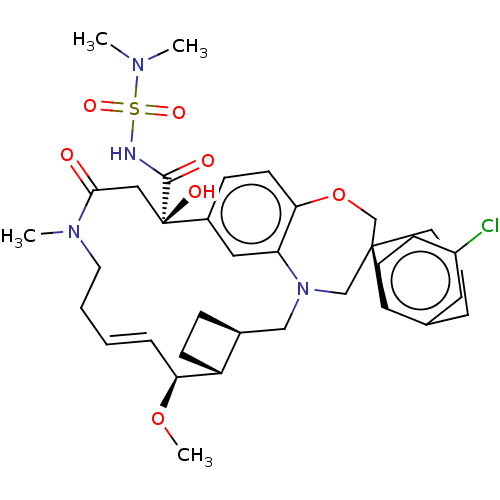
(CHEMBL4528051 | US11274105, Example 5)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C36H47ClN4O7S/c1-39(2)49(45,46)38-34(43)36(44)20-33(42)40(3)17-6-5-9-31(47-4)28-13-10-25(28)21-41-22-35(23-48-32-15-11-26(36)19-30(32)41)16-7-8-24-18-27(37)12-14-29(24)35/h5,9,11-12,14-15,18-19,25,28,31,44H,6-8,10,13,16-17,20-23H2,1-4H3,(H,38,43)/b9-5+/t25-,28+,31-,35-,36+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514204
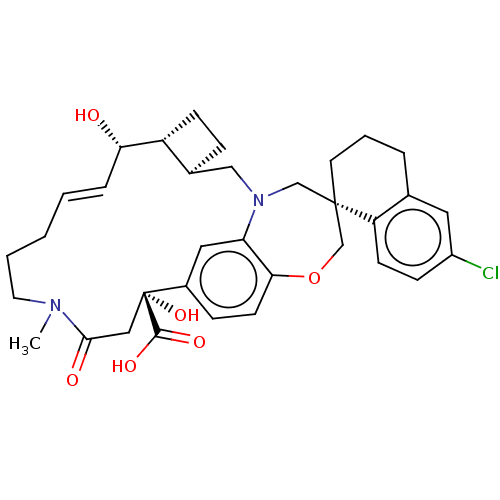
(CHEMBL4437832)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-2-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38,42H,2,4-6,8,11,14-15,18-21H2,1H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50381654

(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M1 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229
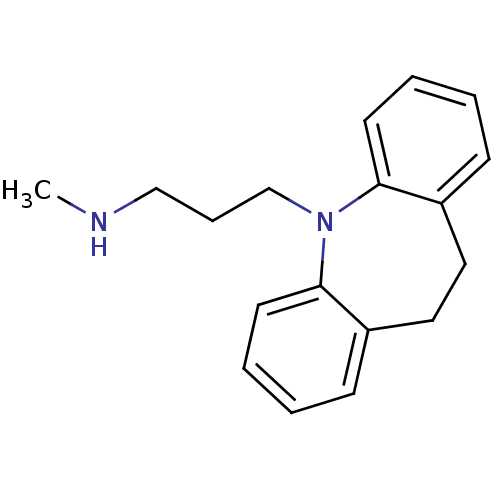
(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398598
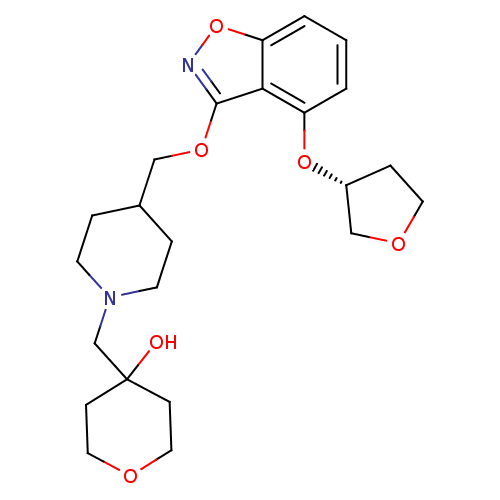
(CHEMBL2152922)Show SMILES OC1(CN2CCC(COc3noc4cccc(O[C@@H]5CCOC5)c34)CC2)CCOCC1 |r| Show InChI InChI=1S/C23H32N2O6/c26-23(7-12-27-13-8-23)16-25-9-4-17(5-10-25)14-29-22-21-19(30-18-6-11-28-15-18)2-1-3-20(21)31-24-22/h1-3,17-18,26H,4-16H2/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50381654

(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M3 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50382491
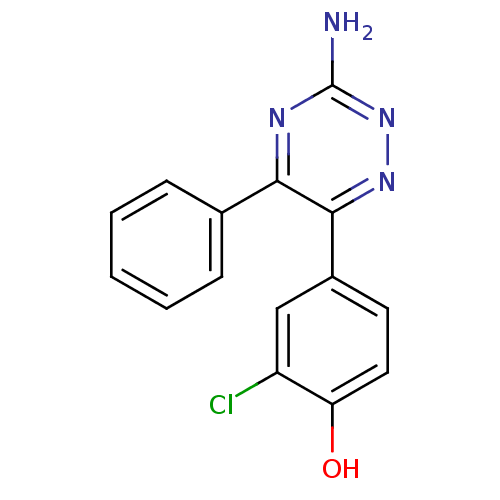
(CHEMBL2024114 | US10988455, Example 1(xlii))Show InChI InChI=1S/C15H11ClN4O/c16-11-8-10(6-7-12(11)21)14-13(18-15(17)20-19-14)9-4-2-1-3-5-9/h1-8,21H,(H2,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells after 1 hr by liquid scintillation counting |
J Med Chem 55: 1898-903 (2012)
Article DOI: 10.1021/jm201376w
BindingDB Entry DOI: 10.7270/Q2S46SZG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31592
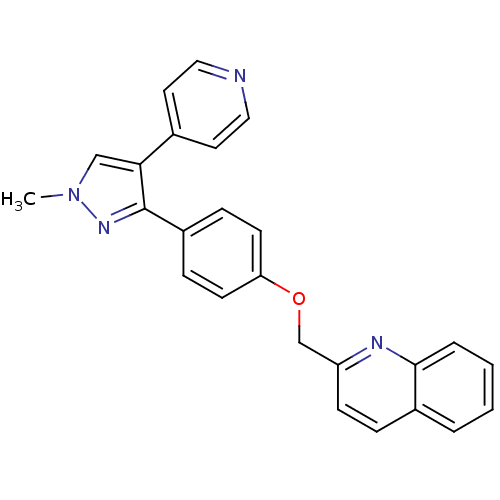
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum |
J Med Chem 55: 4776-87 (2012)
Article DOI: 10.1021/jm3002372
BindingDB Entry DOI: 10.7270/Q2WM1FFG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM82071

(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514217

(CHEMBL4452794 | US11274105, Example 196)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCC(F)(F)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C42H56ClF2N5O7S/c1-47(2)58(54,55)46-39(52)42(53)25-38(51)48(3)18-5-4-8-36(56-22-21-49-19-16-41(44,45)17-20-49)33-12-9-30(33)26-50-27-40(28-57-37-14-10-31(42)24-35(37)50)15-6-7-29-23-32(43)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,53H,5-7,9,12,15-22,25-28H2,1-3H3,(H,46,52)/b8-4+/t30-,33+,36-,40-,42+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50241132
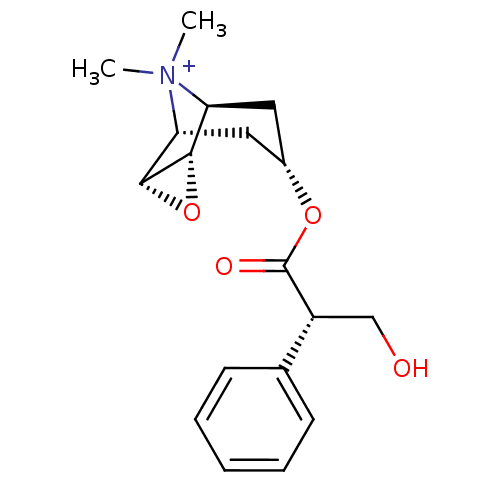
(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute for Medical Research
Curated by PDSP Ki Database
| |
Annu Rev Pharmacol Toxicol 30: 633-73 (1990)
Article DOI: 10.1146/annurev.pa.30.040190.003221
BindingDB Entry DOI: 10.7270/Q21Z42WK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM82071

(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50336931
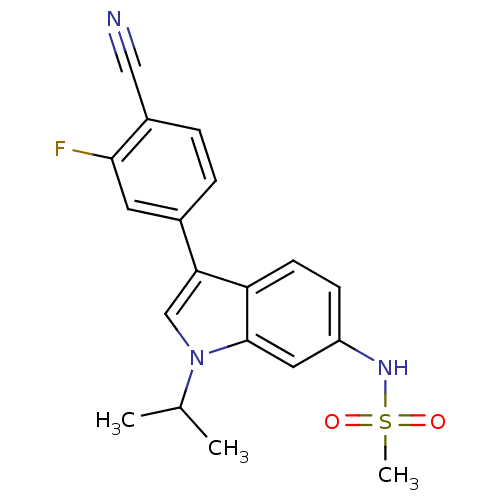
(CHEMBL1672547 | N-(3-(4-cyano-3-fluorophenyl)-1-is...)Show SMILES CC(C)n1cc(-c2ccc(C#N)c(F)c2)c2ccc(NS(C)(=O)=O)cc12 Show InChI InChI=1S/C19H18FN3O2S/c1-12(2)23-11-17(13-4-5-14(10-21)18(20)8-13)16-7-6-15(9-19(16)23)22-26(3,24)25/h4-9,11-12,22H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from human progesterone receptor expressed in HEK293 cells |
ACS Med Chem Lett 2: 148-153 (2011)
Article DOI: 10.1021/ml100220b
BindingDB Entry DOI: 10.7270/Q2Z038G1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute for Medical Research
Curated by PDSP Ki Database
| |
Annu Rev Pharmacol Toxicol 30: 633-73 (1990)
Article DOI: 10.1146/annurev.pa.30.040190.003221
BindingDB Entry DOI: 10.7270/Q21Z42WK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398597
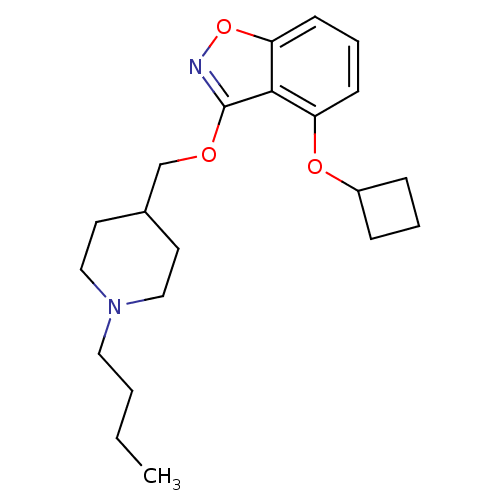
(CHEMBL2179584)Show InChI InChI=1S/C21H30N2O3/c1-2-3-12-23-13-10-16(11-14-23)15-24-21-20-18(25-17-6-4-7-17)8-5-9-19(20)26-22-21/h5,8-9,16-17H,2-4,6-7,10-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514208
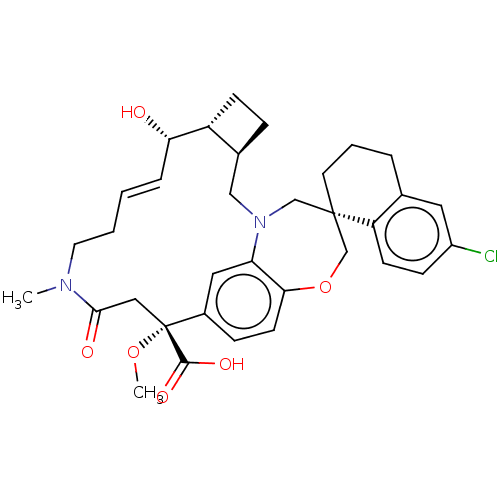
(CHEMBL4469850 | US11274105, Example 61)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](OC)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42-2,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38H,4-6,8,11,14-15,18-21H2,1-2H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398593
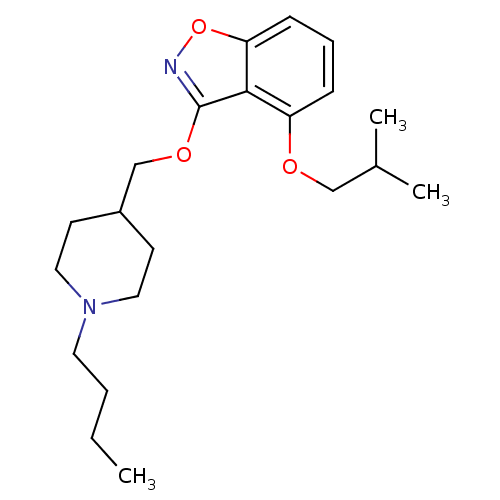
(CHEMBL2179587)Show InChI InChI=1S/C21H32N2O3/c1-4-5-11-23-12-9-17(10-13-23)15-25-21-20-18(24-14-16(2)3)7-6-8-19(20)26-22-21/h6-8,16-17H,4-5,9-15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50336933

(CHEMBL1672541 | N-(3-(4-cyano-3-methylphenyl)-1-is...)Show SMILES CC(C)n1cc(-c2ccc(C#N)c(C)c2)c2ccc(NS(C)(=O)=O)cc12 Show InChI InChI=1S/C20H21N3O2S/c1-13(2)23-12-19(15-5-6-16(11-21)14(3)9-15)18-8-7-17(10-20(18)23)22-26(4,24)25/h5-10,12-13,22H,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from human progesterone receptor expressed in HEK293 cells |
ACS Med Chem Lett 2: 148-153 (2011)
Article DOI: 10.1021/ml100220b
BindingDB Entry DOI: 10.7270/Q2Z038G1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50398598

(CHEMBL2152922)Show SMILES OC1(CN2CCC(COc3noc4cccc(O[C@@H]5CCOC5)c34)CC2)CCOCC1 |r| Show InChI InChI=1S/C23H32N2O6/c26-23(7-12-27-13-8-23)16-25-9-4-17(5-10-25)14-29-22-21-19(30-18-6-11-28-15-18)2-1-3-20(21)31-24-22/h1-3,17-18,26H,4-16H2/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228078
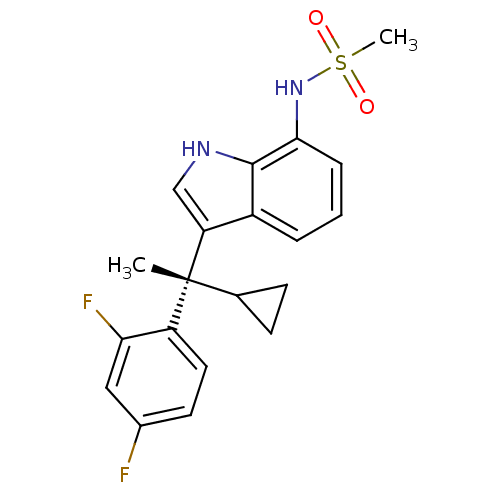
((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.319 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from human mineralocorticoid receptor expressed in HEK293 cells |
ACS Med Chem Lett 2: 148-153 (2011)
Article DOI: 10.1021/ml100220b
BindingDB Entry DOI: 10.7270/Q2Z038G1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398598

(CHEMBL2152922)Show SMILES OC1(CN2CCC(COc3noc4cccc(O[C@@H]5CCOC5)c34)CC2)CCOCC1 |r| Show InChI InChI=1S/C23H32N2O6/c26-23(7-12-27-13-8-23)16-25-9-4-17(5-10-25)14-29-22-21-19(30-18-6-11-28-15-18)2-1-3-20(21)31-24-22/h1-3,17-18,26H,4-16H2/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50336930
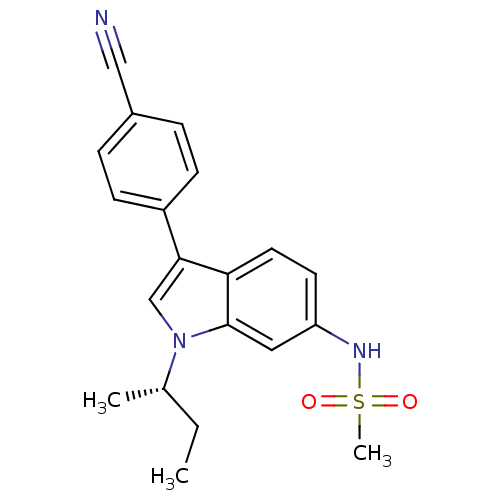
((S)-N-(1-sec-butyl-3-(4-cyanophenyl)-1H-indol-6-yl...)Show SMILES CC[C@H](C)n1cc(-c2ccc(cc2)C#N)c2ccc(NS(C)(=O)=O)cc12 |r| Show InChI InChI=1S/C20H21N3O2S/c1-4-14(2)23-13-19(16-7-5-15(12-21)6-8-16)18-10-9-17(11-20(18)23)22-26(3,24)25/h5-11,13-14,22H,4H2,1-3H3/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from human progesterone receptor expressed in HEK293 cells |
ACS Med Chem Lett 2: 148-153 (2011)
Article DOI: 10.1021/ml100220b
BindingDB Entry DOI: 10.7270/Q2Z038G1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute for Medical Research
Curated by PDSP Ki Database
| |
Annu Rev Pharmacol Toxicol 30: 633-73 (1990)
Article DOI: 10.1146/annurev.pa.30.040190.003221
BindingDB Entry DOI: 10.7270/Q21Z42WK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398596

(CHEMBL2179589)Show SMILES CC(C)COc1cccc2onc(OCC3CCN(CC4(O)CCOCC4)CC3)c12 Show InChI InChI=1S/C23H34N2O5/c1-17(2)14-28-19-4-3-5-20-21(19)22(24-30-20)29-15-18-6-10-25(11-7-18)16-23(26)8-12-27-13-9-23/h3-5,17-18,26H,6-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398598

(CHEMBL2152922)Show SMILES OC1(CN2CCC(COc3noc4cccc(O[C@@H]5CCOC5)c34)CC2)CCOCC1 |r| Show InChI InChI=1S/C23H32N2O6/c26-23(7-12-27-13-8-23)16-25-9-4-17(5-10-25)14-29-22-21-19(30-18-6-11-28-15-18)2-1-3-20(21)31-24-22/h1-3,17-18,26H,4-16H2/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data