 Found 113 hits with Last Name = 'lee' and Initial = 'ej'
Found 113 hits with Last Name = 'lee' and Initial = 'ej' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50069055

((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...)Show SMILES CCCc1ccc(cc1)S(=O)(=O)N[C@@H](Cc1ccc(CN)cc1)C(=O)N(C)C1CCCC1 Show InChI InChI=1S/C25H35N3O3S/c1-3-6-19-13-15-23(16-14-19)32(30,31)27-24(17-20-9-11-21(18-26)12-10-20)25(29)28(2)22-7-4-5-8-22/h9-16,22,24,27H,3-8,17-18,26H2,1-2H3/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069053
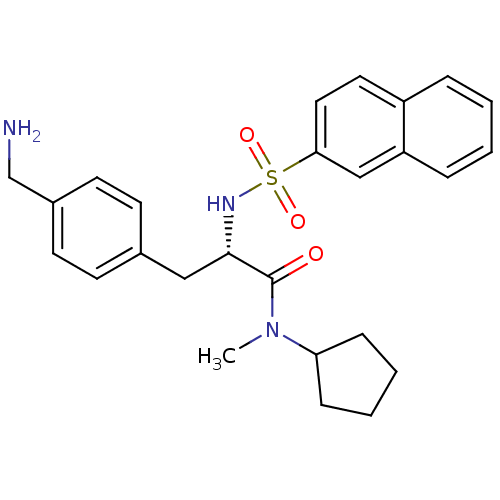
((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(CN)cc1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H31N3O3S/c1-29(23-8-4-5-9-23)26(30)25(16-19-10-12-20(18-27)13-11-19)28-33(31,32)24-15-14-21-6-2-3-7-22(21)17-24/h2-3,6-7,10-15,17,23,25,28H,4-5,8-9,16,18,27H2,1H3/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069052

(CHEMBL164661 | Naphthalene-2-sulfonic acid [(S)-1-...)Show SMILES NCc1ccc(C[C@H](NS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCC2)cc1 Show InChI InChI=1S/C24H27N3O3S/c25-17-19-9-7-18(8-10-19)15-23(24(28)27-13-3-4-14-27)26-31(29,30)22-12-11-20-5-1-2-6-21(20)16-22/h1-2,5-12,16,23,26H,3-4,13-15,17,25H2/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069051

((S)-3-(4-Aminomethyl-phenyl)-N-isopropyl-N-methyl-...)Show SMILES CC(C)N(C)C(=O)[C@H](Cc1ccc(CN)cc1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C24H29N3O3S/c1-17(2)27(3)24(28)23(14-18-8-10-19(16-25)11-9-18)26-31(29,30)22-13-12-20-6-4-5-7-21(20)15-22/h4-13,15,17,23,26H,14,16,25H2,1-3H3/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069057

((S)-3-(4-Aminomethyl-phenyl)-N,N-dimethyl-2-(napht...)Show SMILES CN(C)C(=O)[C@H](Cc1ccc(CN)cc1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H25N3O3S/c1-25(2)22(26)21(13-16-7-9-17(15-23)10-8-16)24-29(27,28)20-12-11-18-5-3-4-6-19(18)14-20/h3-12,14,21,24H,13,15,23H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069056
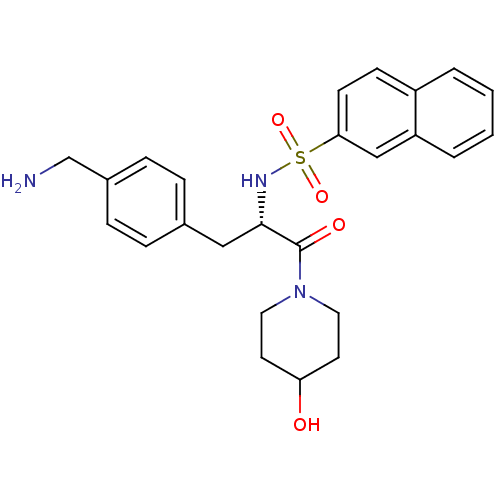
(CHEMBL350751 | Naphthalene-2-sulfonic acid [(S)-1-...)Show SMILES NCc1ccc(C[C@H](NS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCC(O)CC2)cc1 Show InChI InChI=1S/C25H29N3O4S/c26-17-19-7-5-18(6-8-19)15-24(25(30)28-13-11-22(29)12-14-28)27-33(31,32)23-10-9-20-3-1-2-4-21(20)16-23/h1-10,16,22,24,27,29H,11-15,17,26H2/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069054

((S)-3-(4-Aminomethyl-phenyl)-N-isopropyl-2-(naphth...)Show SMILES CC(C)NC(=O)[C@H](Cc1ccc(CN)cc1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H27N3O3S/c1-16(2)25-23(27)22(13-17-7-9-18(15-24)10-8-17)26-30(28,29)21-12-11-19-5-3-4-6-20(19)14-21/h3-12,14,16,22,26H,13,15,24H2,1-2H3,(H,25,27)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069050
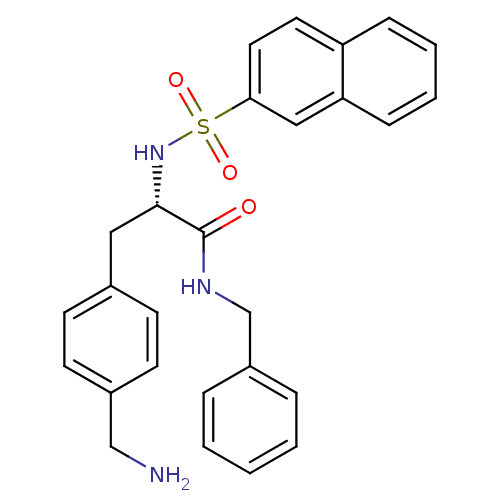
((S)-3-(4-Aminomethyl-phenyl)-N-benzyl-2-(naphthale...)Show SMILES NCc1ccc(C[C@H](NS(=O)(=O)c2ccc3ccccc3c2)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C27H27N3O3S/c28-18-21-12-10-20(11-13-21)16-26(27(31)29-19-22-6-2-1-3-7-22)30-34(32,33)25-15-14-23-8-4-5-9-24(23)17-25/h1-15,17,26,30H,16,18-19,28H2,(H,29,31)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin |
Bioorg Med Chem Lett 8: 735-8 (1999)
BindingDB Entry DOI: 10.7270/Q2668C95 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317169
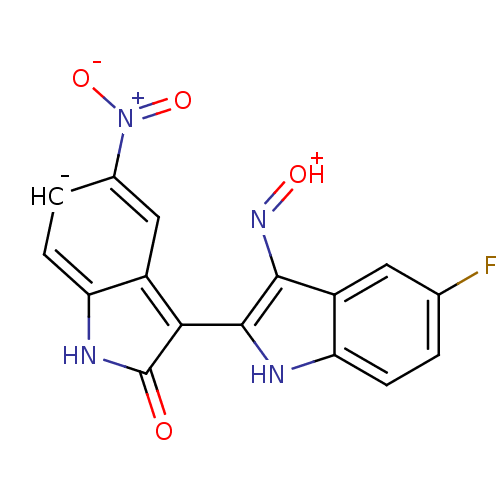
((2'Z,3'E)-5-Nitro-5'-fluoro-indirubin-3'-oxime | C...)Show SMILES [O-][N+](=O)c1ccc2[nH]c(=O)[c-](-c3[nH]c4ccc(F)cc4c3N=[OH+])c2c1 |(19.84,4.61,;18.7,3.57,;17.23,4.04,;19.03,2.07,;20.5,1.6,;20.83,.09,;19.69,-.94,;19.69,-2.48,;18.22,-2.96,;17.74,-4.42,;17.32,-1.71,;15.78,-1.71,;14.87,-2.97,;13.39,-2.49,;12.06,-3.26,;10.72,-2.49,;10.73,-.94,;9.39,-.17,;12.05,-.17,;13.39,-.93,;14.87,-.45,;15.34,1.01,;16.85,1.33,;18.22,-.46,;17.9,1.03,)| Show InChI InChI=1S/C16H8FN4O4/c17-7-1-3-12-10(5-7)14(20-23)15(18-12)13-9-6-8(21(24)25)2-4-11(9)19-16(13)22/h1-6,18H,(H,19,22)/q-1/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317171
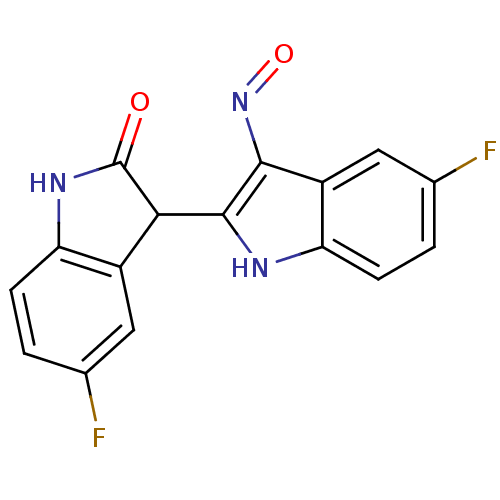
((2'Z,3'E)-5-Fluoro-5'-fluoro-indirubin-3'-oxime | ...)Show InChI InChI=1S/C16H9F2N3O2/c17-7-1-3-11-9(5-7)13(16(22)20-11)15-14(21-23)10-6-8(18)2-4-12(10)19-15/h1-6,13,19H,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317161

((2'Z,3'E)-5-Nitro-5'-hydroxy-indirubin-3'-oxime | ...)Show SMILES Oc1ccc2[nH]c(c(N=[OH+])c2c1)-[c-]1c2cc(ccc2[nH]c1=O)[N+]([O-])=O |(-8.68,.59,;-7.35,-.18,;-7.35,-1.72,;-6.01,-2.49,;-4.68,-1.72,;-3.21,-2.2,;-2.29,-.95,;-3.21,.31,;-2.73,1.77,;-1.22,2.09,;-4.68,-.17,;-6.02,.59,;-.75,-.95,;.15,.3,;-.18,1.8,;.96,2.83,;2.43,2.36,;2.75,.85,;1.61,-.18,;1.61,-1.72,;.15,-2.19,;-.33,-3.66,;.63,4.33,;1.77,5.37,;-.84,4.8,)| Show InChI InChI=1S/C16H9N4O5/c21-8-2-4-12-10(6-8)14(19-23)15(17-12)13-9-5-7(20(24)25)1-3-11(9)18-16(13)22/h1-6,17,21H,(H,18,22)/q-1/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317163

((2'Z,3'E)-5-Fluoro-5'-hydroxy-indirubin-3'-oxime |...)Show SMILES Oc1ccc2[nH]c(C3C(=O)Nc4ccc(F)cc34)c(N=O)c2c1 Show InChI InChI=1S/C16H10FN3O3/c17-7-1-3-11-9(5-7)13(16(22)19-11)15-14(20-23)10-6-8(21)2-4-12(10)18-15/h1-6,13,18,21H,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317162

((2'Z,3'E)-5-Chloro-5'-hydroxy-indirubin-3'-oxime |...)Show SMILES Oc1ccc2[nH]c(C3C(=O)Nc4ccc(Cl)cc34)c(N=O)c2c1 Show InChI InChI=1S/C16H10ClN3O3/c17-7-1-3-11-9(5-7)13(16(22)19-11)15-14(20-23)10-6-8(21)2-4-12(10)18-15/h1-6,13,18,21H,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624845
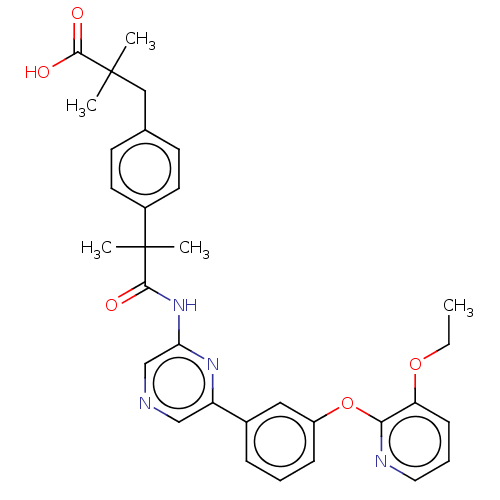
(US20230322706, Example 29)Show SMILES CCOc1cccnc1Oc1cccc(c1)-c1cncc(NC(=O)C(C)(C)c2ccc(CC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624843

(US20230322706, Example 27 | US20230322706, Example...)Show SMILES CCOc1cccnc1Oc1cccc(c1)-c1cncc(NC(=O)Cc2ccc(CC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317177

(5-nitroindirubin-3'-oxime | CHEMBL369303)Show SMILES Oc1[nH]c2ccc(cc2c1-c1[nH]c2ccccc2c1N=O)[N+]([O-])=O |(-.11,-4.55,;.8,-3.31,;2.35,-3.31,;2.82,-1.84,;4.22,-1.22,;4.38,.32,;3.13,1.23,;1.73,.6,;1.58,-.94,;.33,-1.84,;-1.21,-1.82,;-2.13,-3.07,;-3.6,-2.58,;-4.91,-3.35,;-6.24,-2.58,;-6.24,-1.05,;-4.91,-.26,;-3.58,-1.03,;-2.11,-.57,;-1.62,.9,;-2.65,2.04,;3.29,2.75,;4.69,3.38,;2.05,3.65,)| Show InChI InChI=1S/C16H9N4O4/c21-16-13(10-7-8(20(23)24)5-6-12(10)18-16)15-14(19-22)9-3-1-2-4-11(9)17-15/h1-7,17H,(H,18,21)/q-1/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317170
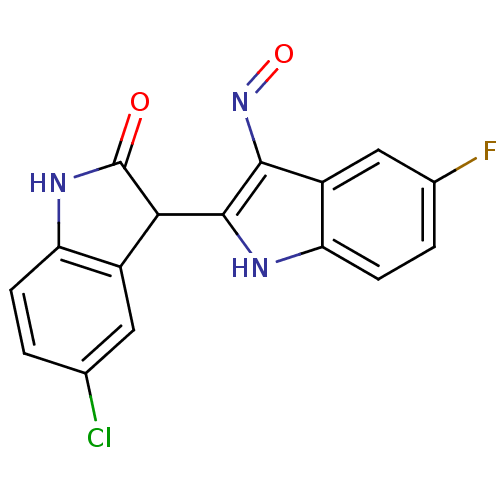
((2'Z,3'E)-5-Chloro-5'-fluoro-indirubin-3'-oxime | ...)Show SMILES Fc1ccc2[nH]c(C3C(=O)Nc4ccc(Cl)cc34)c(N=O)c2c1 Show InChI InChI=1S/C16H9ClFN3O2/c17-7-1-3-11-9(5-7)13(16(22)20-11)15-14(21-23)10-6-8(18)2-4-12(10)19-15/h1-6,13,19H,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317164
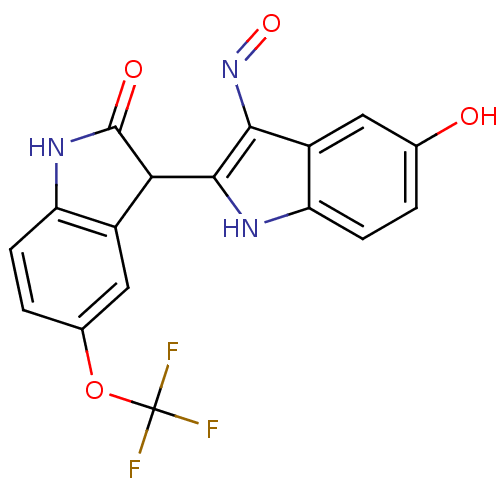
((2'Z,3'E)-5-Trifluoromethoxy-5'-hydroxy-indirubin-...)Show SMILES Oc1ccc2[nH]c(C3C(=O)Nc4ccc(OC(F)(F)F)cc34)c(N=O)c2c1 Show InChI InChI=1S/C17H10F3N3O4/c18-17(19,20)27-8-2-4-11-9(6-8)13(16(25)22-11)15-14(23-26)10-5-7(24)1-3-12(10)21-15/h1-6,13,21,24H,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317173

((2'Z,3'E)-5-Nitro-5'-methyl-indirubin-3'-oxime | C...)Show SMILES Cc1ccc2[nH]c(c(N=[OH+])c2c1)-[c-]1c2cc(ccc2[nH]c1=O)[N+]([O-])=O |(23.35,-22.07,;24.68,-22.84,;24.68,-24.38,;26.01,-25.15,;27.35,-24.38,;28.82,-24.86,;29.73,-23.61,;28.82,-22.35,;29.3,-20.89,;30.8,-20.57,;27.34,-22.83,;26.01,-22.07,;31.27,-23.61,;32.17,-22.36,;31.85,-20.86,;32.98,-19.83,;34.46,-20.3,;34.78,-21.81,;33.64,-22.84,;33.64,-24.38,;32.18,-24.85,;31.7,-26.32,;32.66,-18.33,;33.79,-17.29,;31.19,-17.86,)| Show InChI InChI=1S/C17H11N4O4/c1-8-2-4-13-11(6-8)15(20-23)16(18-13)14-10-7-9(21(24)25)3-5-12(10)19-17(14)22/h2-7,18H,1H3,(H,19,22)/q-1/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317167
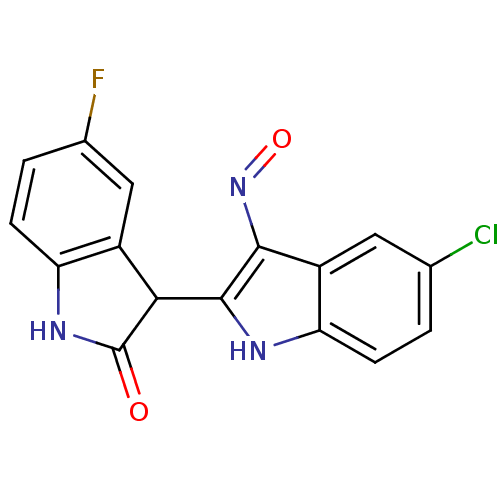
((2'Z,3'E)-5-Fluoro-5'-chloro-indirubin-3'-oxime | ...)Show SMILES Fc1ccc2NC(=O)C(c2c1)c1[nH]c2ccc(Cl)cc2c1N=O Show InChI InChI=1S/C16H9ClFN3O2/c17-7-1-3-12-10(5-7)14(21-23)15(19-12)13-9-6-8(18)2-4-11(9)20-16(13)22/h1-6,13,19H,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317166
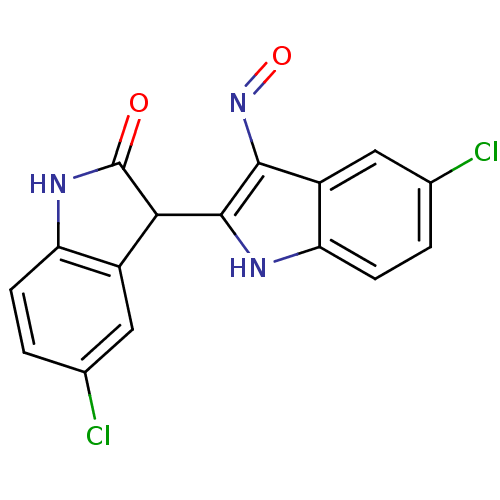
((2'Z,3'E)-5-Chloro-5'-chloro-indirubin-3'-oxime | ...)Show SMILES Clc1ccc2NC(=O)C(c2c1)c1[nH]c2ccc(Cl)cc2c1N=O Show InChI InChI=1S/C16H9Cl2N3O2/c17-7-1-3-11-9(5-7)13(16(22)20-11)15-14(21-23)10-6-8(18)2-4-12(10)19-15/h1-6,13,19H,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624819
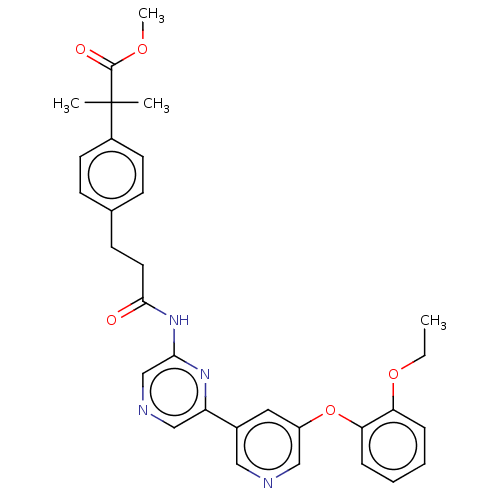
(US20230322706, Example 5)Show SMILES CCOc1ccccc1Oc1cncc(c1)-c1cncc(NC(=O)CCc2ccc(cc2)C(C)(C)C(=O)OC)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50317161

((2'Z,3'E)-5-Nitro-5'-hydroxy-indirubin-3'-oxime | ...)Show SMILES Oc1ccc2[nH]c(c(N=[OH+])c2c1)-[c-]1c2cc(ccc2[nH]c1=O)[N+]([O-])=O |(-8.68,.59,;-7.35,-.18,;-7.35,-1.72,;-6.01,-2.49,;-4.68,-1.72,;-3.21,-2.2,;-2.29,-.95,;-3.21,.31,;-2.73,1.77,;-1.22,2.09,;-4.68,-.17,;-6.02,.59,;-.75,-.95,;.15,.3,;-.18,1.8,;.96,2.83,;2.43,2.36,;2.75,.85,;1.61,-.18,;1.61,-1.72,;.15,-2.19,;-.33,-3.66,;.63,4.33,;1.77,5.37,;-.84,4.8,)| Show InChI InChI=1S/C16H9N4O5/c21-8-2-4-12-10(6-8)14(19-23)15(17-12)13-9-5-7(20(24)25)1-3-11(9)18-16(13)22/h1-6,17,21H,(H,18,22)/q-1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclin B after 40 mins by liquid scintillation counting |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624829
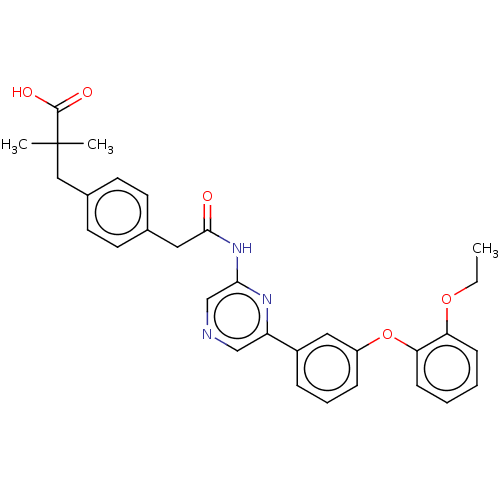
(US20230322706, Example 15)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)Cc2ccc(CC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624824
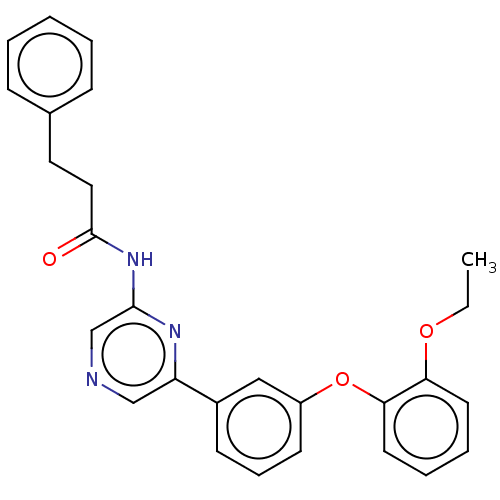
(US20230322706, Example 10)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)CCc2ccccc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624830
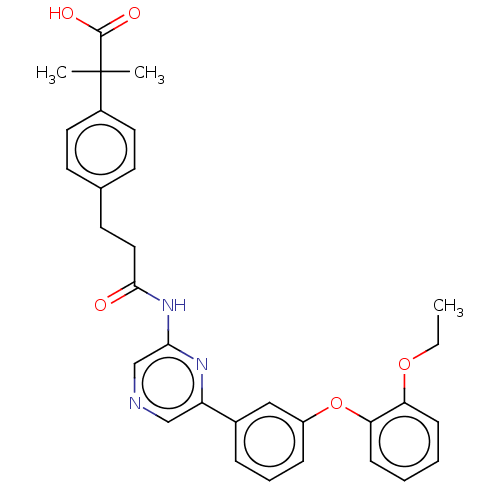
(US20230322706, Example 16)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)CCc2ccc(cc2)C(C)(C)C(O)=O)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624841
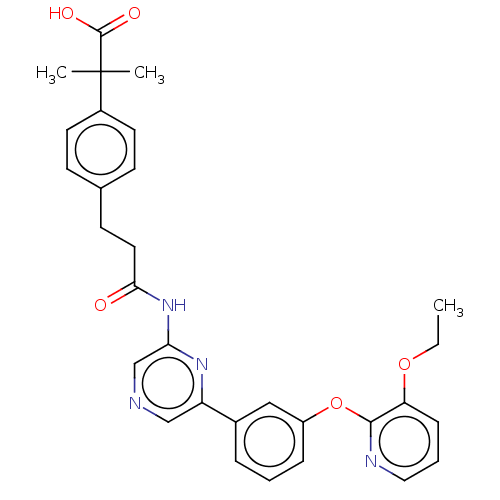
(US20230322706, Example 26)Show SMILES CCOc1cccnc1Oc1cccc(c1)-c1cncc(NC(=O)CCc2ccc(cc2)C(C)(C)C(O)=O)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624832
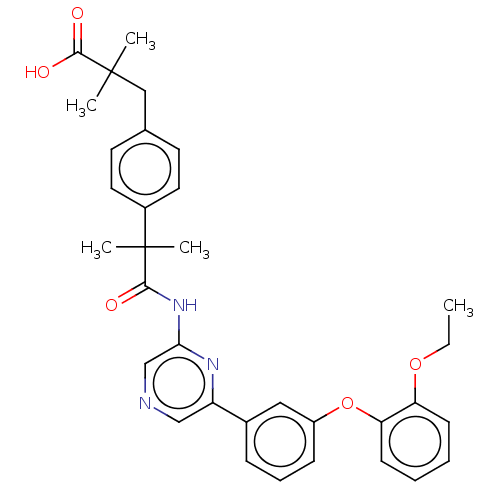
(US20230322706, Example 18)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)C(C)(C)c2ccc(CC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317165
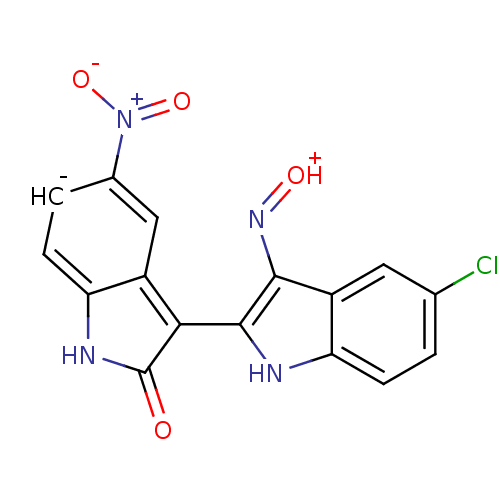
((2'Z,3'E)-5-Nitro-5'-chloro-indirubin-3'-oxime | C...)Show SMILES [O-][N+](=O)c1ccc2[nH]c(=O)[c-](-c3[nH]c4ccc(Cl)cc4c3N=[OH+])c2c1 |(18.15,-5.7,;17.01,-6.74,;15.55,-6.27,;17.34,-8.24,;18.82,-8.71,;19.14,-10.22,;18,-11.25,;18,-12.79,;16.53,-13.26,;16.06,-14.73,;15.63,-12.02,;14.09,-12.02,;13.18,-13.27,;11.7,-12.79,;10.37,-13.56,;9.04,-12.79,;9.04,-11.25,;7.7,-10.48,;10.37,-10.48,;11.7,-11.24,;13.18,-10.76,;13.65,-9.3,;15.16,-8.98,;16.53,-10.77,;16.21,-9.27,)| Show InChI InChI=1S/C16H8ClN4O4/c17-7-1-3-12-10(5-7)14(20-23)15(18-12)13-9-6-8(21(24)25)2-4-11(9)19-16(13)22/h1-6,18H,(H,19,22)/q-1/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624812
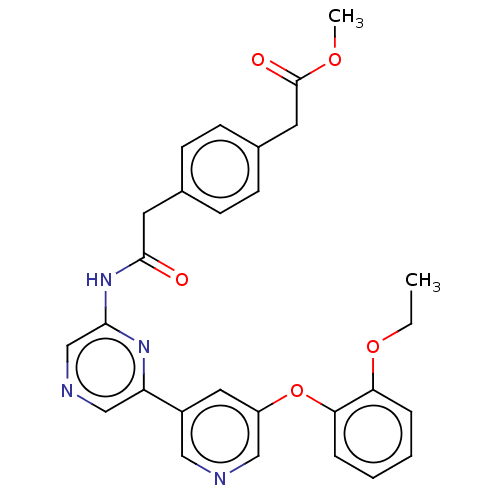
(US20230322706, Example 2)Show SMILES CCOc1ccccc1Oc1cncc(c1)-c1cncc(NC(=O)Cc2ccc(CC(=O)OC)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624811
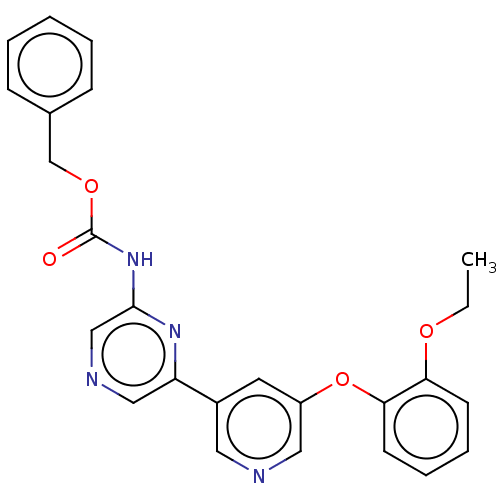
(US20230322706, Example 1)Show SMILES CCOc1ccccc1Oc1cncc(c1)-c1cncc(NC(=O)OCc2ccccc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624821
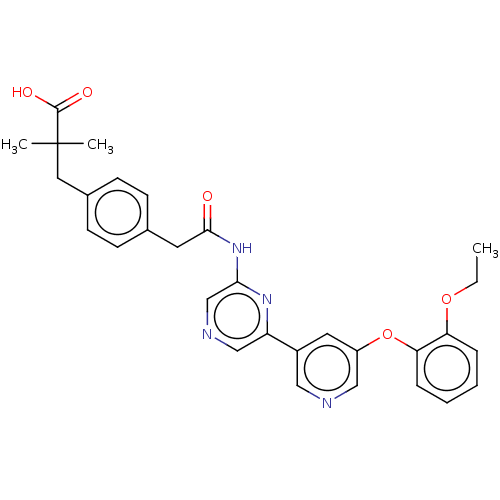
(US20230322706, Example 7)Show SMILES CCOc1ccccc1Oc1cncc(c1)-c1cncc(NC(=O)Cc2ccc(CC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317172

((2'Z,3'E)-5-Trifluoromethoxy-5'-fluoro-indirubin-3...)Show SMILES Fc1ccc2[nH]c(C3C(=O)Nc4ccc(OC(F)(F)F)cc34)c(N=O)c2c1 Show InChI InChI=1S/C17H9F4N3O3/c18-7-1-3-12-10(5-7)14(24-26)15(22-12)13-9-6-8(27-17(19,20)21)2-4-11(9)23-16(13)25/h1-6,13,22H,(H,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624838
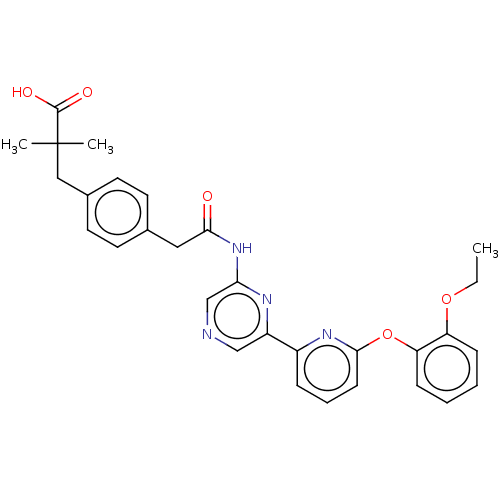
(US20230322706, Example 23)Show SMILES CCOc1ccccc1Oc1cccc(n1)-c1cncc(NC(=O)Cc2ccc(CC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624848
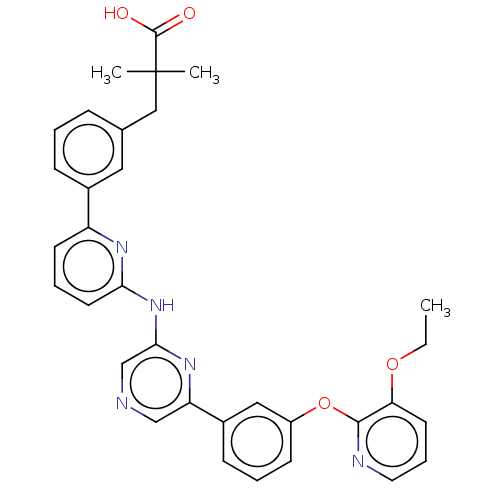
(US20230322706, Example 32)Show SMILES CCOc1cccnc1Oc1cccc(c1)-c1cncc(Nc2cccc(n2)-c2cccc(CC(C)(C)C(O)=O)c2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50317168
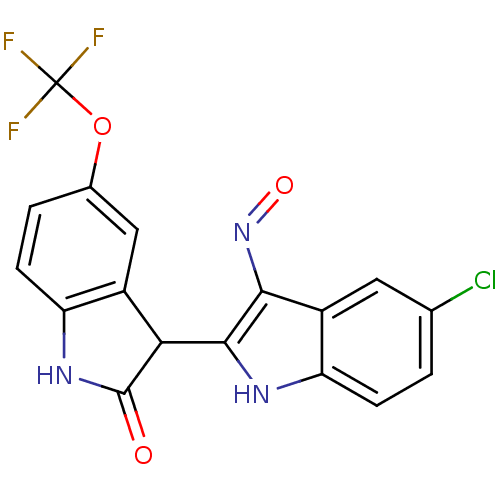
((2'Z,3'E)-5-Trifluoromethoxy-5'-chloro-indirubin-3...)Show SMILES FC(F)(F)Oc1ccc2NC(=O)C(c2c1)c1[nH]c2ccc(Cl)cc2c1N=O Show InChI InChI=1S/C17H9ClF3N3O3/c18-7-1-3-12-10(5-7)14(24-26)15(22-12)13-9-6-8(27-17(19,20)21)2-4-11(9)23-16(13)25/h1-6,13,22H,(H,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624820

(US20230322706, Example 6)Show SMILES CCOC(=O)C(F)(F)c1ccc(CC(=O)Nc2cncc(n2)-c2cncc(Oc3ccccc3OCC)c2)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624823
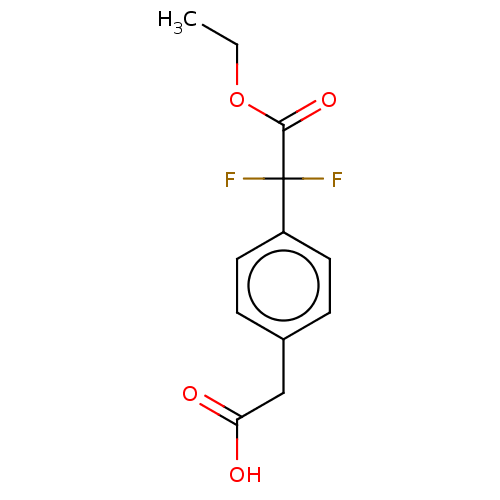
(US20230322706, Example 9) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624826
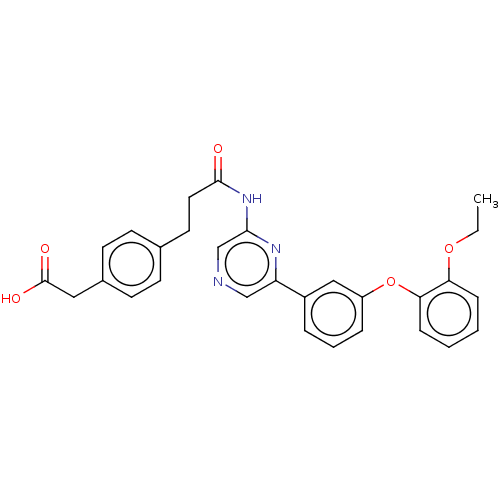
(US20230322706, Example 12)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)CCc2ccc(CC(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50405260
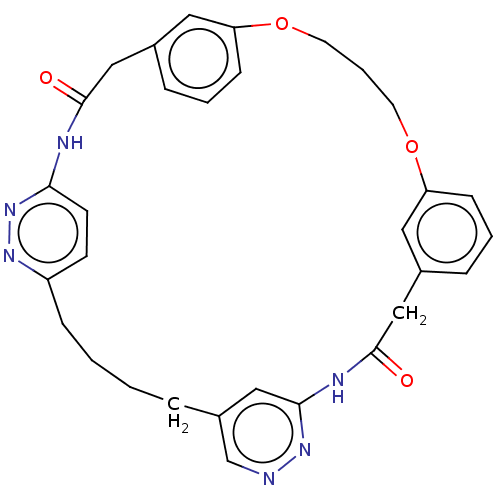
(CHEMBL5288019)Show InChI InChI=1S/C17H21NO4/c19-14(9-8-13-5-2-1-3-6-13)10-11-16(20)18-12-4-7-15(18)17(21)22/h1-3,5-6,15H,4,7-12H2,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50405259
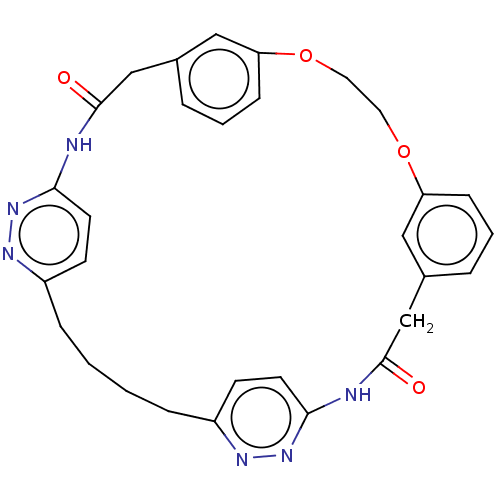
(CHEMBL5277719)Show SMILES CC(NC(C1CCCN1C(=O)NC(Cc1ccccc1)C(O)=O)C(O)=O)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C24H32N4O8/c1-14(20(29)27-11-6-10-18(27)22(32)33)25-19(23(34)35)17-9-5-12-28(17)24(36)26-16(21(30)31)13-15-7-3-2-4-8-15/h2-4,7-8,14,16-19,25H,5-6,9-13H2,1H3,(H,26,36)(H,30,31)(H,32,33)(H,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50317165

((2'Z,3'E)-5-Nitro-5'-chloro-indirubin-3'-oxime | C...)Show SMILES [O-][N+](=O)c1ccc2[nH]c(=O)[c-](-c3[nH]c4ccc(Cl)cc4c3N=[OH+])c2c1 |(18.15,-5.7,;17.01,-6.74,;15.55,-6.27,;17.34,-8.24,;18.82,-8.71,;19.14,-10.22,;18,-11.25,;18,-12.79,;16.53,-13.26,;16.06,-14.73,;15.63,-12.02,;14.09,-12.02,;13.18,-13.27,;11.7,-12.79,;10.37,-13.56,;9.04,-12.79,;9.04,-11.25,;7.7,-10.48,;10.37,-10.48,;11.7,-11.24,;13.18,-10.76,;13.65,-9.3,;15.16,-8.98,;16.53,-10.77,;16.21,-9.27,)| Show InChI InChI=1S/C16H8ClN4O4/c17-7-1-3-12-10(5-7)14(20-23)15(18-12)13-9-6-8(21(24)25)2-4-11(9)19-16(13)22/h1-6,18H,(H,19,22)/q-1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclin B after 40 mins by liquid scintillation counting |
J Med Chem 53: 3696-706 (2010)
Article DOI: 10.1021/jm100080z
BindingDB Entry DOI: 10.7270/Q2X92BFT |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM109086

(US10793535, Cmpd ID 727 | US8604016, 670 | US99382...)Show SMILES FC(F)(F)Oc1cccc(CC(=O)Nc2ccc(CCCCc3nnc(NC(=O)Cc4ccccn4)s3)nn2)c1 Show InChI InChI=1S/C26H24F3N7O3S/c27-26(28,29)39-20-9-5-6-17(14-20)15-22(37)31-21-12-11-18(33-34-21)7-1-2-10-24-35-36-25(40-24)32-23(38)16-19-8-3-4-13-30-19/h3-6,8-9,11-14H,1-2,7,10,15-16H2,(H,31,34,37)(H,32,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from Candida albicans |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624844
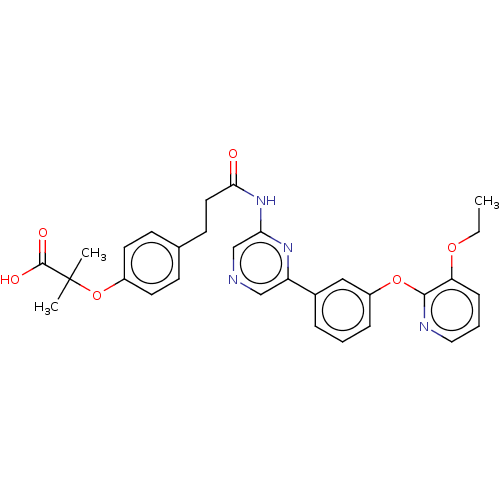
(US20230322706, Example 28)Show SMILES CCOc1cccnc1Oc1cccc(c1)-c1cncc(NC(=O)CCc2ccc(OC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624831

(US20230322706, Example 17)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)\C=C\c2ccc(cc2)C(C)(C)C(O)=O)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624843

(US20230322706, Example 27 | US20230322706, Example...)Show SMILES CCOc1cccnc1Oc1cccc(c1)-c1cncc(NC(=O)Cc2ccc(CC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624818
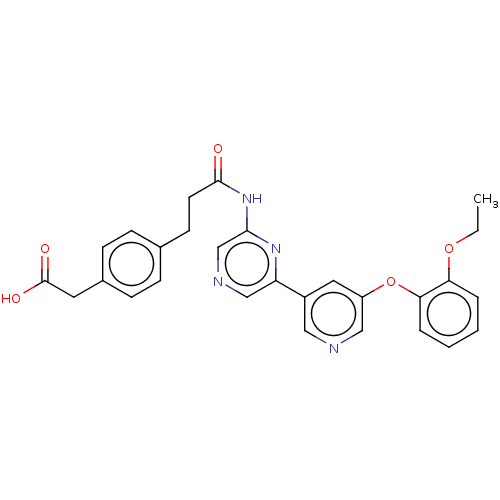
(US20230322706, Example 4)Show SMILES CCOc1ccccc1Oc1cncc(c1)-c1cncc(NC(=O)CCc2ccc(CC(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50405240

(CHEMBL5271248)Show SMILES [#6]-[#6]\[#6](-[#6]-[#6])=[#7]\[#8]-[#6]-[#6](-[#8])-[#6]-[#7]C([#6])([#6])[#6] Show InChI InChI=1S/C12H26N2O2/c1-6-10(7-2)14-16-9-11(15)8-13-12(3,4)5/h11,13,15H,6-9H2,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from rat |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624827

(US20230322706, Example 13)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)CCc2ccc(OC(C)(C)C(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM624825
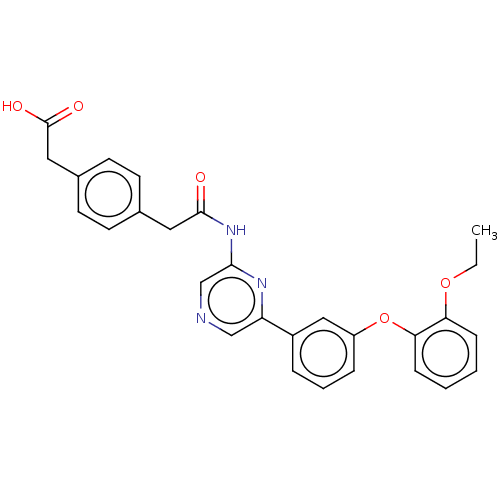
(US20230322706, Example 11)Show SMILES CCOc1ccccc1Oc1cccc(c1)-c1cncc(NC(=O)Cc2ccc(CC(O)=O)cc2)n1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data