

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423299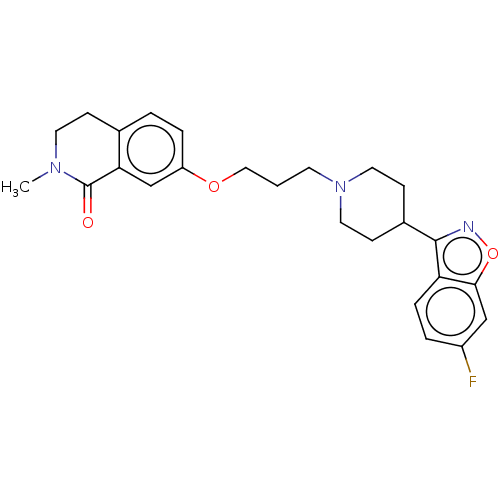 (US10501452, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)8-OH-DPAT from human 5HT1A receptor expressed in human HeLa cells measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | DrugBank Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]raclopride from human D2 long receptor expressed in CHO cells measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7840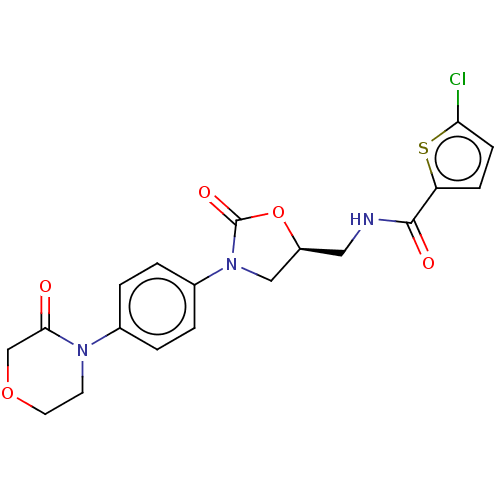 (RIVAROXABAN | US8822458, 44 | US8822458, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO-K1 cells measured after 20 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423299 (US10501452, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50469504 (CHEMBL4293999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5HT2A receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423301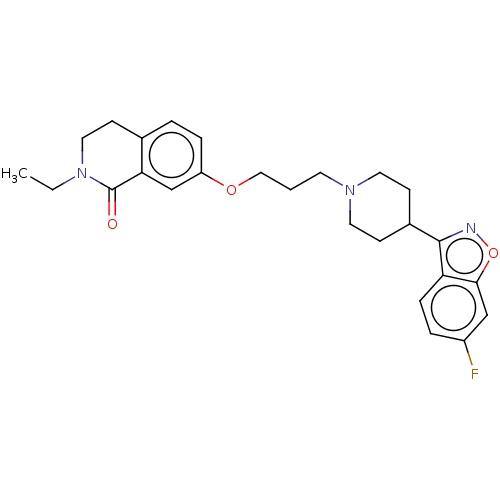 (US10501452, Compound 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443853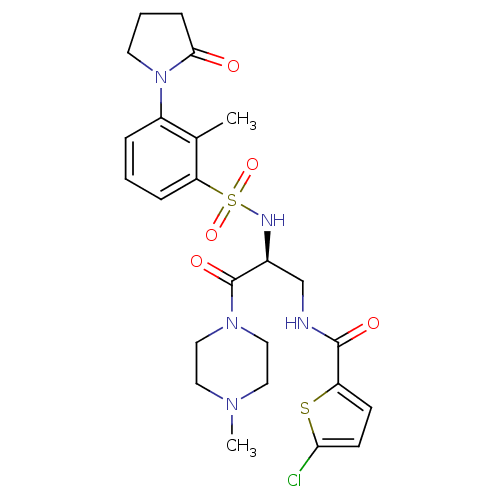 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM423299 (US10501452, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50469504 (CHEMBL4293999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to D3 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423301 (US10501452, Compound 7) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423299 (US10501452, Compound 5) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423302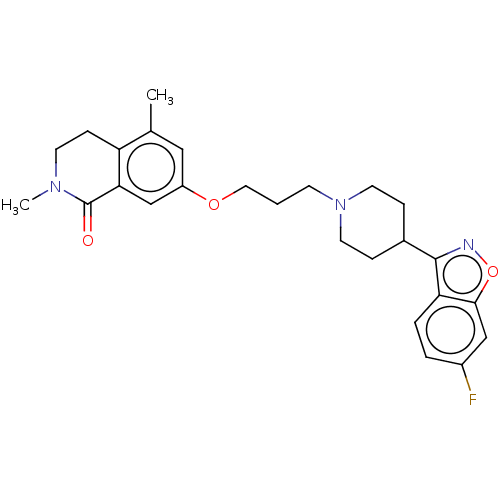 (US10501452, Compound 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423302 (US10501452, Compound 8) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50469504 (CHEMBL4293999) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to D2 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM423303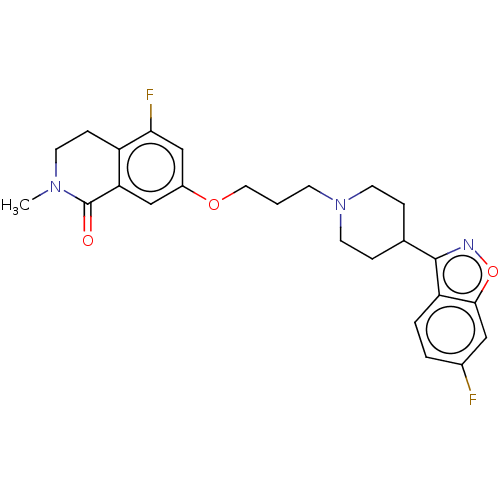 (US10501452, Compound 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]prazosin from rat cerebral cortex alpha1 adrenergic receptor measured after 60 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423316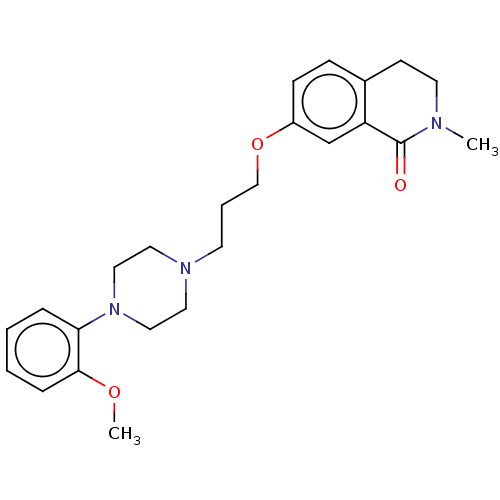 (US10501452, Compound 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50556105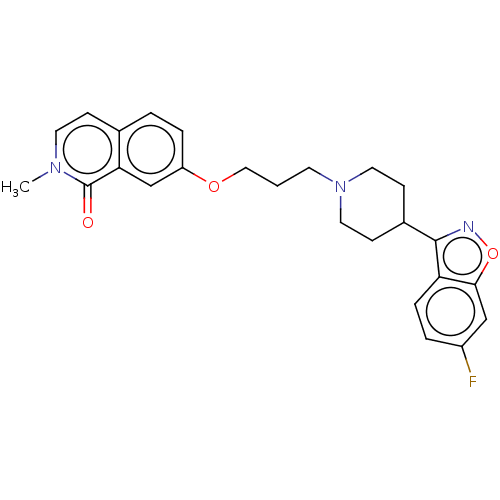 (CHEMBL4743324) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509922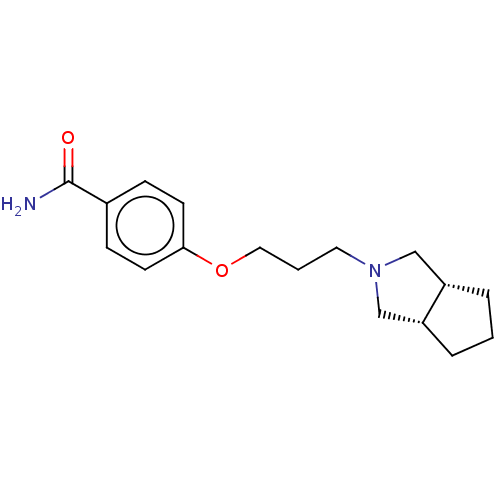 (CHEMBL4575319) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423305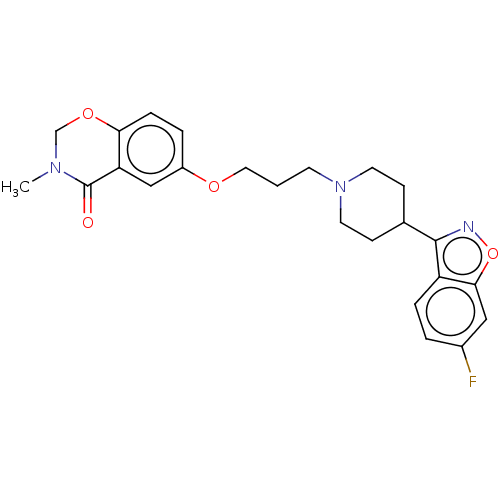 (US10501452, Compound 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423307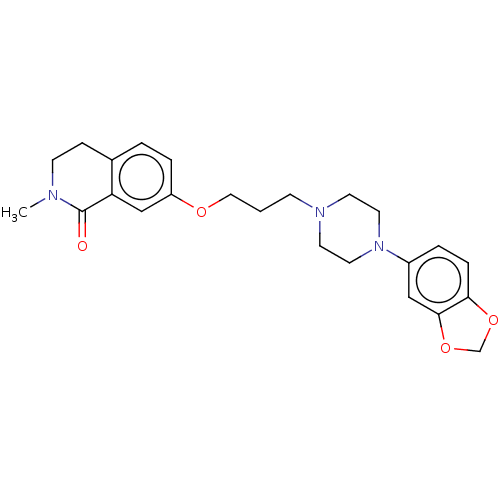 (US10501452, Compound 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]spiperone from D2 receptor in rat striatum measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423303 (US10501452, Compound 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423298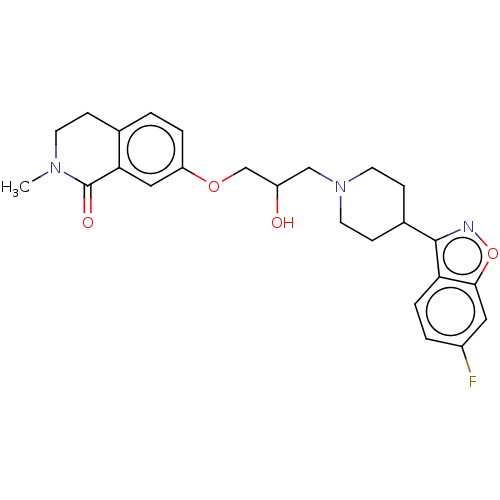 (US10501452, Compound 4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423303 (US10501452, Compound 9) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423304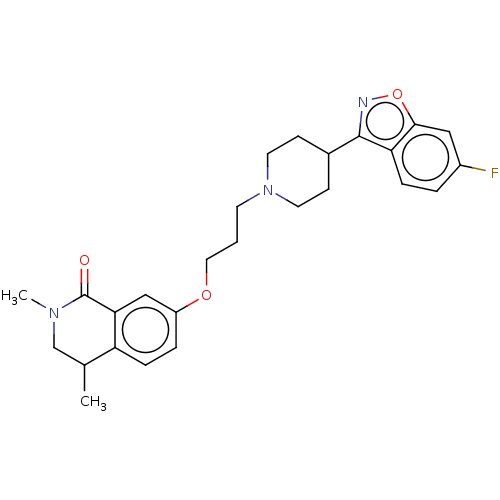 (US10501452, Compound 10) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM423299 (US10501452, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]5-CT from rat cerebral cortex 5HT7 receptor measured after 30 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423306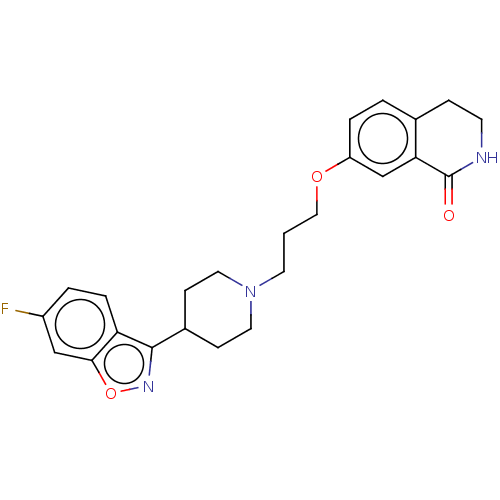 (US10501452, Compound 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM423330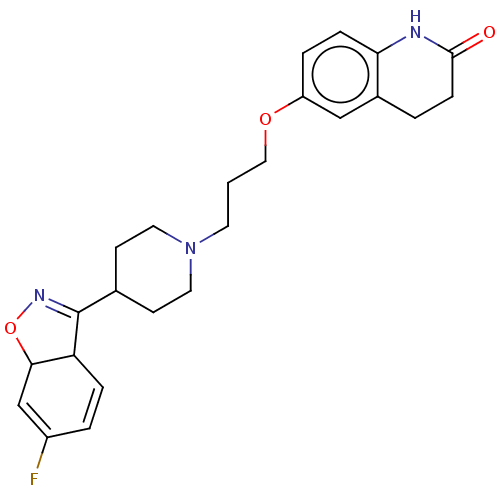 (US10501452, Compound E) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50469504 (CHEMBL4293999) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5HT6 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423322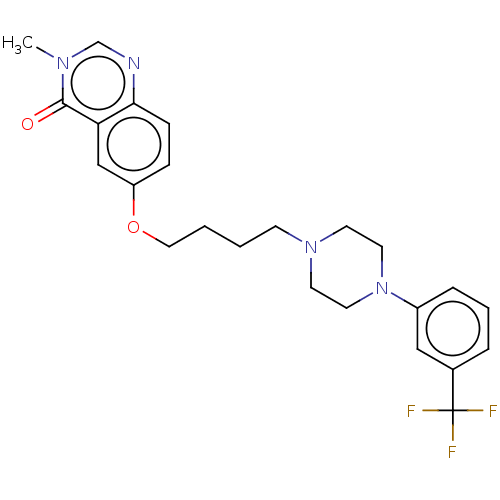 (US10501452, Compound 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM423304 (US10501452, Compound 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM423299 (US10501452, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423316 (US10501452, Compound 22) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509934 (CHEMBL4557586) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM423302 (US10501452, Compound 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM423308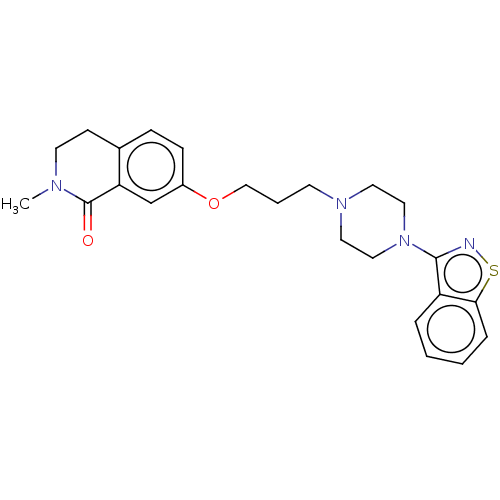 (US10501452, Compound 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM423299 (US10501452, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]spiperone from D2 receptor in rat striatum measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423308 (US10501452, Compound 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM423322 (US10501452, Compound 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423318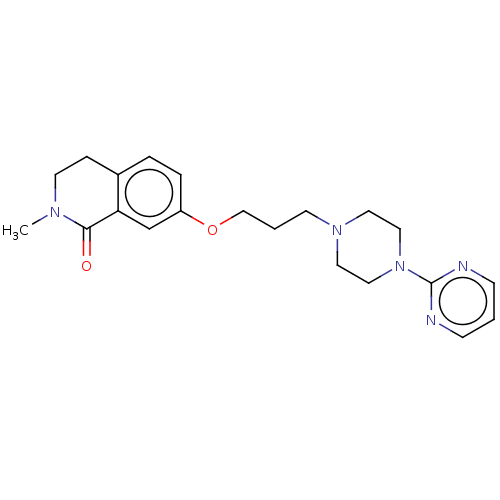 (US10501452, Compound 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM423303 (US10501452, Compound 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM423325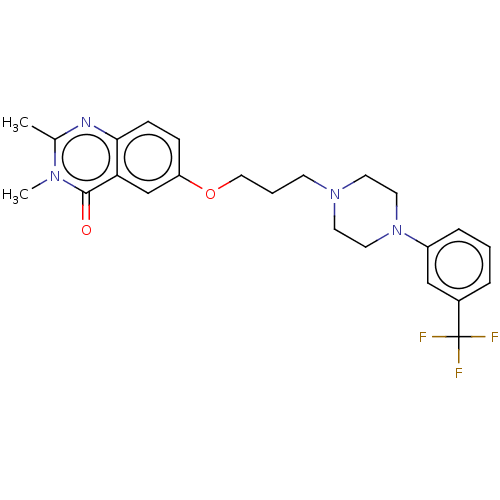 (US10501452, Compound 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM423304 (US10501452, Compound 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50469504 (CHEMBL4293999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5HT1A receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM423302 (US10501452, Compound 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 748 total ) | Next | Last >> |