

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of recombinant ALK (unknown origin) | J Med Chem 59: 3392-408 (2016) Article DOI: 10.1021/acs.jmedchem.6b00064 BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033839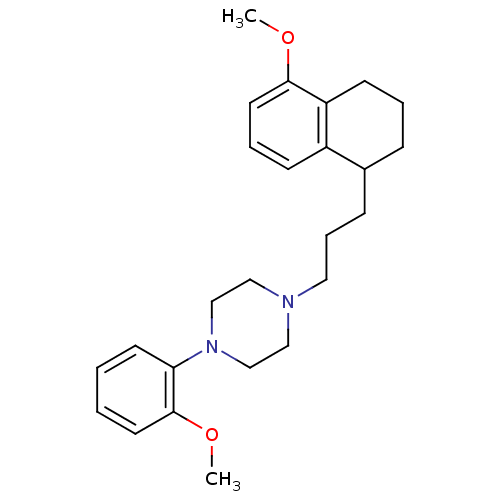 (1-(2-Methoxy-phenyl)-4-[3-(5-methoxy-1,2,3,4-tetra...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033843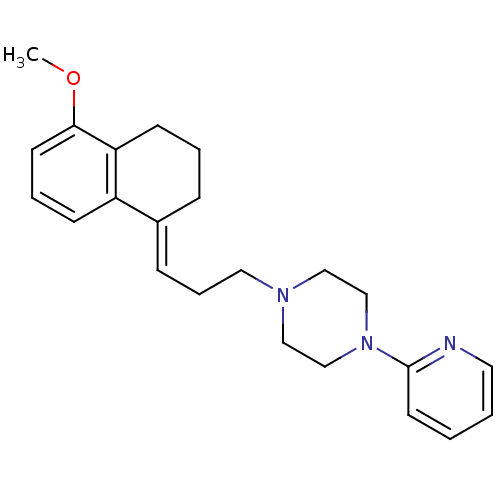 (1-[3-(5-Methoxy-3,4-dihydro-2H-naphthalen-1-yliden...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033857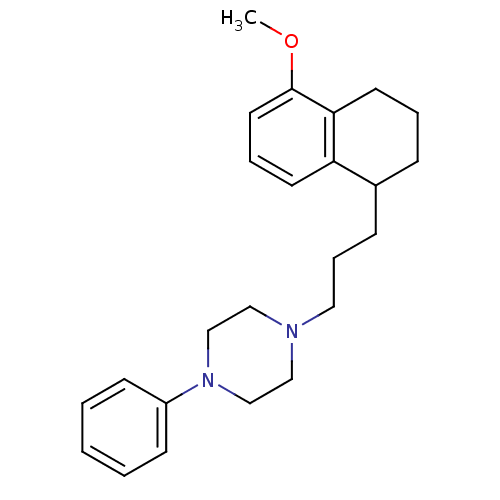 (1-[3-(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033851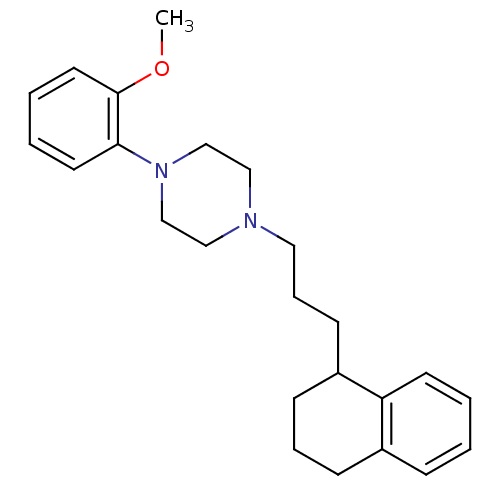 (1-(2-Methoxy-phenyl)-4-[3-(1,2,3,4-tetrahydro-naph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description Inhibition of binding of radioligand [3H]-spiroperidol to dopamine D2 receptor in rat striatal membranes | J Med Chem 37: 99-104 (1994) BindingDB Entry DOI: 10.7270/Q2NP23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on dopamine receptor D2 using [3H]-spiroperidol as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033833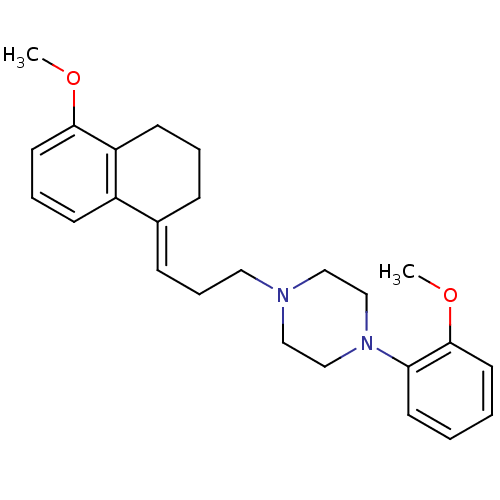 (1-{3-[5-Methoxy-3,4-dihydro-2H-naphthalen-(1E)-yli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033852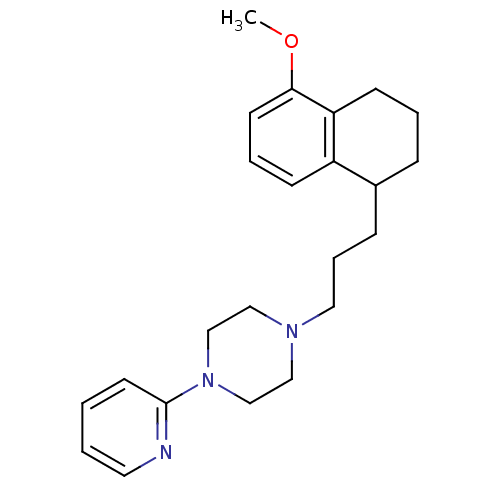 (1-[3-(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM31541 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307546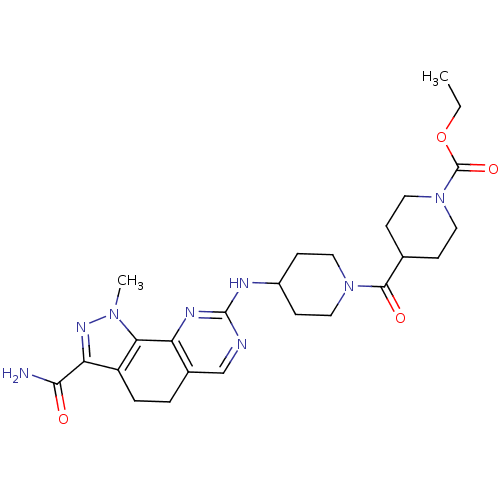 (CHEMBL598401 | Ethyl 4-[(3-Carbamoyl-1-methyl-4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of TRKA (unknown origin) in presence of gamma33-ATP | J Med Chem 59: 3392-408 (2016) Article DOI: 10.1021/acs.jmedchem.6b00064 BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307543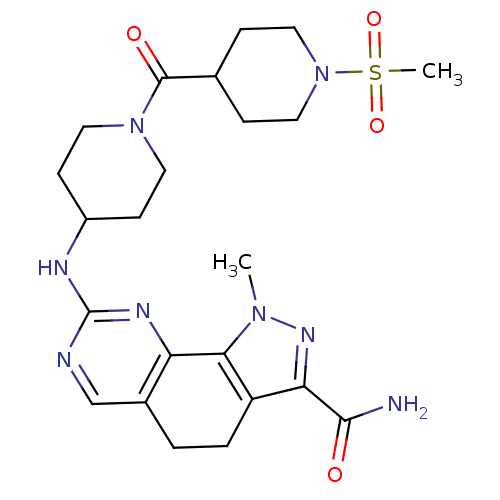 (1-Methyl-8-{[1-(methylsulfonyl)piperidin-4-yl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033836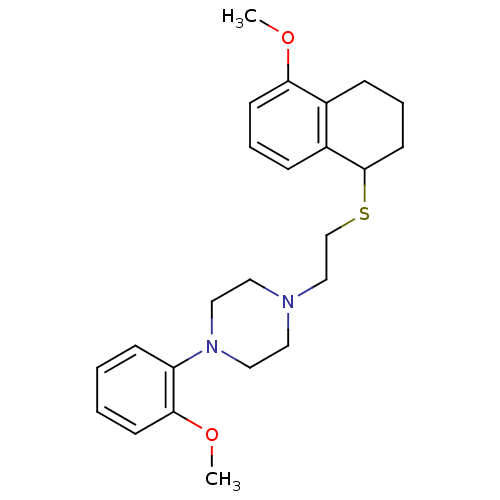 (1-(2-Methoxy-phenyl)-4-[2-(5-methoxy-1,2,3,4-tetra...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit binding of radioligand [3H]-8-OH-DPAT to 5-hydroxytryptamine 1A receptor in rat cerebral cortex | J Med Chem 37: 99-104 (1994) BindingDB Entry DOI: 10.7270/Q2NP23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033859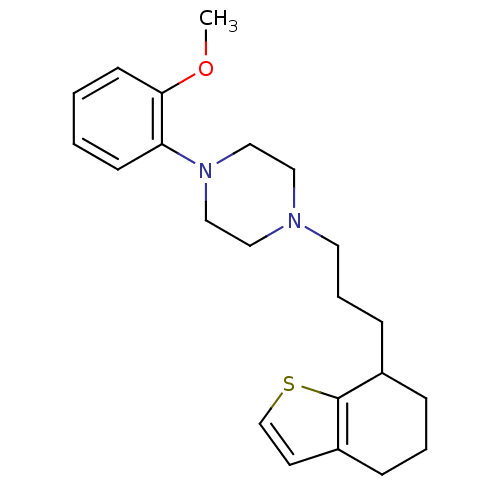 (1-(2-Methoxy-phenyl)-4-[3-(4,5,6,7-tetrahydro-benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50043891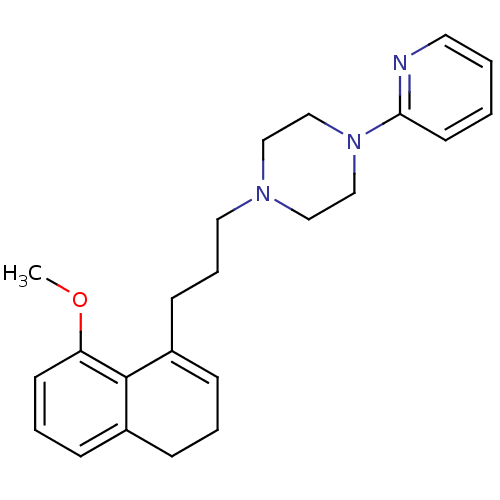 (1-[3-(8-Methoxy-3,4-dihydro-naphthalen-1-yl)-propy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit binding of radioligand [3H]-8-OH-DPAT to 5-hydroxytryptamine 1A receptor in rat cerebral cortex | J Med Chem 37: 99-104 (1994) BindingDB Entry DOI: 10.7270/Q2NP23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033853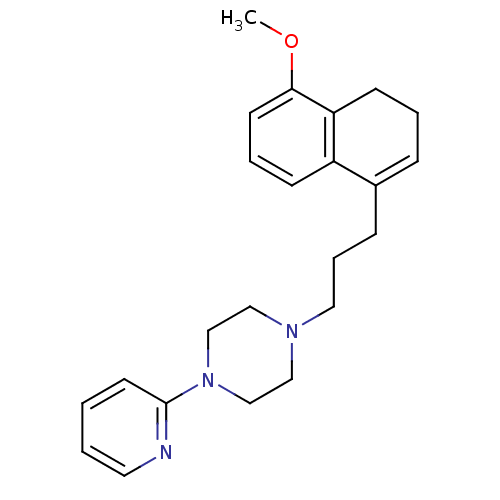 (1-[3-(5-Methoxy-3,4-dihydro-naphthalen-1-yl)-propy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033858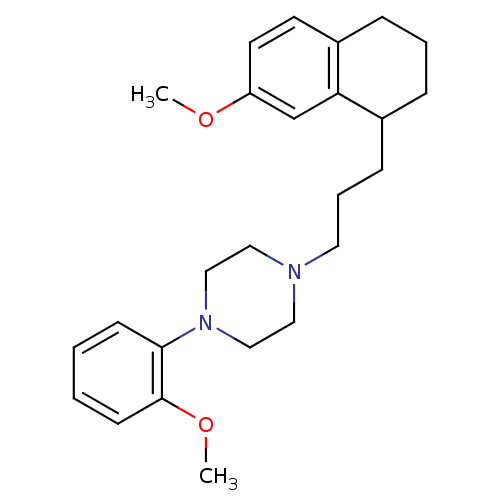 (1-(2-Methoxy-phenyl)-4-[3-(7-methoxy-1,2,3,4-tetra...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033831 (1-[4-(7-Methoxy-3,4-dihydro-naphthalen-1-yl)-butyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033835 (1-(2-Methoxy-phenyl)-4-[4-(5-methoxy-1,2,3,4-tetra...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033844 (1-{3-[3,4-Dihydro-2H-naphthalen-(1E)-ylidene]-prop...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307507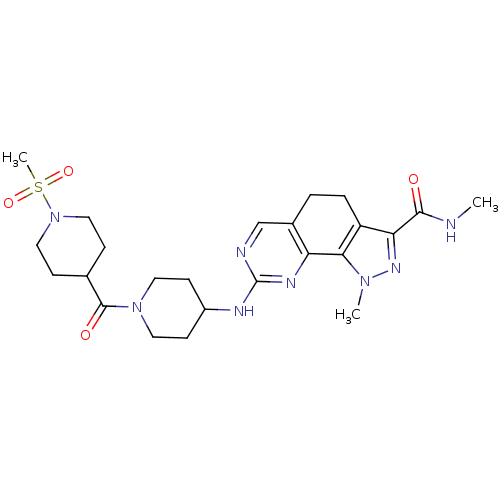 (CHEMBL597754 | N-1-Dimethyl-8-{[1-(methylsulfonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7111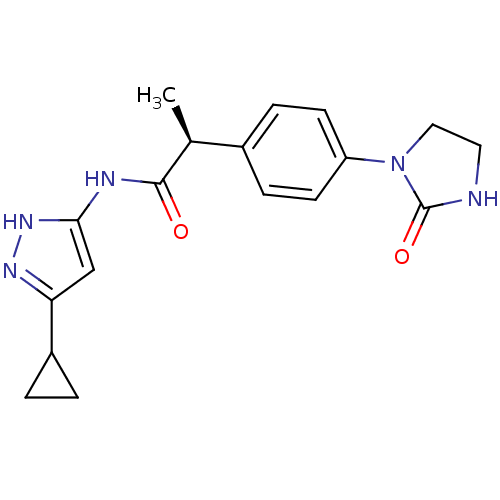 ((2S)-N-(5-Cyclopropyl-1H-pyrazol-3-yl)-2-[4-(2-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 48: 2944-56 (2005) Article DOI: 10.1021/jm0408870 BindingDB Entry DOI: 10.7270/Q2WQ020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7107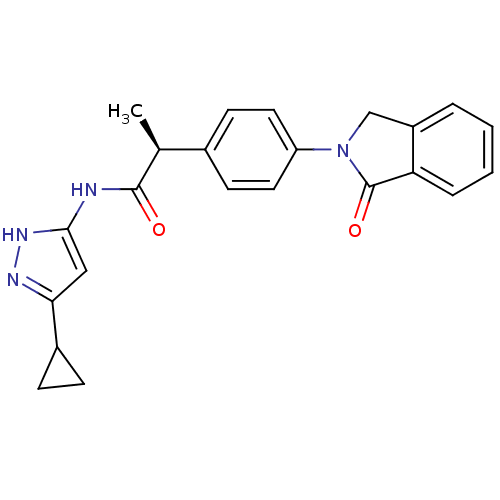 ((2S)-N-(5-Cyclopropyl-1H-pyrazol-3-yl)-2-[4-(1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 48: 2944-56 (2005) Article DOI: 10.1021/jm0408870 BindingDB Entry DOI: 10.7270/Q2WQ020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM31539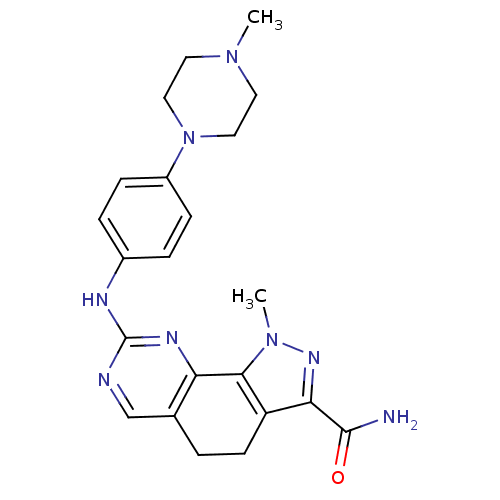 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM31532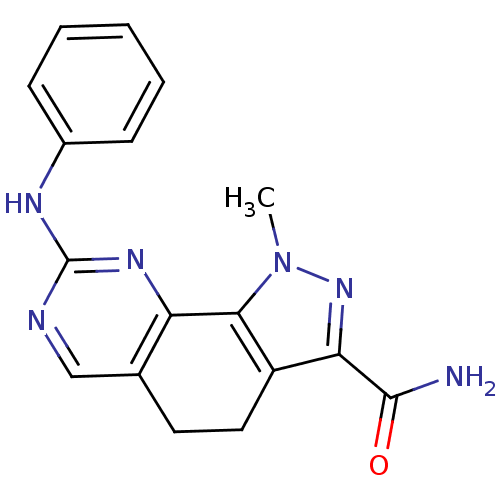 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27371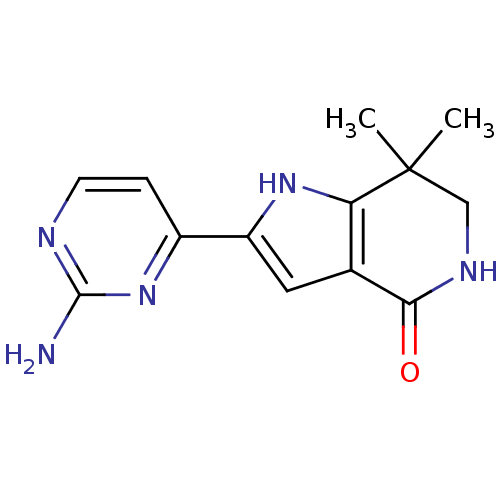 (2-(2-aminopyrimidin-4-yl)-7,7-dimethyl-1H,4H,5H,6H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50329419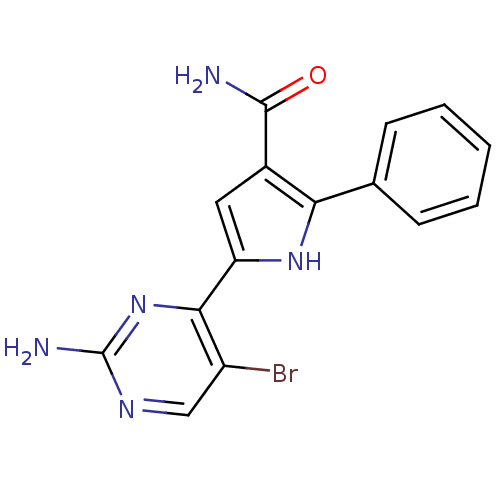 (5-(2-amino-5-bromopyrimidin-4-yl)-2-p-tolyl-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Cdc7 | J Med Chem 53: 7296-315 (2010) Article DOI: 10.1021/jm100504d BindingDB Entry DOI: 10.7270/Q24T6JM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27362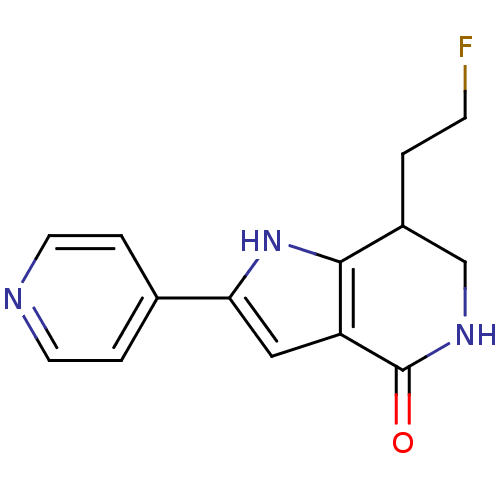 (7-(2-fluoroethyl)-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50343559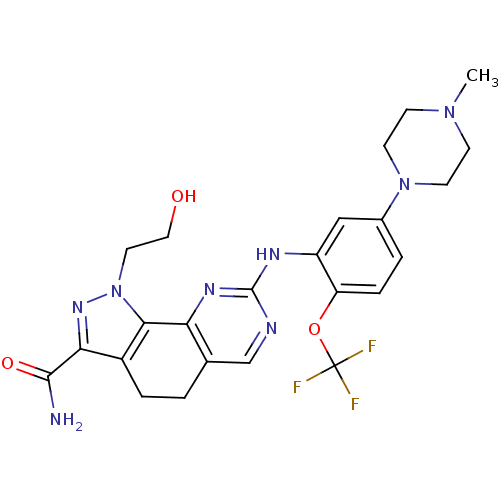 (1-(2-HYDROXYETHYL)-8-[[5-(4-METHYLPIPERAZIN-1-YL)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of PLK1 assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting | Bioorg Med Chem Lett 21: 2969-74 (2011) Article DOI: 10.1016/j.bmcl.2011.03.054 BindingDB Entry DOI: 10.7270/Q27M088W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27359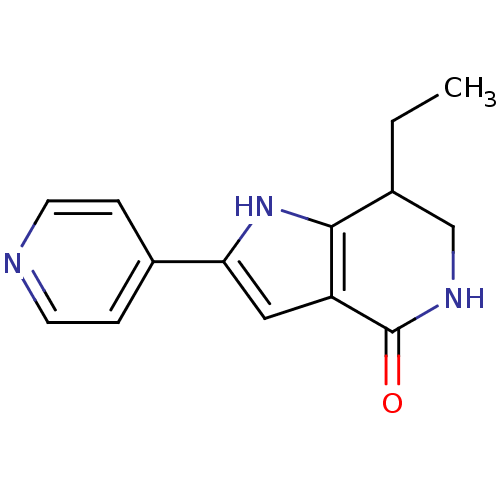 (7-ethyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50329915 (1-methyl-8-(5-(piperazin-1-yl)-2-(trifluoromethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of PLK1 | Bioorg Med Chem Lett 20: 6489-94 (2010) Article DOI: 10.1016/j.bmcl.2010.09.060 BindingDB Entry DOI: 10.7270/Q2VT1T39 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27380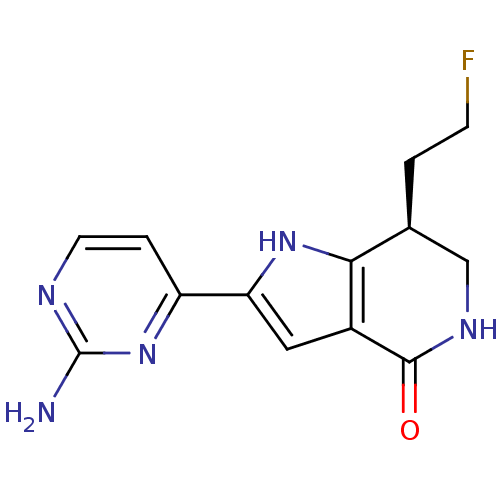 ((7S)-2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307535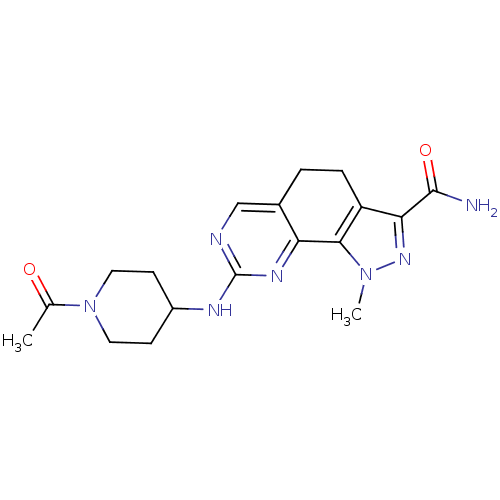 (8-[(1-Acetylpiperidin-4-yl)amino]-1-methyl-4,5-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307522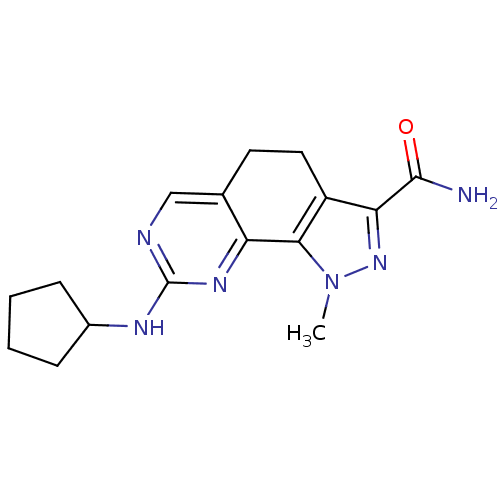 (8-(Cyclopentylamino)-1-methyl-4,5-dihydro-1H-pyraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50033834 (1-[3-(7-Methoxy-3,4-dihydro-naphthalen-1-yl)-propy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro binding affinity measured on serotonin 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand. | J Med Chem 38: 942-9 (1995) BindingDB Entry DOI: 10.7270/Q2SQ8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50043882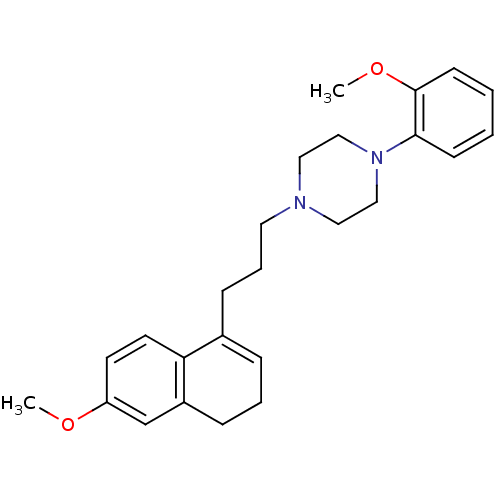 (1-[3-(6-Methoxy-3,4-dihydro-naphthalen-1-yl)-propy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit binding of radioligand [3H]-8-OH-DPAT to 5-hydroxytryptamine 1A receptor in rat cerebral cortex | J Med Chem 37: 99-104 (1994) BindingDB Entry DOI: 10.7270/Q2NP23GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of human TEL (336 residues) fused-TRKA (440 to 796 residues) (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibi... | J Med Chem 59: 3392-408 (2016) Article DOI: 10.1021/acs.jmedchem.6b00064 BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of human TEL (336 residues) fused-TRKB (455 to 822 residues) (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibi... | J Med Chem 59: 3392-408 (2016) Article DOI: 10.1021/acs.jmedchem.6b00064 BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7163 (3-Phenylacetamidoaminopyrazole deriv. 40 | CS10 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 47: 3367-80 (2004) Article DOI: 10.1021/jm031145u BindingDB Entry DOI: 10.7270/Q2RX998G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50329914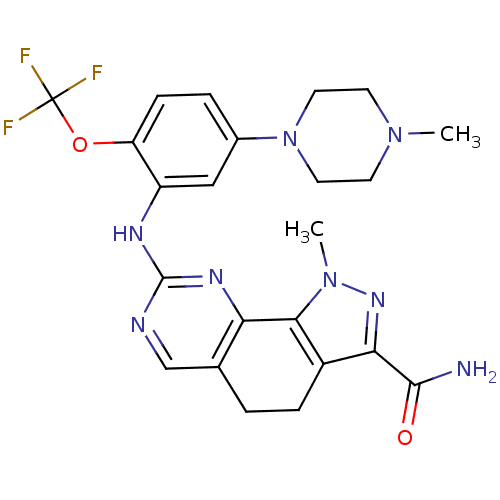 (1-methyl-8-(5-(4-methylpiperazin-1-yl)-2-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of PLK1 assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting | Bioorg Med Chem Lett 21: 2969-74 (2011) Article DOI: 10.1016/j.bmcl.2011.03.054 BindingDB Entry DOI: 10.7270/Q27M088W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50329914 (1-methyl-8-(5-(4-methylpiperazin-1-yl)-2-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of PLK1 | Bioorg Med Chem Lett 20: 6489-94 (2010) Article DOI: 10.1016/j.bmcl.2010.09.060 BindingDB Entry DOI: 10.7270/Q2VT1T39 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50170106 (CHEMBL3805643) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of recombinant ALK (unknown origin) in presence of gamma33-ATP | J Med Chem 59: 3392-408 (2016) Article DOI: 10.1021/acs.jmedchem.6b00064 BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27370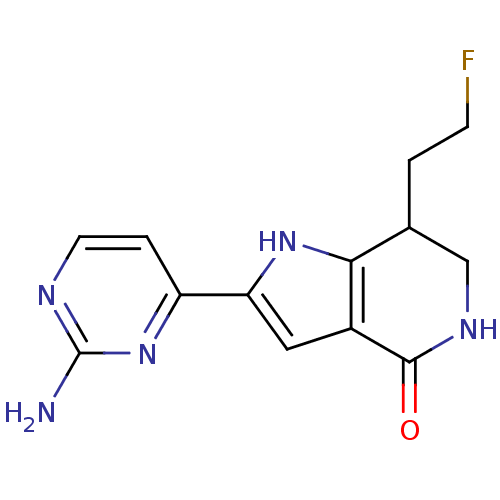 (2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1H,4H,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27363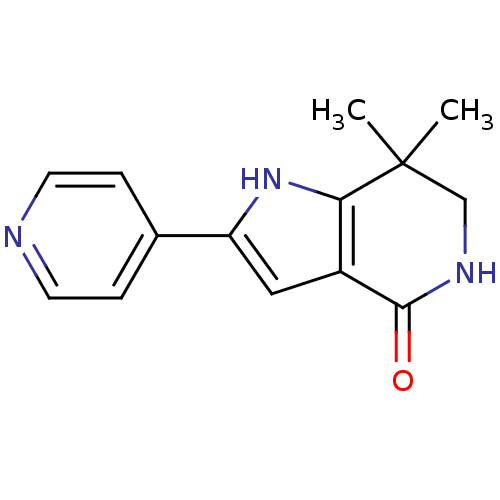 (7,7-dimethyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27361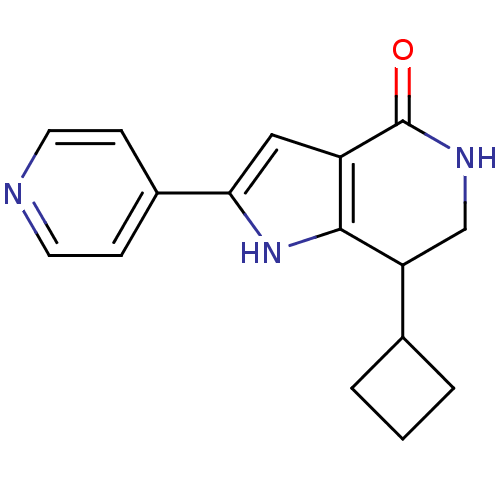 (7-cyclobutyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50343568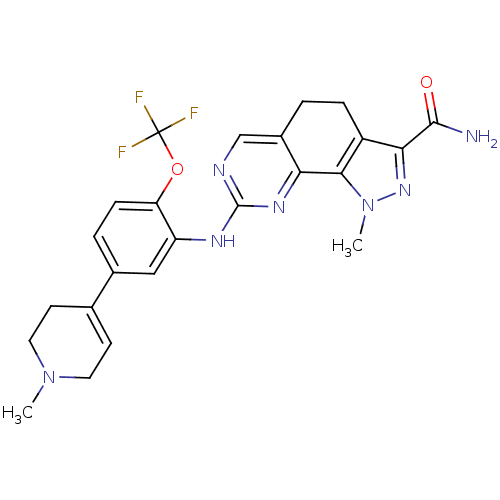 (1-methyl-8-(5-(1-methyl-1,2,3,6-tetrahydropyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of PLK1 assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting | Bioorg Med Chem Lett 21: 2969-74 (2011) Article DOI: 10.1016/j.bmcl.2011.03.054 BindingDB Entry DOI: 10.7270/Q27M088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of human TEL (336 residues) fused-TRKC (454 to 825 residues) (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibi... | J Med Chem 59: 3392-408 (2016) Article DOI: 10.1021/acs.jmedchem.6b00064 BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1240 total ) | Next | Last >> |