

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde oxidase (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive type inhibition of human AOX assessed as inhibition constant using vanillin as substrate by HPLC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01125 BindingDB Entry DOI: 10.7270/Q2C82F55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldehyde oxidase (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive type inhibition of human AOX assessed as inhibition constant using phthalazine as substrate preincubated for 30 mins followed by substr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01125 BindingDB Entry DOI: 10.7270/Q2C82F55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldehyde oxidase (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive type inhibition of human AOX assessed as inhibition constant using nicotine-1(S)-iminium ion as substrate incubated for 2 mins by HPLC-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01125 BindingDB Entry DOI: 10.7270/Q2C82F55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50019056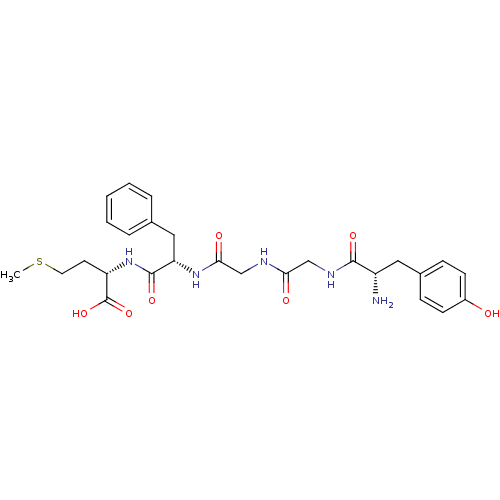 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50233416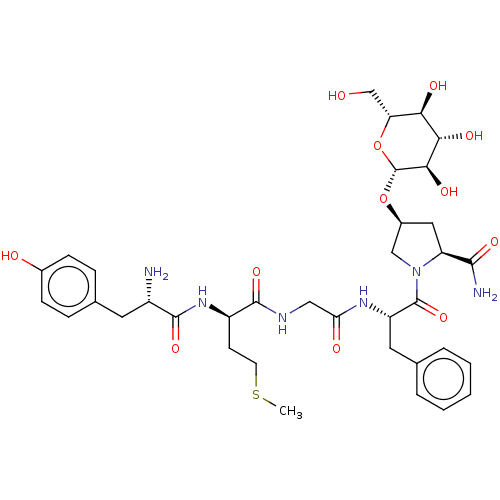 (CHEMBL4065055) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50233418 (CHEMBL4101712) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50233419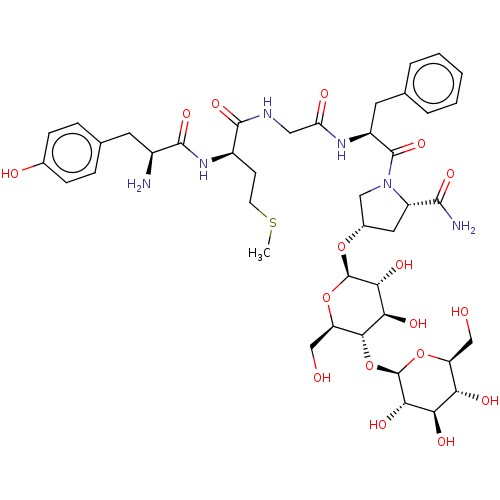 (CHEMBL4083712) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50233415 (CHEMBL4064725) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50233414 (CHEMBL4095111) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50233414 (CHEMBL4095111) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50233416 (CHEMBL4065055) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50233417 (CHEMBL4102609) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 383 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50233416 (CHEMBL4065055) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50233417 (CHEMBL4102609) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50233417 (CHEMBL4102609) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 519 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50233419 (CHEMBL4083712) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 528 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50233415 (CHEMBL4064725) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 536 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50233418 (CHEMBL4101712) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 559 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50233414 (CHEMBL4095111) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 566 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50233415 (CHEMBL4064725) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 609 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50233418 (CHEMBL4101712) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 635 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50019056 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 684 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor homologue (Danio rerio) | BDBM50233419 (CHEMBL4083712) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 843 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Danio rerio) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish mu opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oprd1b protein (Danio rerio) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Avanzada de Catalu£a (IQAC-CSIC) Curated by ChEMBL | Assay Description Displacement of [3H]DPN from zebrafish delta 1b opioid receptor expressed in HEK293 cell membranes after 4 hrs by scintillation counting method | Bioorg Med Chem 25: 2260-2265 (2017) Article DOI: 10.1016/j.bmc.2017.02.052 BindingDB Entry DOI: 10.7270/Q2QR50CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50298570 (2-(Acetylamino)-6-chloro-7-(benzyl{6-azido-3,4-di-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci£ncias da Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of BChE in human serum assessed as hydrolysis of butyrylthiocholine by Ellman's method | Bioorg Med Chem 17: 5106-16 (2009) Article DOI: 10.1016/j.bmc.2009.05.057 BindingDB Entry DOI: 10.7270/Q2V40V8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci£ncias da Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of BChE in human serum assessed as hydrolysis of butyrylthiocholine by Ellman's method | Bioorg Med Chem 17: 5106-16 (2009) Article DOI: 10.1016/j.bmc.2009.05.057 BindingDB Entry DOI: 10.7270/Q2V40V8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382508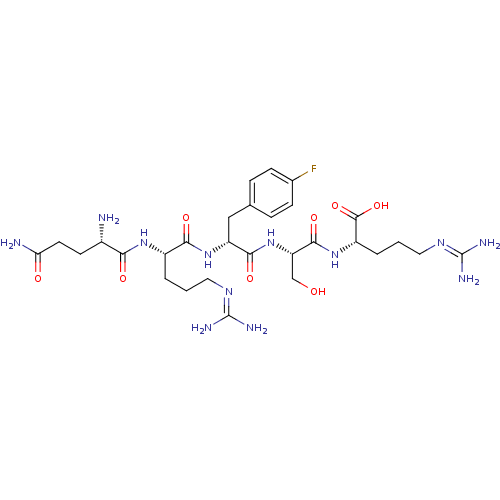 (CHEMBL2022234) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382512 (CHEMBL2022238) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50298569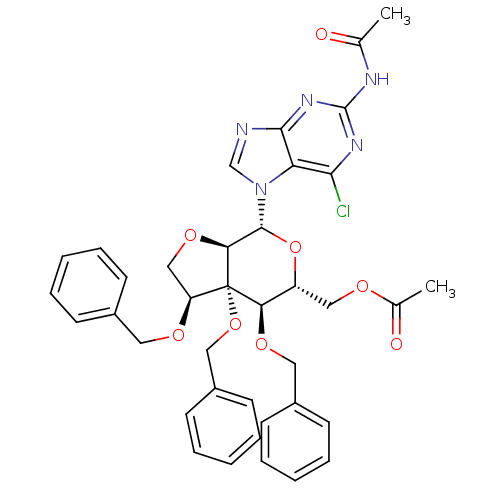 (2-(acetylamino)-6-chloro-7-{6-O-acetyl-3,4-di-Oben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci£ncias da Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of BChE in human serum assessed as hydrolysis of butyrylthiocholine by Ellman's method | Bioorg Med Chem 17: 5106-16 (2009) Article DOI: 10.1016/j.bmc.2009.05.057 BindingDB Entry DOI: 10.7270/Q2V40V8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50298568 (Benzyl{methyl 6-azido-3,4-di-O-benzyl-3-C,2-O-[(1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci£ncias da Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of BChE in human serum assessed as hydrolysis of butyrylthiocholine by Ellman's method | Bioorg Med Chem 17: 5106-16 (2009) Article DOI: 10.1016/j.bmc.2009.05.057 BindingDB Entry DOI: 10.7270/Q2V40V8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382499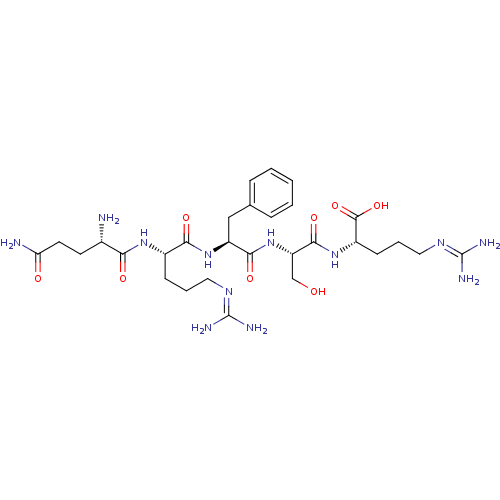 (CHEMBL2022225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382515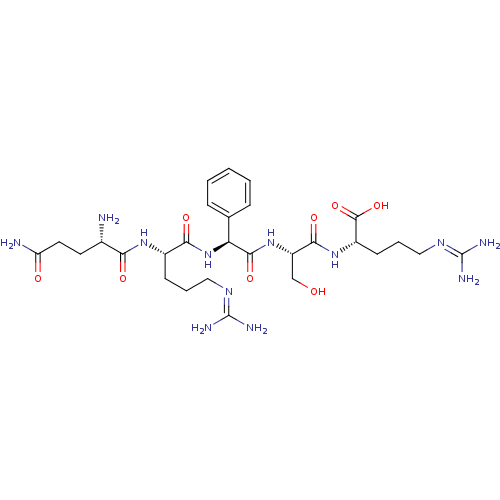 (CHEMBL2024267) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382520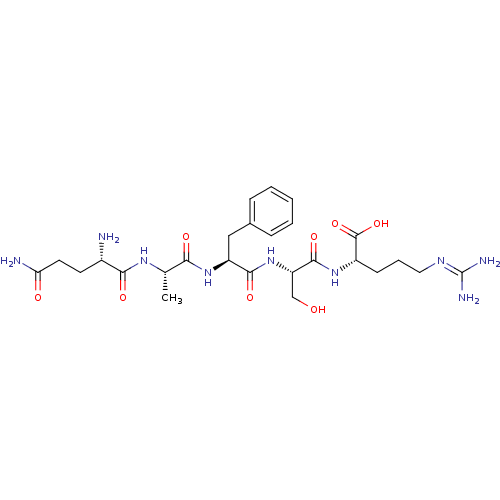 (CHEMBL2022227) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50298567 (Benzyl{methyl 6-azido-3,4-di-O-benzyl-3-C,2-O-[(1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci£ncias da Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of BChE in human serum assessed as hydrolysis of butyrylthiocholine by Ellman's method | Bioorg Med Chem 17: 5106-16 (2009) Article DOI: 10.1016/j.bmc.2009.05.057 BindingDB Entry DOI: 10.7270/Q2V40V8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382507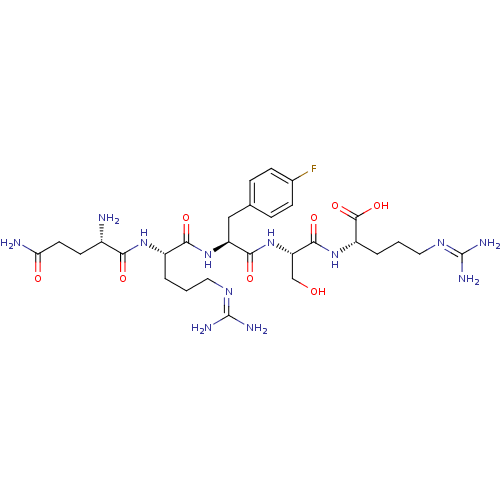 (CHEMBL2021931) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50298566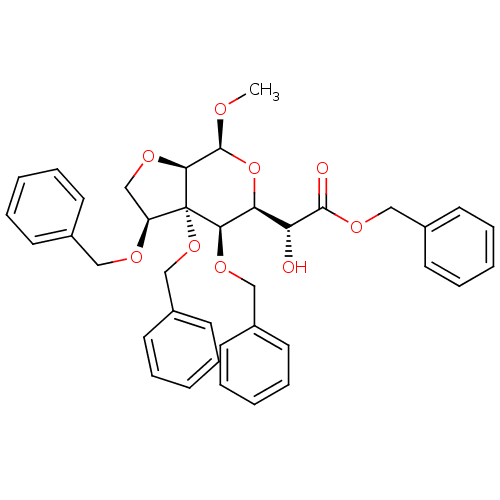 (Benzyl{methyl 3,4-di-O-benzyl-3-C,2-O-[(1S)-1-(ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci£ncias da Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of BChE in human serum assessed as hydrolysis of butyrylthiocholine by Ellman's method | Bioorg Med Chem 17: 5106-16 (2009) Article DOI: 10.1016/j.bmc.2009.05.057 BindingDB Entry DOI: 10.7270/Q2V40V8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131538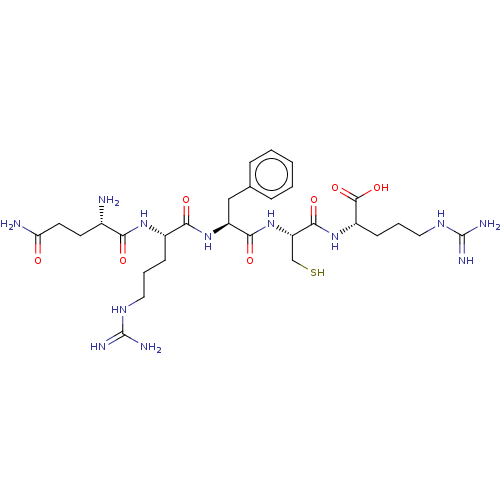 (CHEMBL3633448) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131500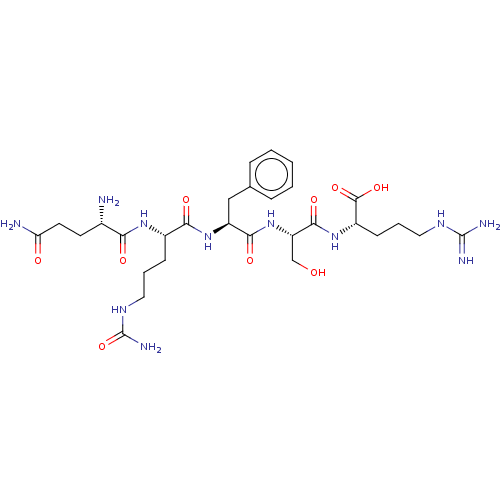 (CHEMBL3633451) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50298565 (2-(Acetylamino)-6-chloro-7-(benzyl{6-azido-3,4-di-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci£ncias da Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of BChE in human serum assessed as hydrolysis of butyrylthiocholine by Ellman's method | Bioorg Med Chem 17: 5106-16 (2009) Article DOI: 10.1016/j.bmc.2009.05.057 BindingDB Entry DOI: 10.7270/Q2V40V8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant neutral endopeptidase using Ala-AMC as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382500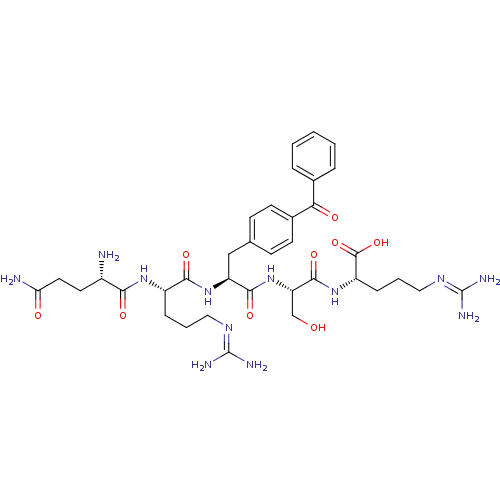 (CHEMBL2024270) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382505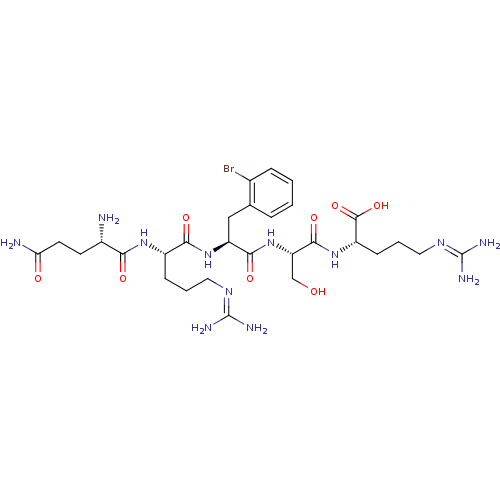 (CHEMBL2022232) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382510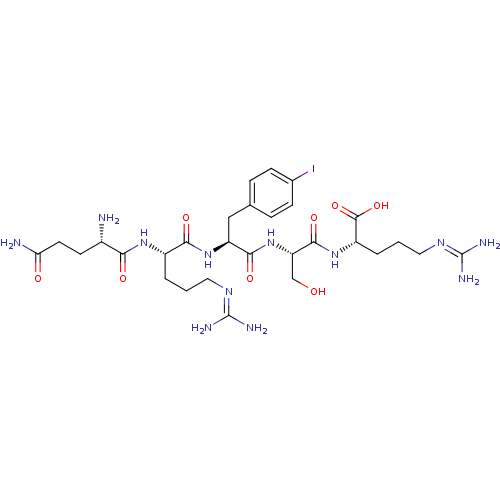 (CHEMBL2022236) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |