 Found 264 hits with Last Name = 'gamo' and Initial = 'fj'
Found 264 hits with Last Name = 'gamo' and Initial = 'fj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23232
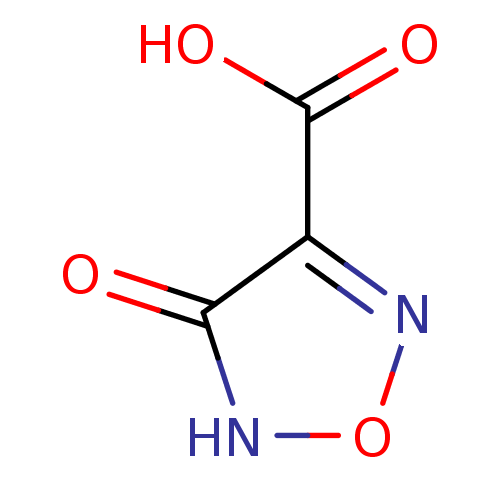
(1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...)Show InChI InChI=1S/C3H2N2O4/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23251
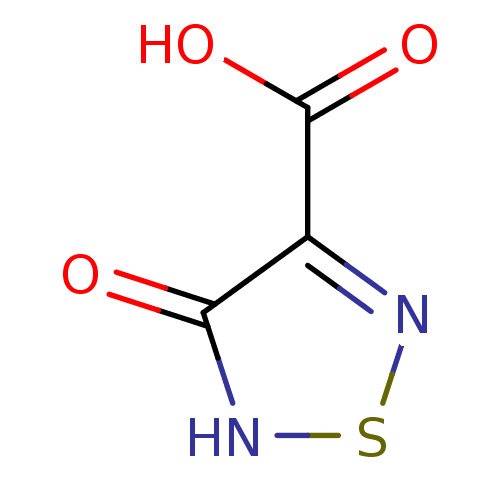
(1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...)Show InChI InChI=1S/C3H2N2O3S/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase
(Plasmodium falciparum) | BDBM23242
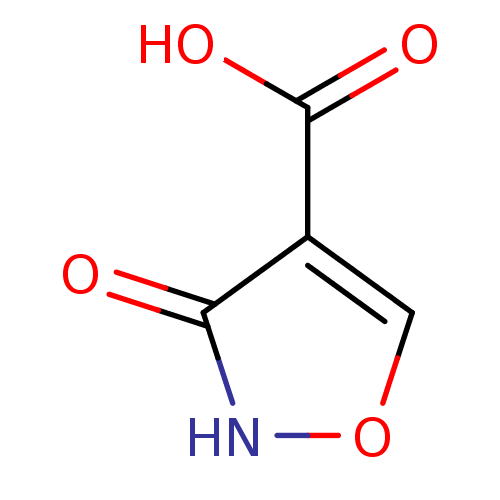
(1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...)Show InChI InChI=1S/C4H3NO4/c6-3-2(4(7)8)1-9-5-3/h1H,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol
| Assay Description
An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... |
J Biol Chem 279: 31429-39 (2004)
Article DOI: 10.1074/jbc.M402433200
BindingDB Entry DOI: 10.7270/Q2CR5RN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603975

(CHEMBL5201780) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603974

(CHEMBL5201904)Show SMILES COc1ccc(cn1)-c1cnc2sc(N[C@@H]3CC[C@H](N)CC3)nn12 |r,wU:15.15,18.19,(-7.26,4.65,;-5.72,4.65,;-4.95,3.32,;-5.72,1.98,;-4.96,.65,;-3.42,.65,;-2.64,1.99,;-3.42,3.32,;-2.65,-.68,;-3.1,-2.13,;-1.86,-3.05,;-.65,-2.17,;.9,-2.18,;1.42,-.69,;2.91,-.3,;4,-1.38,;3.6,-2.87,;4.69,-3.96,;6.17,-3.56,;7.26,-4.65,;6.57,-2.07,;5.48,-.99,;.2,.22,;-1.11,-.68,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086051
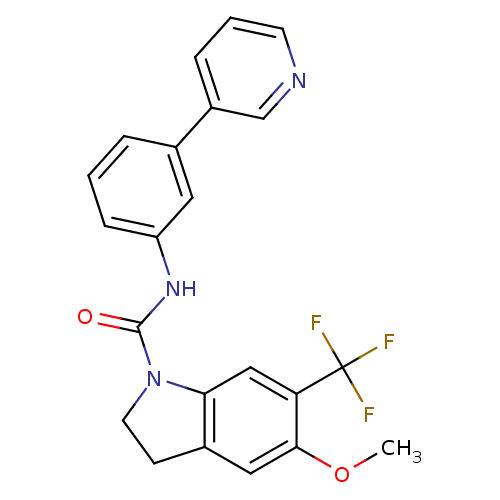
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3cccc(c3)-c3cccnc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H18F3N3O2/c1-30-20-11-15-7-9-28(19(15)12-18(20)22(23,24)25)21(29)27-17-6-2-4-14(10-17)16-5-3-8-26-13-16/h2-6,8,10-13H,7,9H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591317
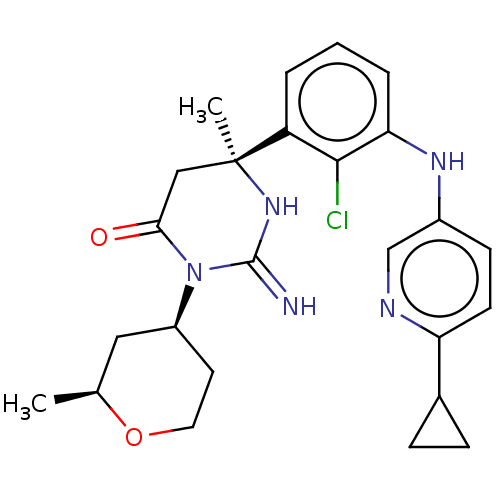
(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591318

(CHEMBL5204196)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2cnc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086062
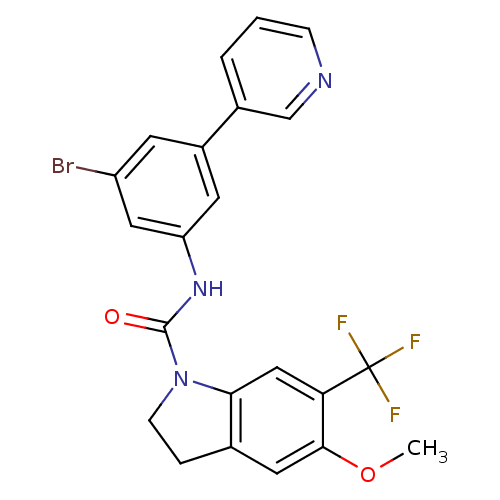
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3cc(Br)cc(c3)-c3cccnc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H17BrF3N3O2/c1-31-20-9-13-4-6-29(19(13)11-18(20)22(24,25)26)21(30)28-17-8-15(7-16(23)10-17)14-3-2-5-27-12-14/h2-3,5,7-12H,4,6H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50396033
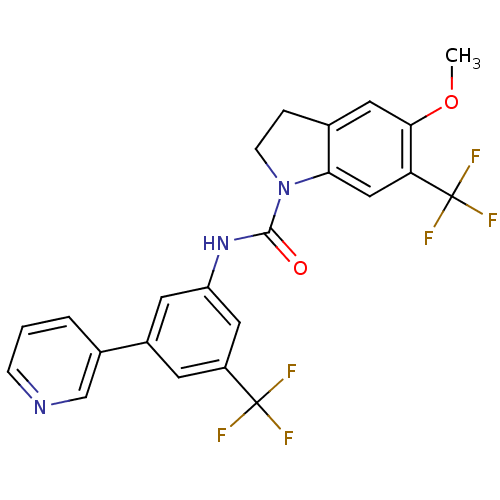
(CHEMBL2169982)Show SMILES COc1cc2CCN(C(=O)Nc3cc(cc(c3)C(F)(F)F)-c3cccnc3)c2cc1C(F)(F)F Show InChI InChI=1S/C23H17F6N3O2/c1-34-20-9-13-4-6-32(19(13)11-18(20)23(27,28)29)21(33)31-17-8-15(14-3-2-5-30-12-14)7-16(10-17)22(24,25)26/h2-3,5,7-12H,4,6H2,1H3,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086056
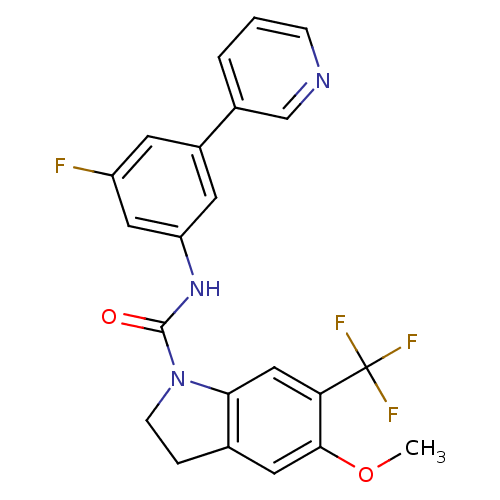
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3cc(F)cc(c3)-c3cccnc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H17F4N3O2/c1-31-20-9-13-4-6-29(19(13)11-18(20)22(24,25)26)21(30)28-17-8-15(7-16(23)10-17)14-3-2-5-27-12-14/h2-3,5,7-12H,4,6H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603992
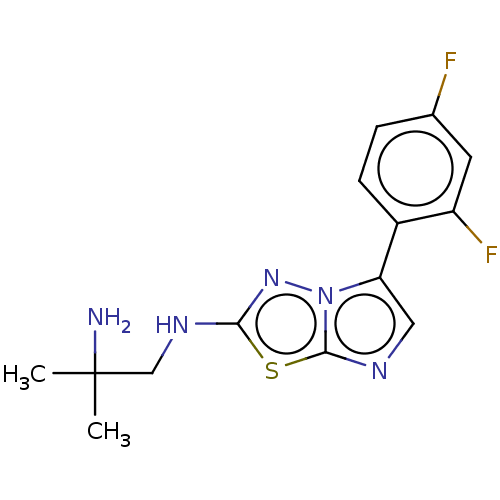
(CHEMBL5179988) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591315
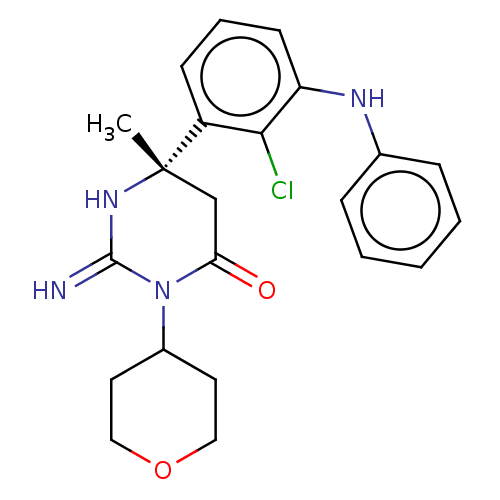
(CHEMBL5172999)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccccc2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591319

(CHEMBL5204856)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(NC(=O)c2ccccc2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603978
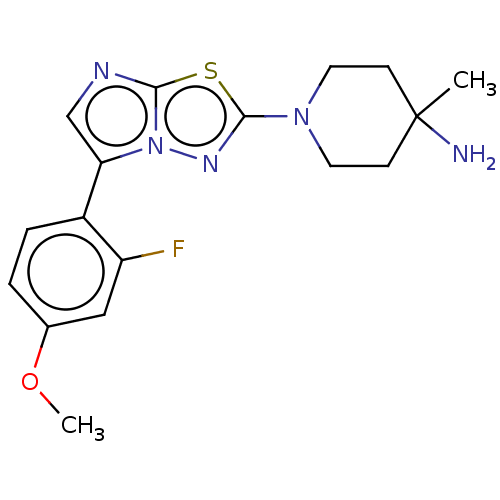
(CHEMBL5187817)Show SMILES COc1ccc(-c2cnc3sc(nn23)N2CCC(C)(N)CC2)c(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Plasmepsin X
(Plasmodium falciparum (isolate 3D7)) | BDBM50591316

(CHEMBL5208335)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603989
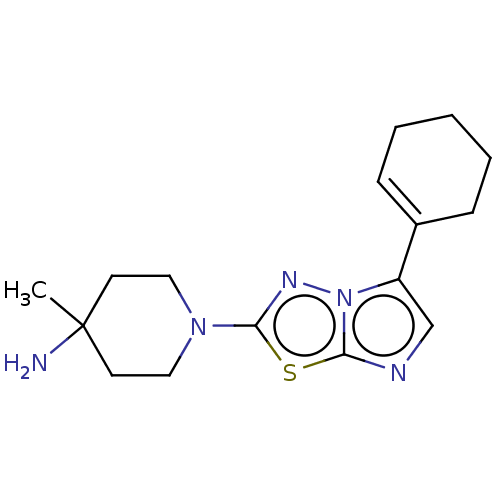
(CHEMBL5192443)Show SMILES CC1(N)CCN(CC1)c1nn2c(cnc2s1)C1=CCCCC1 |t:19| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50396034

(CHEMBL586213 | TCMDC-139046)Show SMILES COc1cc2CCN(C(=O)Nc3cc(OC(F)(F)F)cc(c3)-c3cccnc3)c2cc1C(F)(F)F Show InChI InChI=1S/C23H17F6N3O3/c1-34-20-9-13-4-6-32(19(13)11-18(20)22(24,25)26)21(33)31-16-7-15(14-3-2-5-30-12-14)8-17(10-16)35-23(27,28)29/h2-3,5,7-12H,4,6H2,1H3,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50591315

(CHEMBL5172999)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccccc2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50591317

(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603986

(CHEMBL5187610) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50379156

(CHEMBL2012958 | Genz-669178)Show InChI InChI=1S/C17H14N4OS/c1-10-19-16-11(9-18)3-2-4-13(16)21(10)15-8-7-14(23-15)17(22)20-12-5-6-12/h2-4,7-8,12H,5-6H2,1H3,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health
| Assay Description
Substrate-dependent inhibition of recombinant PfDHODH protein was assessed in an in vitro assay in 384-well clear plates (Corning 3702) as described ... |
J Biol Chem 289: 17980-95 (2014)
Article DOI: 10.1074/jbc.M114.558353
BindingDB Entry DOI: 10.7270/Q27P8X8G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536191

(CHEMBL4584780)Show SMILES C1CN(CCO1)C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H26N6O/c1-2-19(17-25-7-1)21-16-22(27-23(26-21)18-3-8-24-9-4-18)29-10-5-20(6-11-29)28-12-14-30-15-13-28/h1-4,7-9,16-17,20H,5-6,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536193
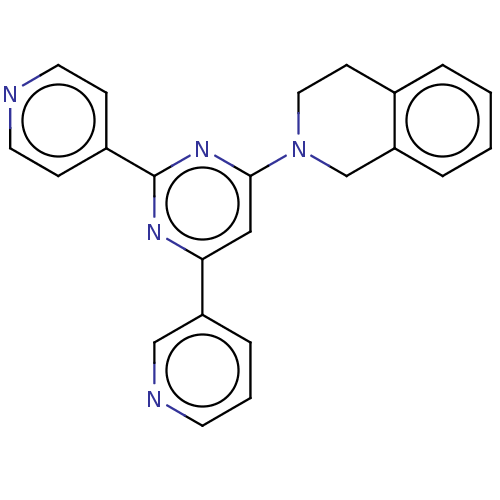
(CHEMBL548646 | GNF-Pf-1447 | TCMDC-125419)Show SMILES C1Cc2ccccc2CN1c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H19N5/c1-2-5-20-16-28(13-9-17(20)4-1)22-14-21(19-6-3-10-25-15-19)26-23(27-22)18-7-11-24-12-8-18/h1-8,10-12,14-15H,9,13,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536194

(CHEMBL4569641)Show SMILES C(C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C24H28N6O/c1-2-21(17-26-7-1)22-16-23(28-24(27-22)20-3-8-25-9-4-20)30-10-5-19(6-11-30)18-29-12-14-31-15-13-29/h1-4,7-9,16-17,19H,5-6,10-15,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50396038

(CHEMBL2169978)Show SMILES COc1ccc2CCN(C(=O)Nc3cc(OC(F)(F)F)cc(c3)-c3cccnc3)c2c1 Show InChI InChI=1S/C22H18F3N3O3/c1-30-18-5-4-14-6-8-28(20(14)12-18)21(29)27-17-9-16(15-3-2-7-26-13-15)10-19(11-17)31-22(23,24)25/h2-5,7,9-13H,6,8H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603988
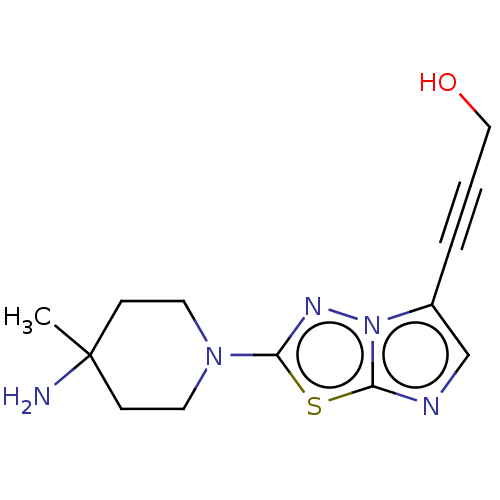
(CHEMBL5170360) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Plasmepsin IX
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin IX
(Plasmodium falciparum (isolate 3D7)) | BDBM50591320

(CHEMBL5190700)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(NC(=O)c2cccc(c2)C#N)c1Cl |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50396037

(CHEMBL2169979)Show SMILES OCc1ccc2CCN(C(=O)Nc3cc(OC(F)(F)F)cc(c3)-c3cccnc3)c2c1 Show InChI InChI=1S/C22H18F3N3O3/c23-22(24,25)31-19-10-17(16-2-1-6-26-12-16)9-18(11-19)27-21(30)28-7-5-15-4-3-14(13-29)8-20(15)28/h1-4,6,8-12,29H,5,7,13H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50591317

(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603984

(CHEMBL5195201)Show SMILES CC1(N)CCN(CC1)c1nn2c(cnc2s1)-c1cccc2cc[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50591318

(CHEMBL5204196)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2cnc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Plasmepsin IX
(Plasmodium falciparum (isolate 3D7)) | BDBM50591317

(CHEMBL5191783)Show SMILES C[C@H]1C[C@H](CCO1)N1C(=O)C[C@](C)(NC1=N)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM28817

(5-methyl-N-[4-(trifluoromethyl)phenyl]-[1,2,4]tria...)Show InChI InChI=1S/C13H10F3N5/c1-8-6-11(21-12(19-8)17-7-18-21)20-10-4-2-9(3-5-10)13(14,15)16/h2-7,20H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health
| Assay Description
Substrate-dependent inhibition of recombinant PfDHODH protein was assessed in an in vitro assay in 384-well clear plates (Corning 3702) as described ... |
J Biol Chem 289: 17980-95 (2014)
Article DOI: 10.1074/jbc.M114.558353
BindingDB Entry DOI: 10.7270/Q27P8X8G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50396032
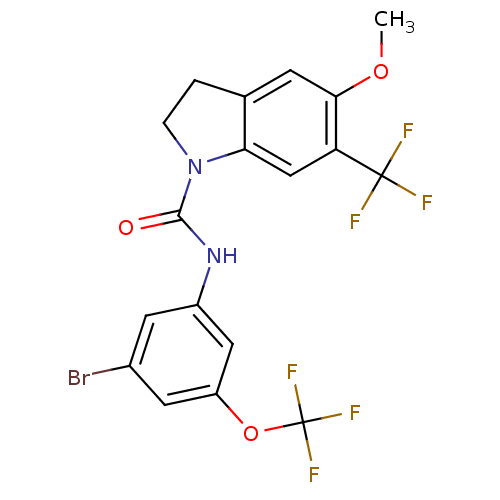
(CHEMBL2169985)Show SMILES COc1cc2CCN(C(=O)Nc3cc(Br)cc(OC(F)(F)F)c3)c2cc1C(F)(F)F Show InChI InChI=1S/C18H13BrF6N2O3/c1-29-15-4-9-2-3-27(14(9)8-13(15)17(20,21)22)16(28)26-11-5-10(19)6-12(7-11)30-18(23,24)25/h4-8H,2-3H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50396030

(CHEMBL2169991)Show SMILES Fc1ccc2CCN(C(=O)Nc3cc(OC(F)(F)F)cc(c3)-c3cccnc3)c2c1 Show InChI InChI=1S/C21H15F4N3O2/c22-16-4-3-13-5-7-28(19(13)10-16)20(29)27-17-8-15(14-2-1-6-26-12-14)9-18(11-17)30-21(23,24)25/h1-4,6,8-12H,5,7H2,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603985
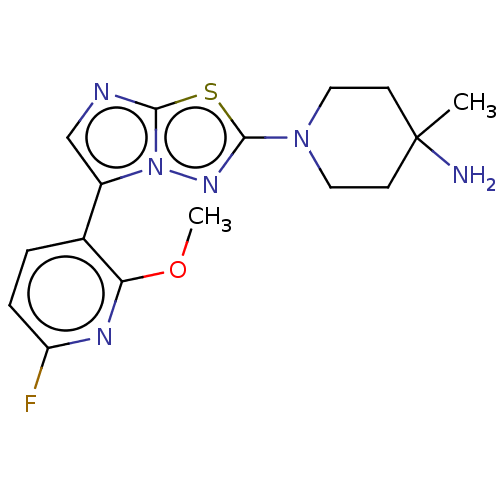
(CHEMBL5207627) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50536194

(CHEMBL4569641)Show SMILES C(C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C24H28N6O/c1-2-21(17-26-7-1)22-16-23(28-24(27-22)20-3-8-25-9-4-20)30-10-5-19(6-11-30)18-29-12-14-31-15-13-29/h1-4,7-9,16-17,19H,5-6,10-15,18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(a... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50536191

(CHEMBL4584780)Show SMILES C1CN(CCO1)C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H26N6O/c1-2-19(17-25-7-1)21-16-22(27-23(26-21)18-3-8-24-9-4-18)29-10-5-20(6-11-29)28-12-14-30-15-13-28/h1-4,7-9,16-17,20H,5-6,10-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(a... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603979
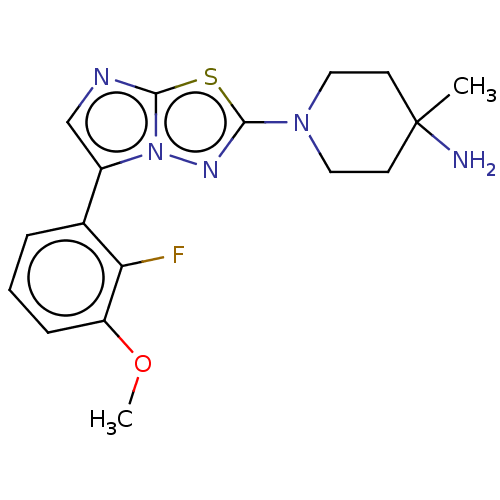
(CHEMBL5204715) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50591316

(CHEMBL5208335)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50591316

(CHEMBL5208335)Show SMILES C[C@]1(CC(=O)N(C2CCOCC2)C(=N)N1)c1cccc(Nc2ccc(nc2)C2CC2)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01336
BindingDB Entry DOI: 10.7270/Q2988C16 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50396031
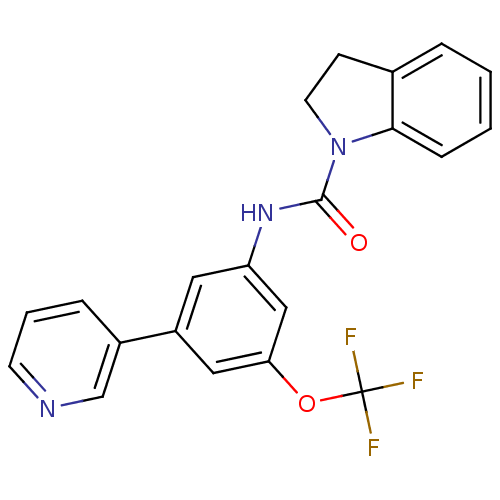
(CHEMBL2169990)Show SMILES FC(F)(F)Oc1cc(NC(=O)N2CCc3ccccc23)cc(c1)-c1cccnc1 Show InChI InChI=1S/C21H16F3N3O2/c22-21(23,24)29-18-11-16(15-5-3-8-25-13-15)10-17(12-18)26-20(28)27-9-7-14-4-1-2-6-19(14)27/h1-6,8,10-13H,7,9H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... |
ACS Med Chem Lett 3: 373-377 (2012)
Article DOI: 10.1021/ml300008j
BindingDB Entry DOI: 10.7270/Q2PZ59X6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603976

(CHEMBL5200247) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603983

(CHEMBL5181699) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM231630
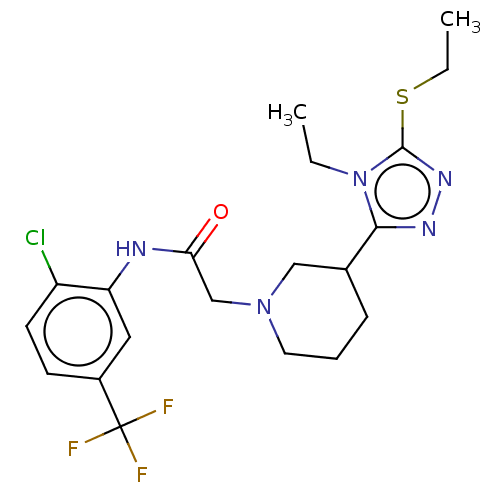
(GSK3)Show SMILES CCSc1nnc(C2CCCN(CC(=O)Nc3cc(ccc3Cl)C(F)(F)F)C2)n1CC Show InChI InChI=1S/C20H25ClF3N5OS/c1-3-29-18(26-27-19(29)31-4-2)13-6-5-9-28(11-13)12-17(30)25-16-10-14(20(22,23)24)7-8-15(16)21/h7-8,10,13H,3-6,9,11-12H2,1-2H3,(H,25,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard School of Public Health
| Assay Description
Substrate-dependent inhibition of recombinant PfDHODH protein was assessed in an in vitro assay in 384-well clear plates (Corning 3702) as described ... |
J Biol Chem 289: 17980-95 (2014)
Article DOI: 10.1074/jbc.M114.558353
BindingDB Entry DOI: 10.7270/Q27P8X8G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data