

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16314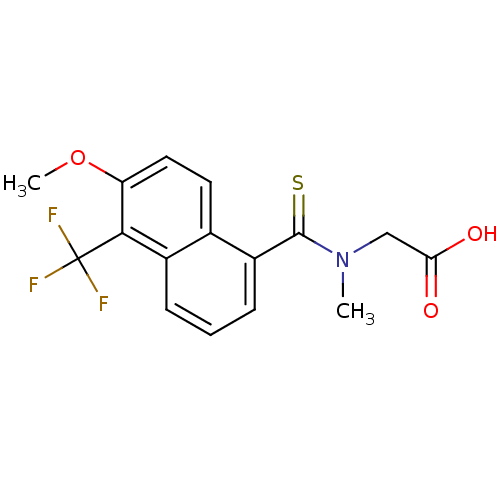 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16313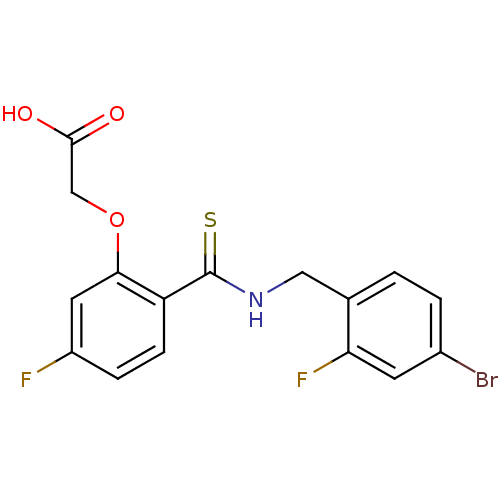 (2-(2-{[(4-bromo-2-fluorophenyl)methyl]carbamothioy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16241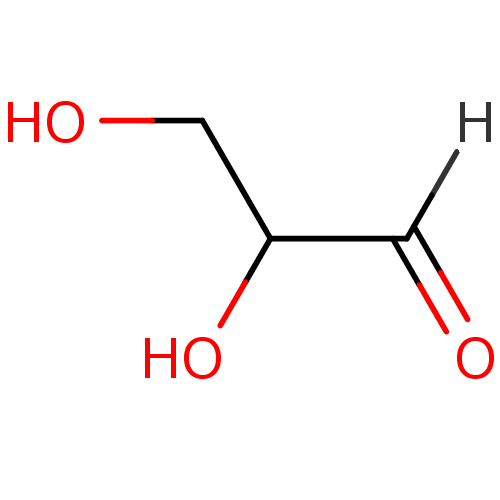 (2,3-dihydroxypropanal | D,L-glyceraldehyde | Glyce...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of glyceraldehyde reduction activity of human AKR1B1 | Proc Natl Acad Sci USA 104: 20764-9 (2007) Article DOI: 10.1073/pnas.0705659105 BindingDB Entry DOI: 10.7270/Q2H132VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase assessed as inhibition of biotin-dUTP incorporation into template | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00268 BindingDB Entry DOI: 10.7270/Q2X06BW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of retinaldehyde reductase activity of human AKR1B10 | Proc Natl Acad Sci USA 104: 20764-9 (2007) Article DOI: 10.1073/pnas.0705659105 BindingDB Entry DOI: 10.7270/Q2H132VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439246 (CHEMBL2418830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439247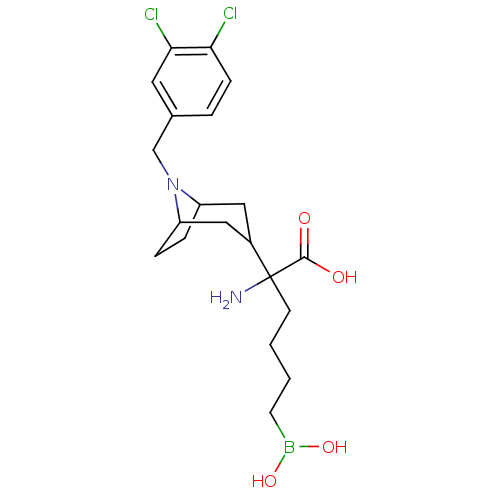 (CHEMBL2418831) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439245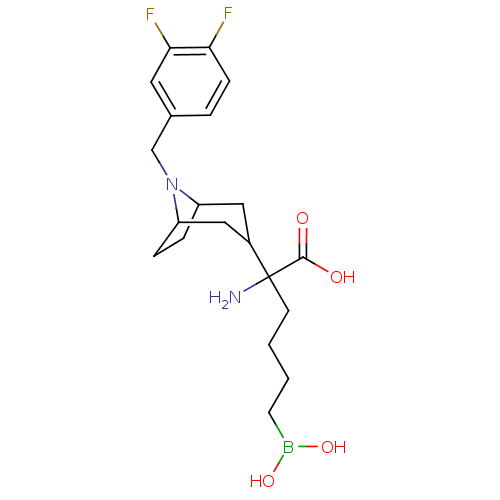 (CHEMBL2418991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439247 (CHEMBL2418831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439244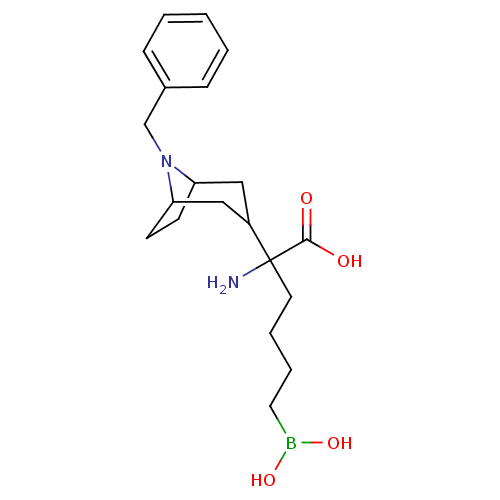 (CHEMBL2418829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM200221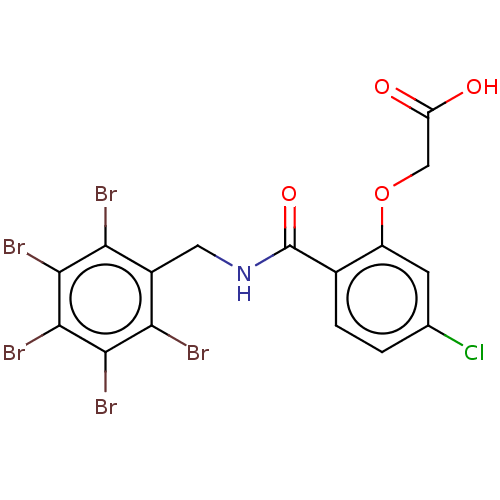 (2-(5-chloro-2-(((perbromophenyl)methyl)carbamoyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439246 (CHEMBL2418830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439245 (CHEMBL2418991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50520394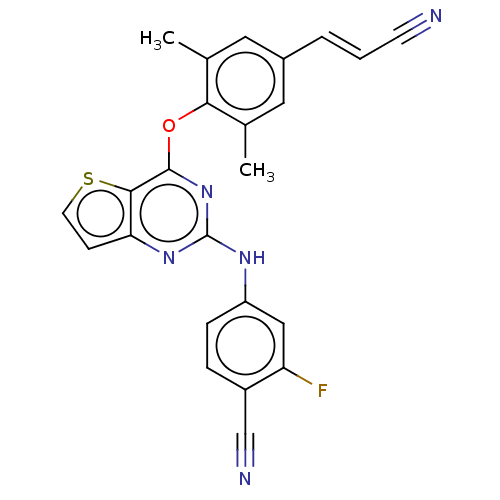 (CHEMBL4442726) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation in to cDNA chain incubated for 1 hr by ELISA | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam substrate in presence of NADPH incubated for 10 mins | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439244 (CHEMBL2418829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439243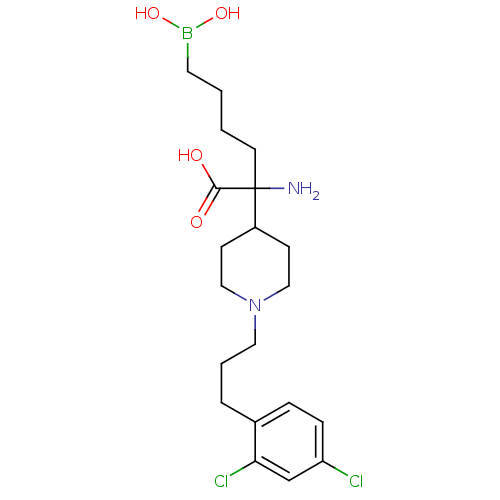 (CHEMBL2418998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50520395 (CHEMBL4526117) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation in to cDNA chain incubated for 1 hr by ELISA | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427899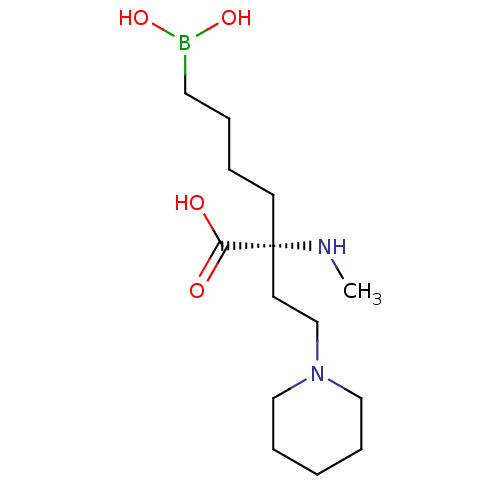 (CHEMBL2326090) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452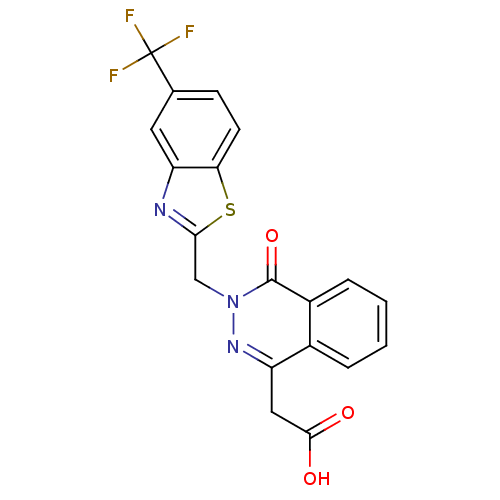 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50427899 (CHEMBL2326090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant fully active truncated form of arginase 2 overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439241 (CHEMBL2418999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50029207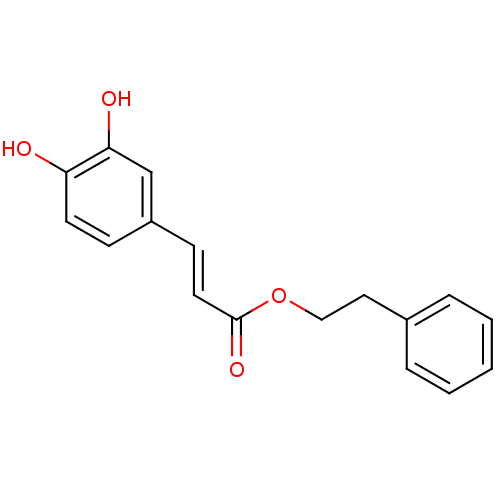 ((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 [K125R,V301L] (Homo sapiens (Human)) | BDBM200221 (2-(5-chloro-2-(((perbromophenyl)methyl)carbamoyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM200221 (2-(5-chloro-2-(((perbromophenyl)methyl)carbamoyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439242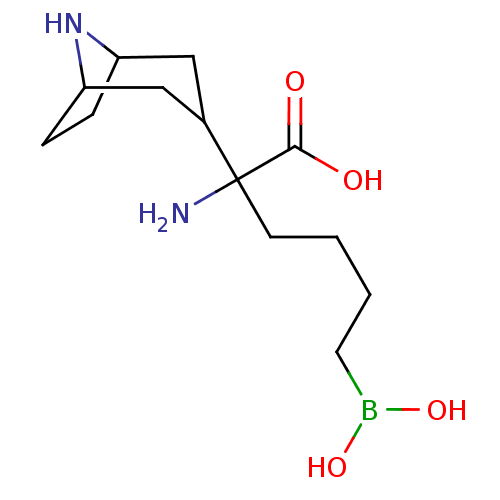 (CHEMBL2418828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439242 (CHEMBL2418828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50575722 (CHEMBL4875952) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase assessed as inhibition of biotin-dUTP incorporation into template | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00268 BindingDB Entry DOI: 10.7270/Q2X06BW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439243 (CHEMBL2418998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427903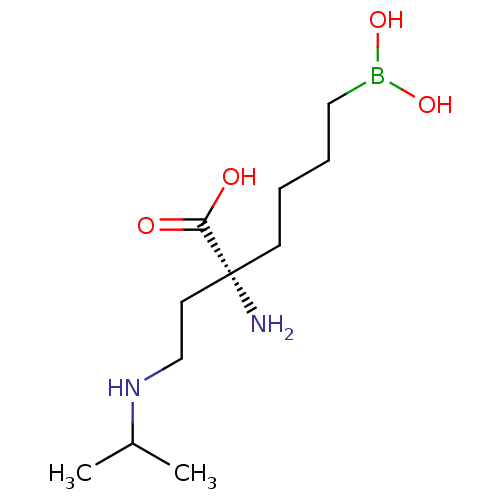 (CHEMBL2326087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50575725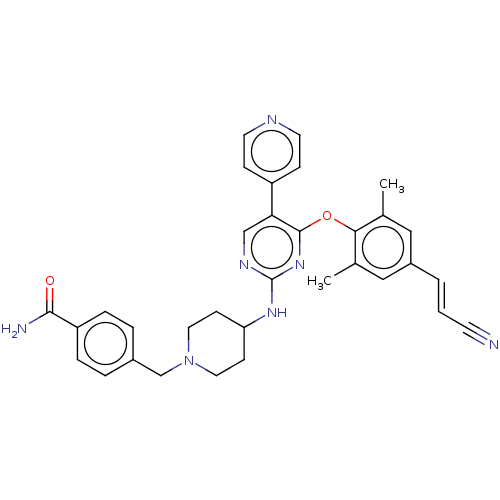 (CHEMBL4858653) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase assessed as inhibition of biotin-dUTP incorporation into template | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00268 BindingDB Entry DOI: 10.7270/Q2X06BW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50575723 (CHEMBL4873054) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase assessed as inhibition of biotin-dUTP incorporation into template | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00268 BindingDB Entry DOI: 10.7270/Q2X06BW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439241 (CHEMBL2418999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50501152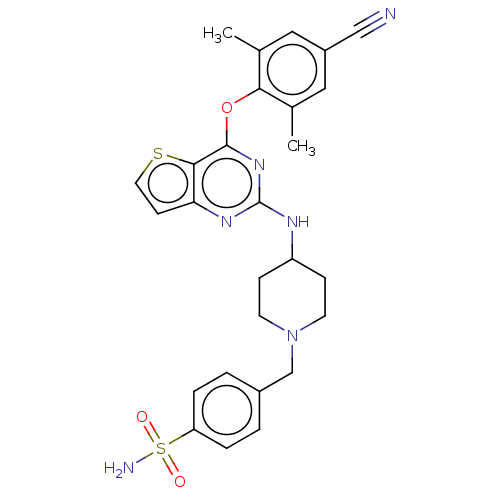 (CHEMBL3929927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in channel tail current by whole cell patch clamp assay | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975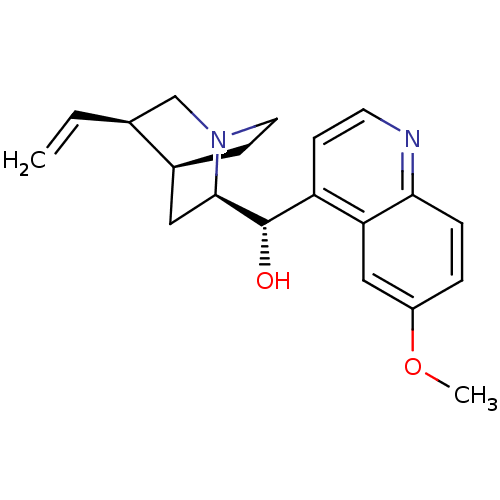 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan substrate in presence of NADPH incubated for 10 mins | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427904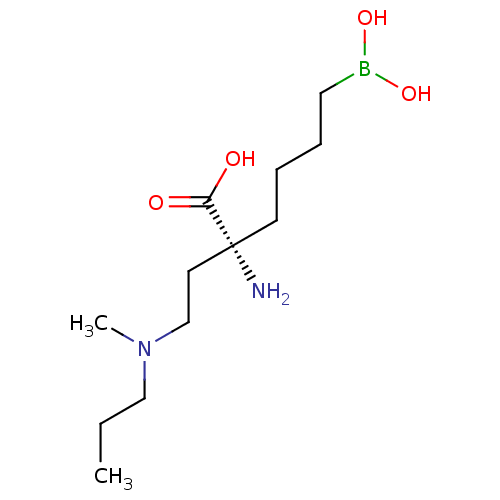 (CHEMBL2326086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50575724 (CHEMBL4855106) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase assessed as inhibition of biotin-dUTP incorporation into template | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00268 BindingDB Entry DOI: 10.7270/Q2X06BW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427911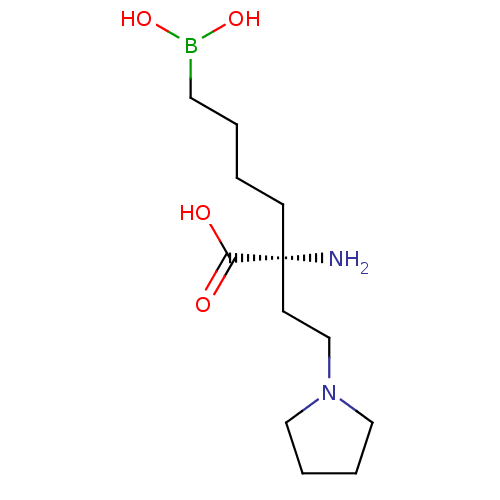 (CHEMBL2326095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439240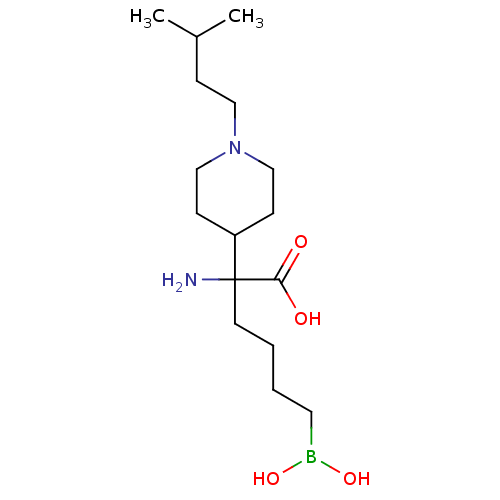 (CHEMBL2418993) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50520396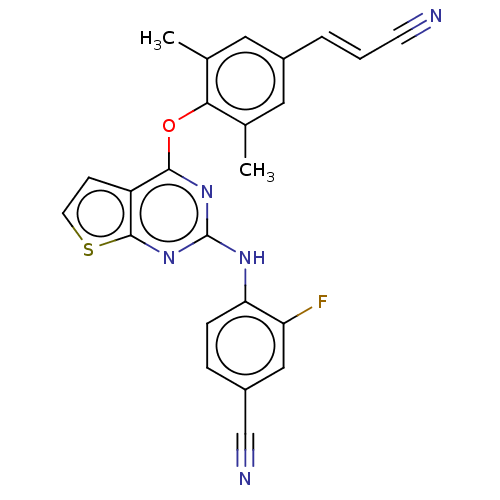 (CHEMBL4520763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in channel tail current by whole cell patch clamp assay | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434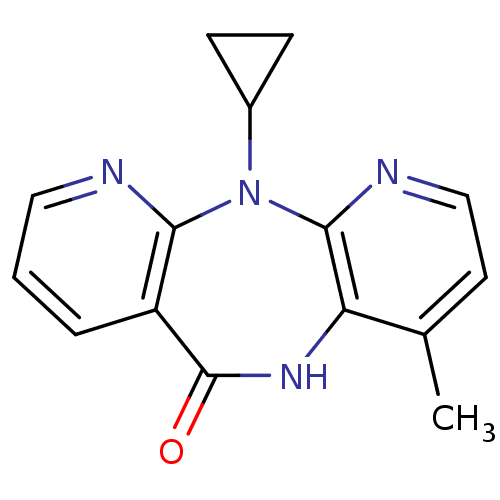 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation in to cDNA chain incubated for 1 hr by ELISA | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50427903 (CHEMBL2326087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant fully active truncated form of arginase 2 overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439238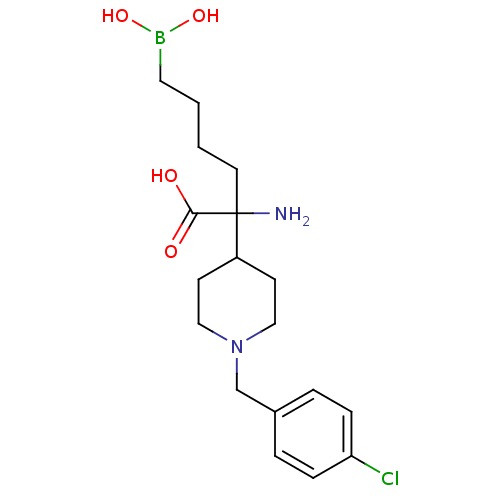 (CHEMBL2418995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01104 BindingDB Entry DOI: 10.7270/Q2KP8660 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439239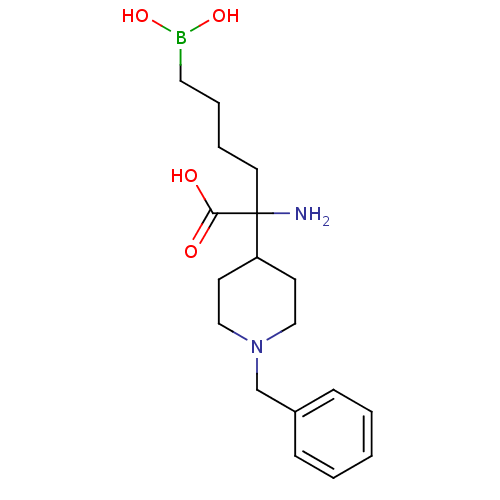 (CHEMBL2418994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439237 (CHEMBL2418996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439236 (CHEMBL2418997) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427909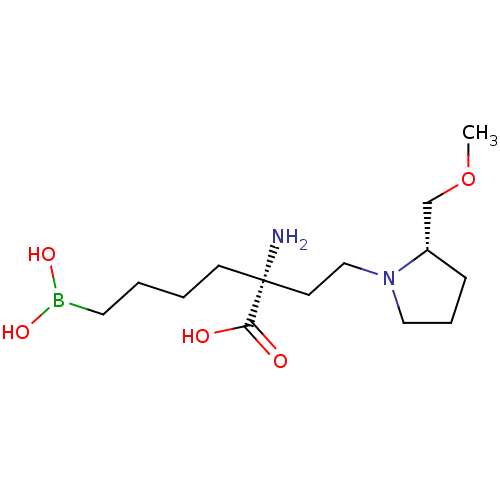 (CHEMBL2326097) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323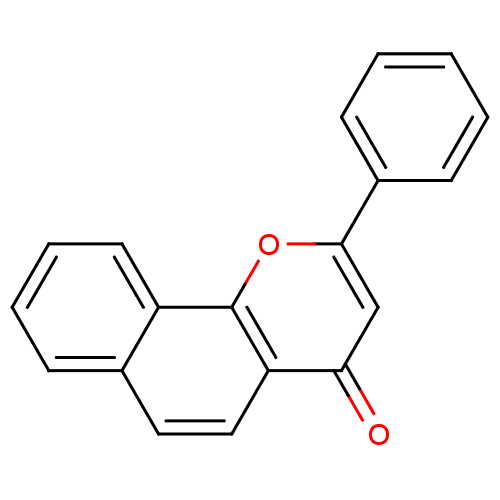 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin substrate in presence of NADPH incubated for 10 mins | J Med Chem 63: 1298-1312 (2020) Article DOI: 10.1021/acs.jmedchem.9b01769 BindingDB Entry DOI: 10.7270/Q2K64NGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 193 total ) | Next | Last >> |