

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479434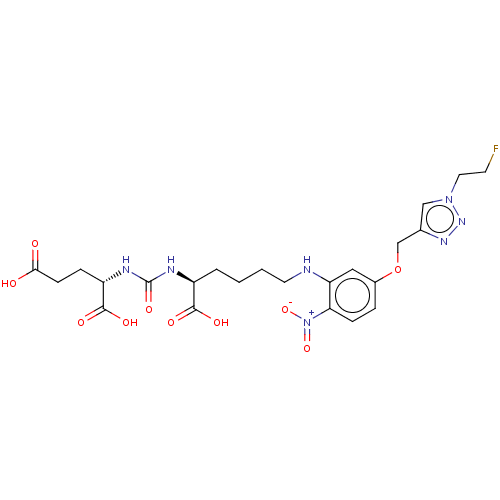 (US10894807, ID P242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50502477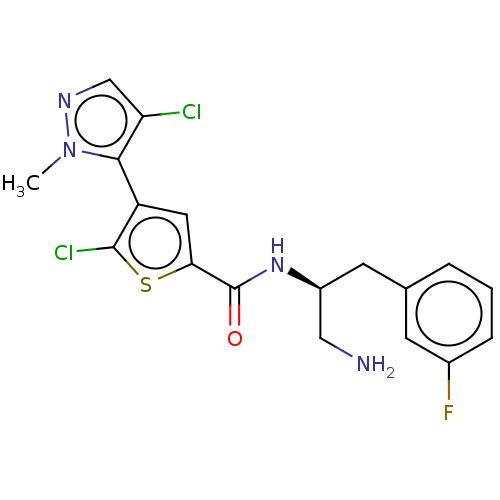 (ASB-183 | ASB183 | Afuresertib | GSK-2110183C | GS...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of Akt1 (unknown origin) | Eur J Med Chem 180: 72-85 (2019) Article DOI: 10.1016/j.ejmech.2019.07.017 BindingDB Entry DOI: 10.7270/Q2Q243H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479437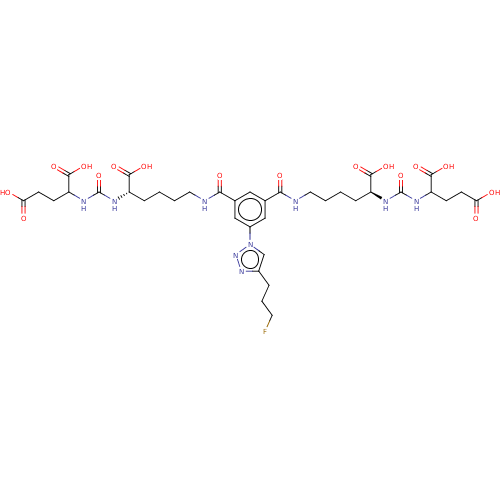 (US10894807, ID P246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355491 (CHEMBL1835870) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of human recombinant FLT3 by radiometric assay | J Med Chem 55: 725-34 (2012) Article DOI: 10.1021/jm201198w BindingDB Entry DOI: 10.7270/Q2GQ6Z6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479444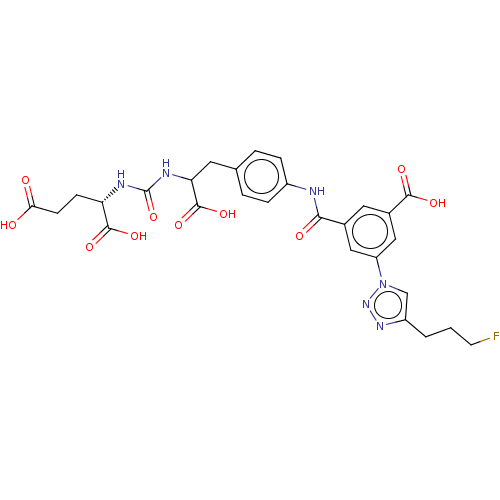 (US10894807, ID P253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479450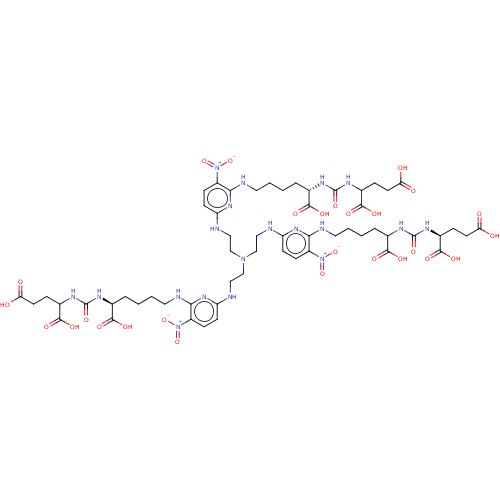 (US10894807, ID P270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479458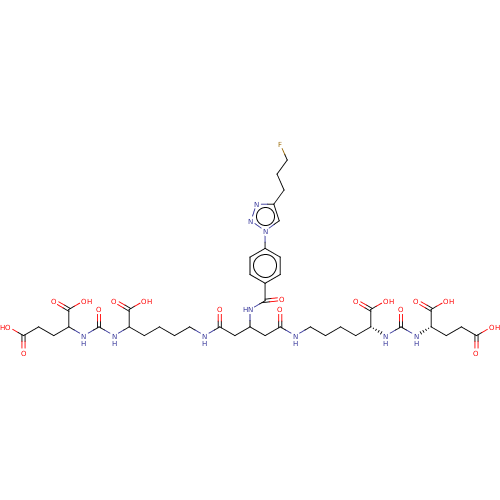 (US10894807, ID P278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479453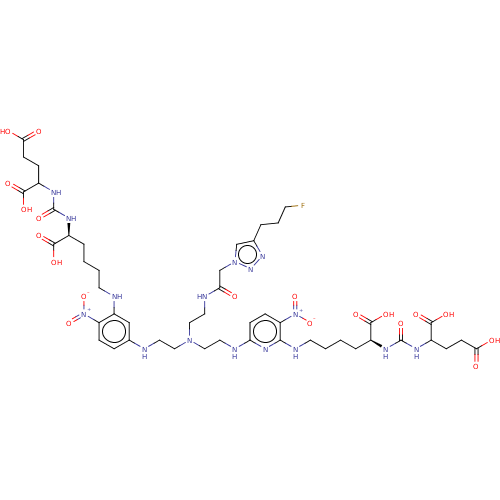 (US10894807, ID P273) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479429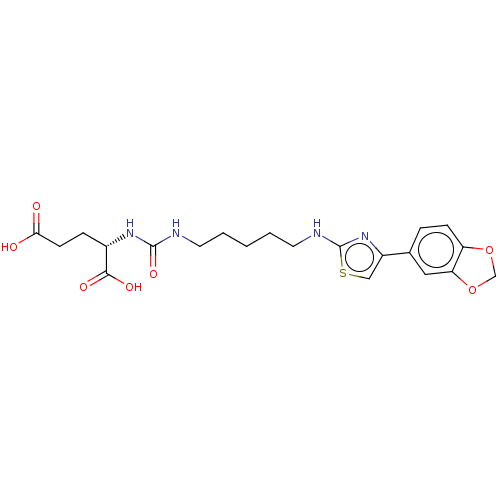 (US10894807, ID P235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479431 (US10894807, ID P238) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479415 (US10894807, ID P200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479449 (US10894807, ID P266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479442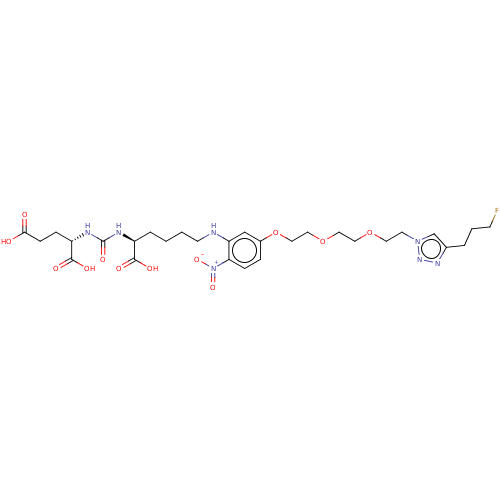 (US10894807, ID P251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479433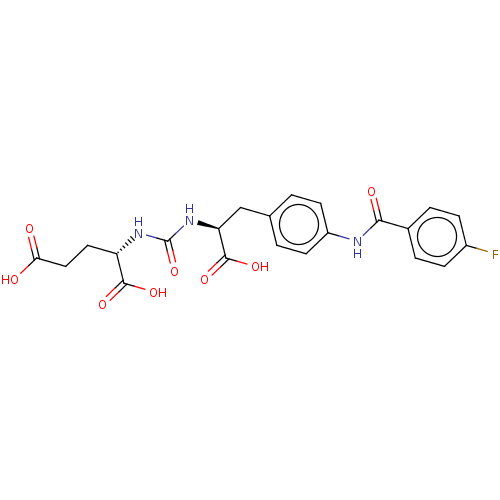 (US10894807, ID P241) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479435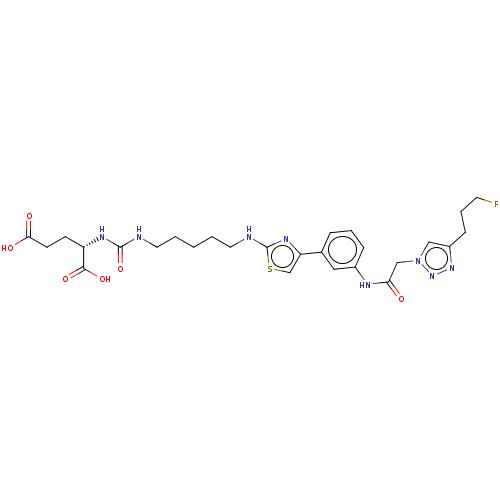 (US10894807, ID P244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479457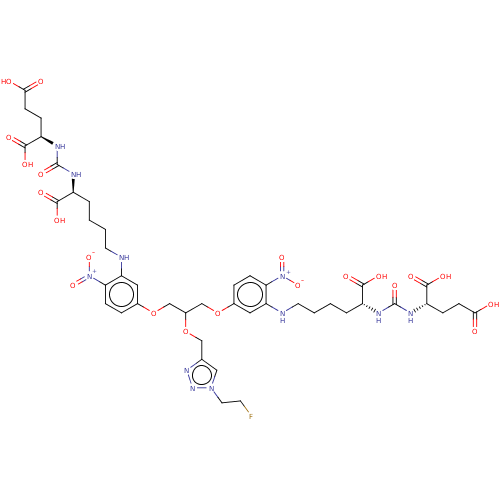 (US10894807, ID P277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479445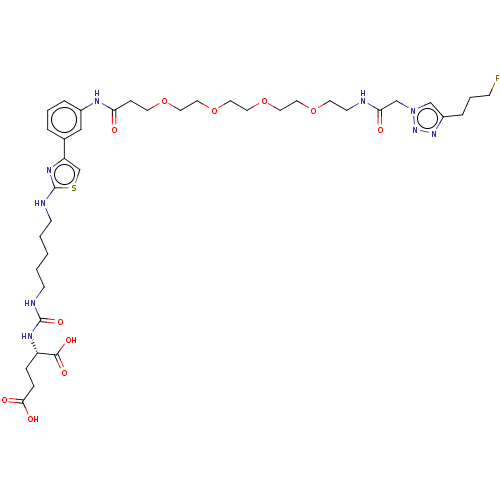 (US10894807, ID P254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396980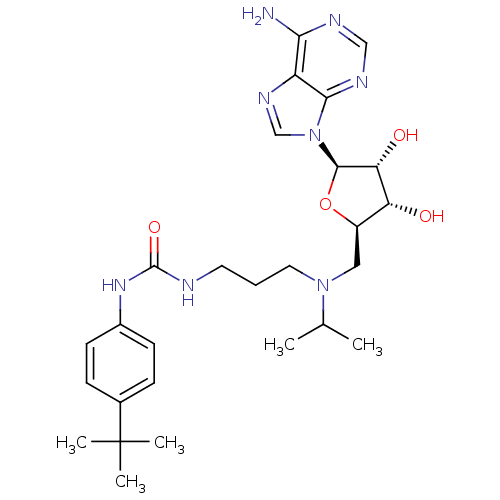 (CHEMBL2171169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter | J Med Chem 55: 8066-74 (2012) Article DOI: 10.1021/jm300917h BindingDB Entry DOI: 10.7270/Q2TD9ZGG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Antagonist activity at 5HT2A receptor | J Med Chem 55: 5749-59 (2012) Article DOI: 10.1021/jm300338m BindingDB Entry DOI: 10.7270/Q2FQ9XQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479464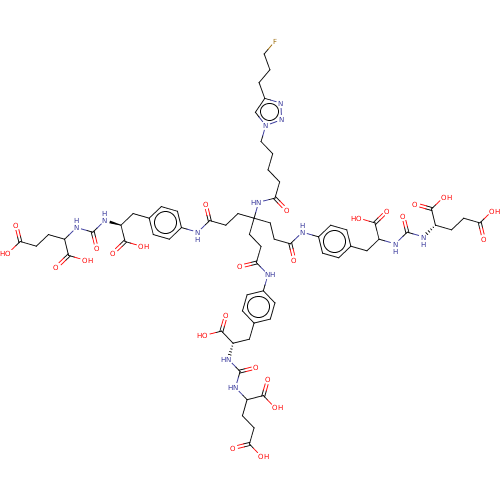 (US10894807, ID P285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479426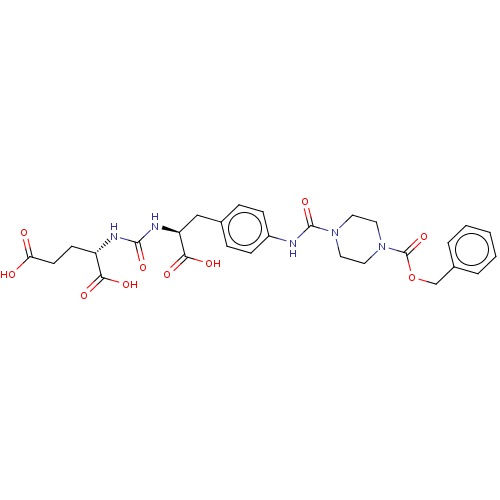 (US10894807, ID P222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479425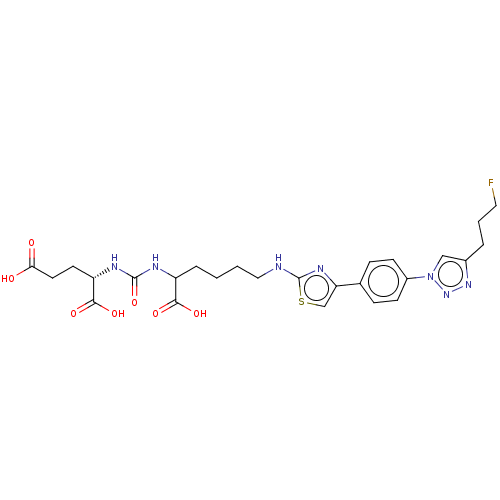 (US10894807, ID P218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479438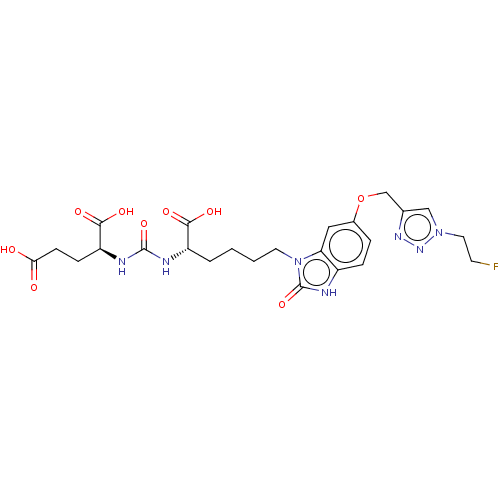 (US10894807, ID P247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479469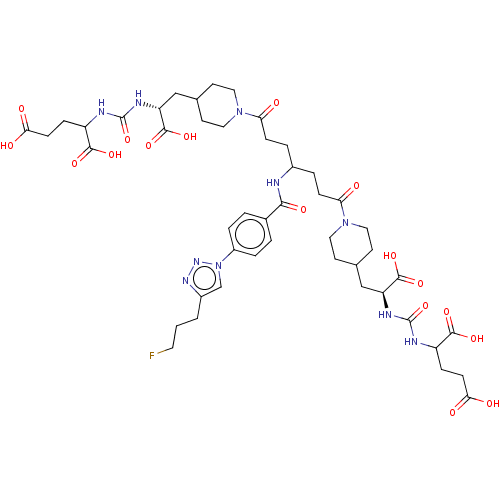 (US10894807, ID P292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396979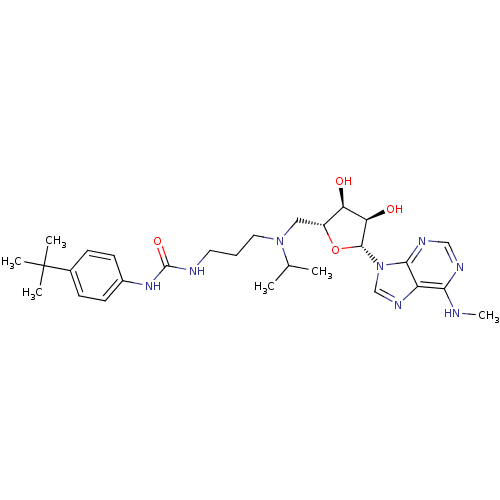 (CHEMBL2171170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter | J Med Chem 55: 8066-74 (2012) Article DOI: 10.1021/jm300917h BindingDB Entry DOI: 10.7270/Q2TD9ZGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479375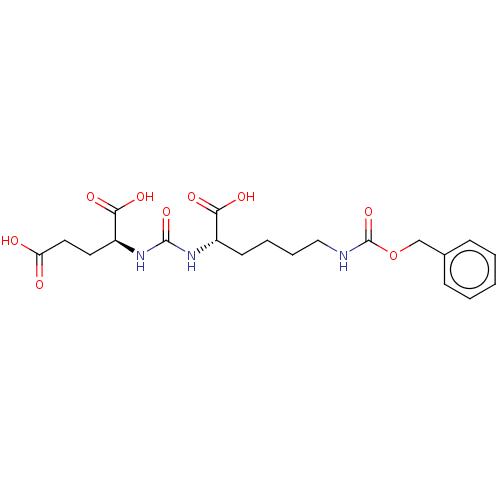 (US10894807, ID P033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479451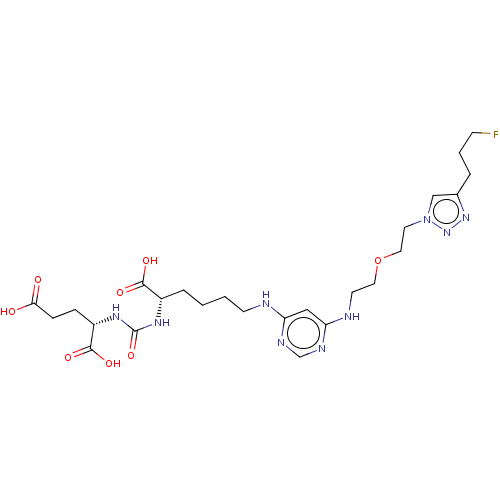 (US10894807, ID P271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479463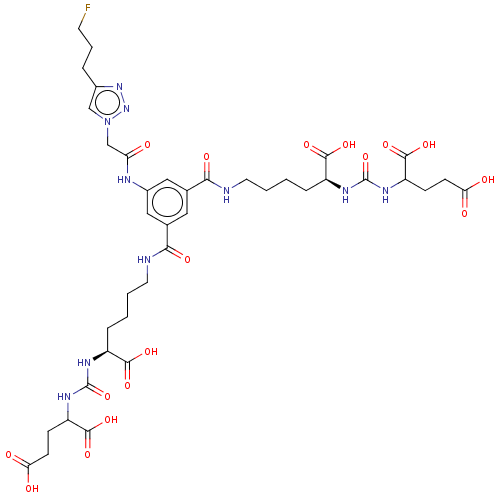 (US10894807, ID P283) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479460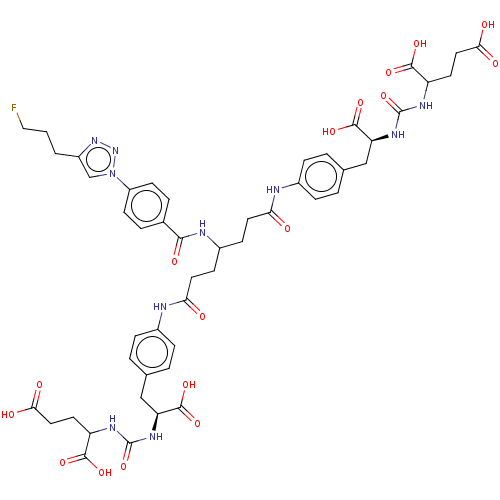 (US10894807, ID P280) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012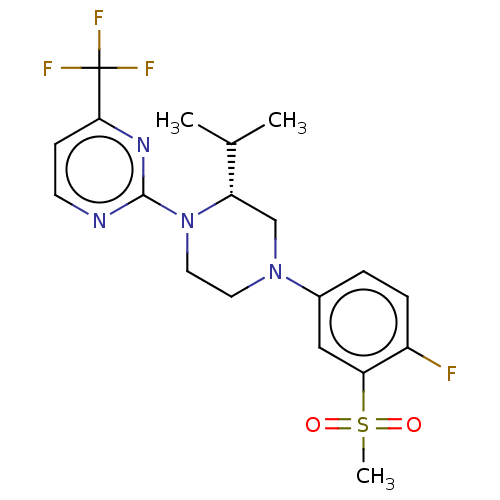 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479466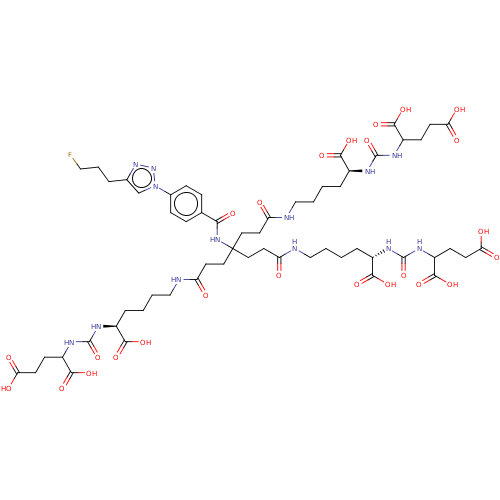 (US10894807, ID P287) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479447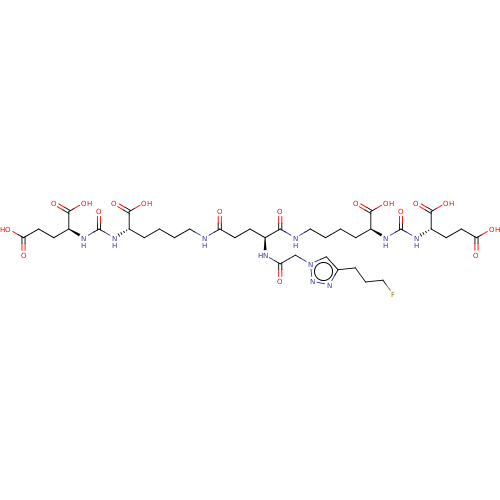 (US10894807, ID P261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355479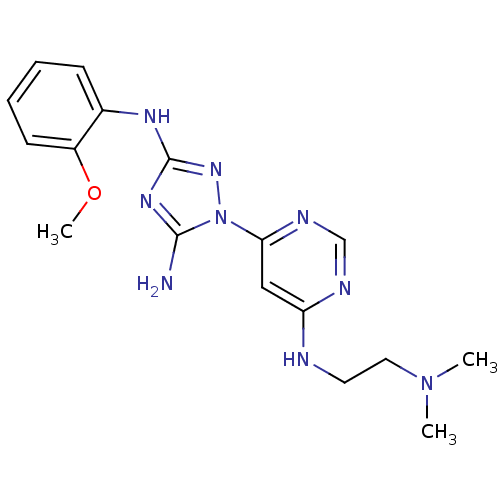 (CHEMBL1835746) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting | J Med Chem 54: 7184-92 (2011) Article DOI: 10.1021/jm200712h BindingDB Entry DOI: 10.7270/Q2DZ08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192752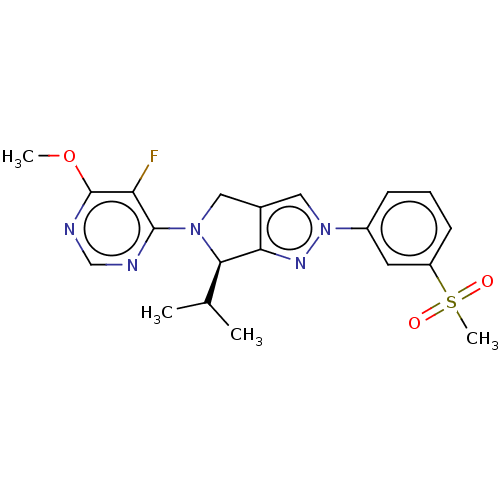 (CHEMBL3905741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177016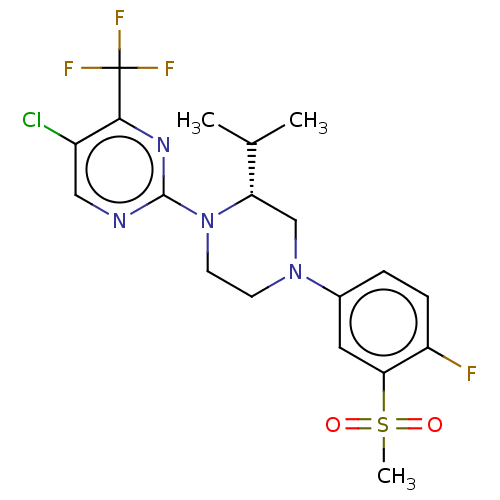 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479427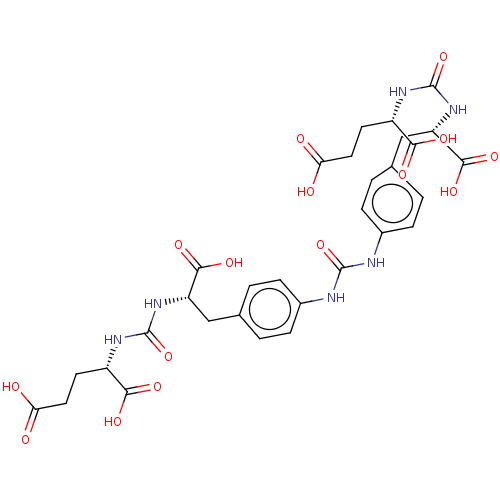 (US10894807, ID P223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479462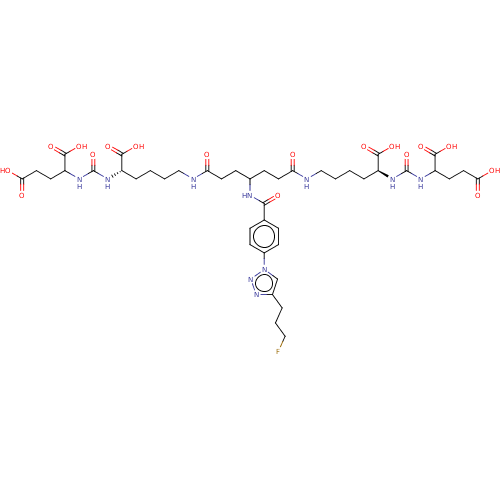 (US10894807, ID P282) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479461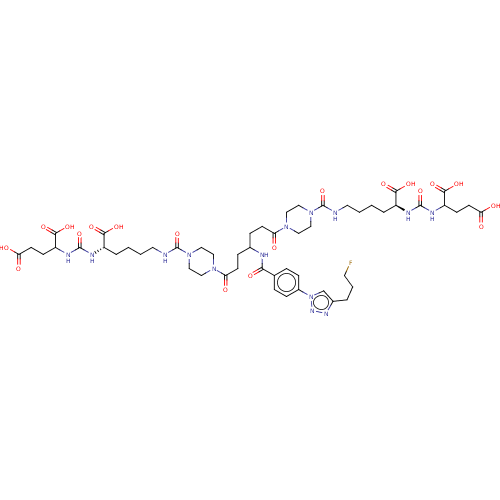 (US10894807, ID P281) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177010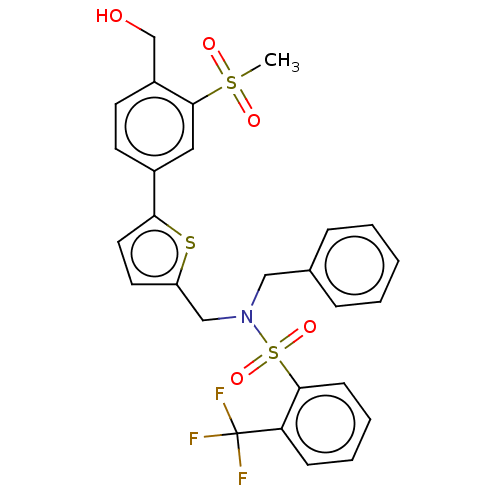 (CHEMBL3814006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479456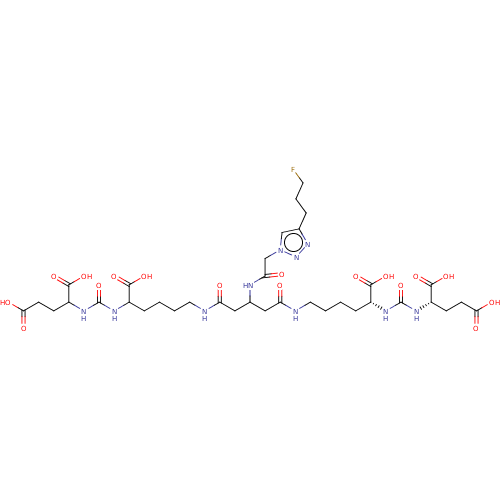 (US10894807, ID P276) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479448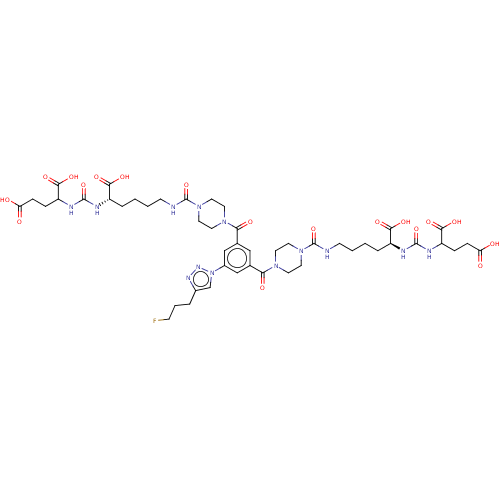 (US10894807, ID P263) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479379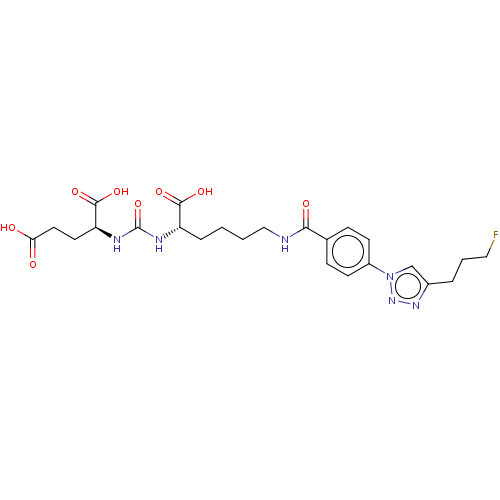 (US10894807, ID P054) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479459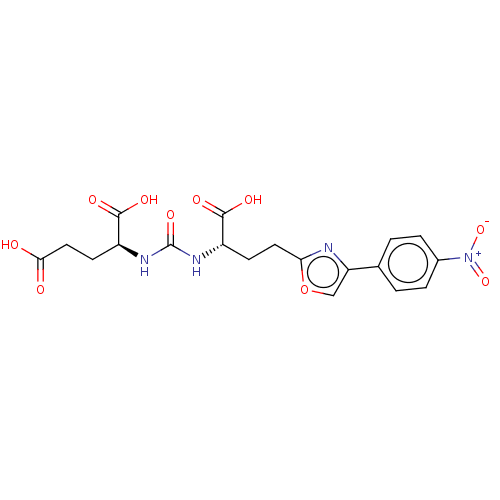 (US10894807, ID P279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35657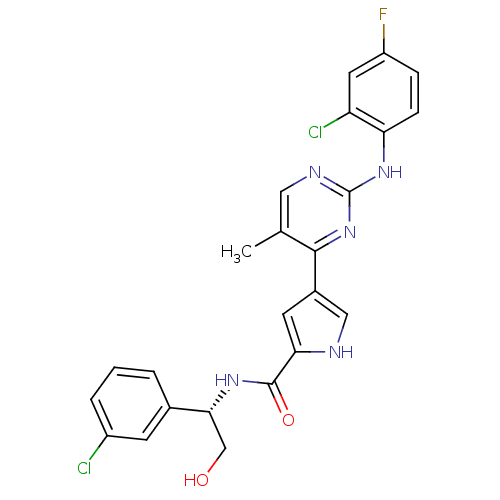 (pyrimidylpyrrole, 11e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35659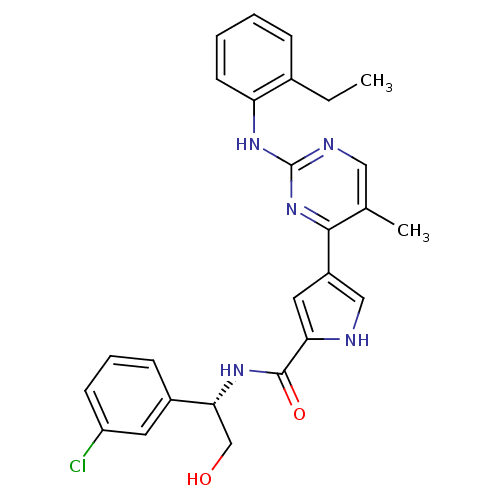 (pyrimidylpyrrole, 11g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM314618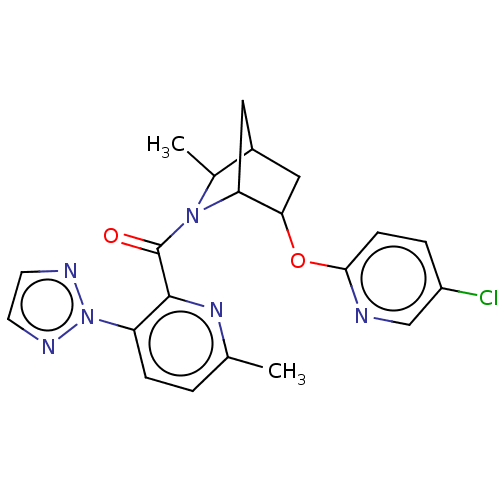 (US9611262, Example 122 | US9611262, Example 196 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description HEK293 stably expressing human orexin 2 receptor (Genebank accession number NM_001526) were grown to confluency in DMEM (Hyclone, cat # SH30022), 10%... | US Patent US9611262 (2017) BindingDB Entry DOI: 10.7270/Q2WS8WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM314681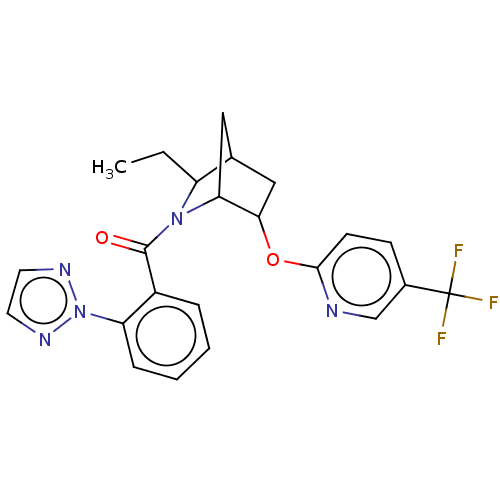 ((R/S)-[2-ethyl-5-[[5-(trifluoromethyl)-2-pyridyl]o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description HEK293 stably expressing human orexin 2 receptor (Genebank accession number NM_001526) were grown to confluency in DMEM (Hyclone, cat # SH30022), 10%... | US Patent US9611262 (2017) BindingDB Entry DOI: 10.7270/Q2WS8WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35660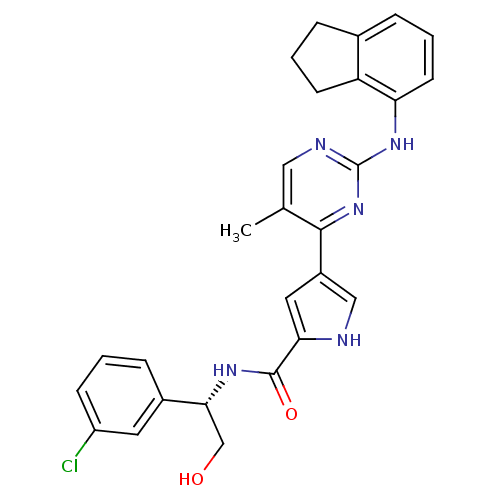 (pyrimidylpyrrole, 11h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35662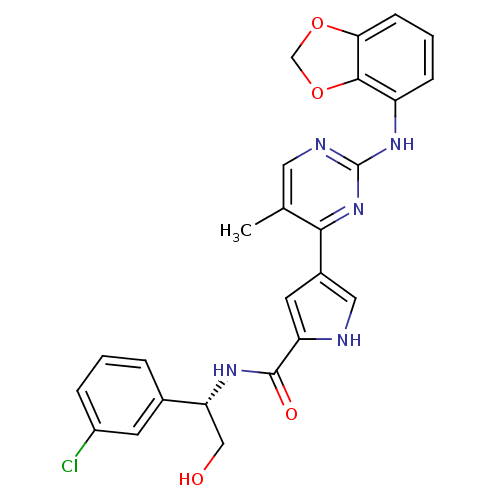 (pyrimidylpyrrole, 11j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35663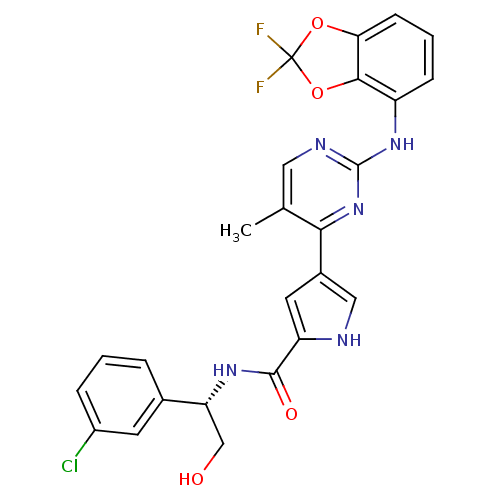 (pyrimidylpyrrole, 11k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8506 total ) | Next | Last >> |