 Found 13051 hits with Last Name = 'lin' and Initial = 'g'
Found 13051 hits with Last Name = 'lin' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50131251
((S)-5-Guanidino-2-{(S)-2-[(S)-3-(3H-imidazol-4-yl)...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CCCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C42H51N11O5/c43-38(55)34(22-29-24-48-32-17-8-7-16-31(29)32)52-39(56)33(18-10-20-47-42(44)45)51-40(57)35(21-28-13-5-2-6-14-28)53-41(58)36(23-30-25-46-26-49-30)50-37(54)19-9-15-27-11-3-1-4-12-27/h1-8,11-14,16-17,24-26,33-36,48H,9-10,15,18-23H2,(H2,43,55)(H,46,49)(H,50,54)(H,51,57)(H,52,56)(H,53,58)(H4,44,45,47)/t33-,34-,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Binding affinity towards human Melanocortin 1 receptor (hMC1R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50388434
(CHEMBL605525)Show SMILES [#6]-[#6@@H](-[#6]-[#6]-[#6]C([#6])([#6])[#8])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)22-12-13-23-21(9-7-15-27(22,23)5)11-10-20-16-24(28)19(2)25(29)17-20/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b21-11+/t18-,22+,23-,24+,25+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417515
(CHEMBL1630755)Show SMILES [#6]-[#6@@H](-[#8])-[#6]-[#6]-[#6]-[#6@H](-[#6])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C26H42O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-18,22-25,27-29H,3,5-9,12-16H2,1-2,4H3/b21-11+/t17-,18+,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50033535
(CHEMBL331883 | N-[(3R,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417519
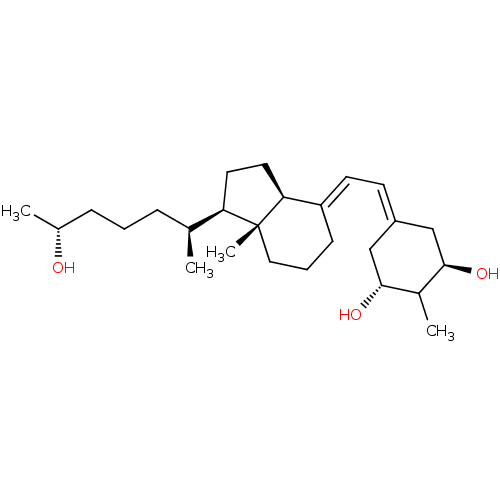
(CHEMBL1630759)Show SMILES C[C@@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,1.1,wD:26.28,(1.96,-41.79,;.6,-42.52,;.6,-44.06,;-.7,-41.71,;-2.06,-42.44,;-3.37,-41.62,;-4.73,-42.35,;-6.07,-41.6,;-4.71,-43.89,;-3.82,-45.14,;-4.73,-46.37,;-6.18,-45.89,;-7.5,-46.66,;-8.85,-45.89,;-8.84,-44.35,;-7.5,-43.58,;-6.17,-44.36,;-6.18,-42.82,;-7.5,-48.2,;-8.84,-48.97,;-8.84,-50.51,;-7.51,-51.29,;-7.51,-52.83,;-6.17,-53.6,;-8.84,-53.59,;-8.84,-55.13,;-10.17,-52.83,;-11.51,-53.6,;-10.17,-51.29,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50021347
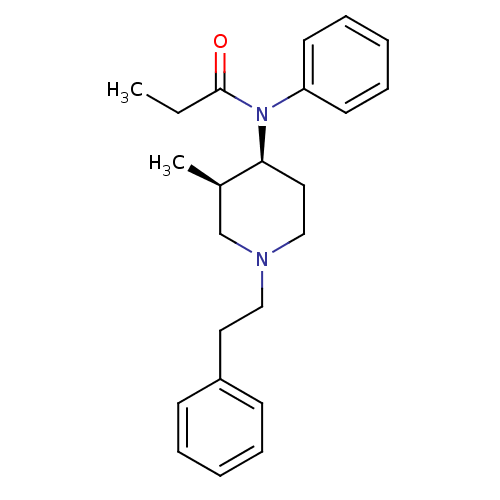
(CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O/c1-3-23(26)25(21-12-8-5-9-13-21)22-15-17-24(18-19(22)2)16-14-20-10-6-4-7-11-20/h4-13,19,22H,3,14-18H2,1-2H3/t19-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50012477
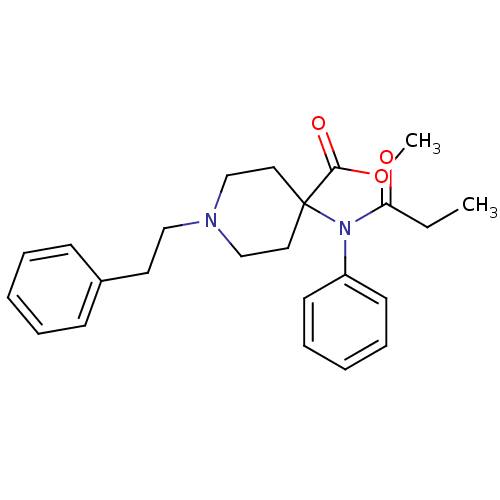
(1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...)Show SMILES CCC(=O)N(c1ccccc1)C1(CCN(CCc2ccccc2)CC1)C(=O)OC Show InChI InChI=1S/C24H30N2O3/c1-3-22(27)26(21-12-8-5-9-13-21)24(23(28)29-2)15-18-25(19-16-24)17-14-20-10-6-4-7-11-20/h4-13H,3,14-19H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470670
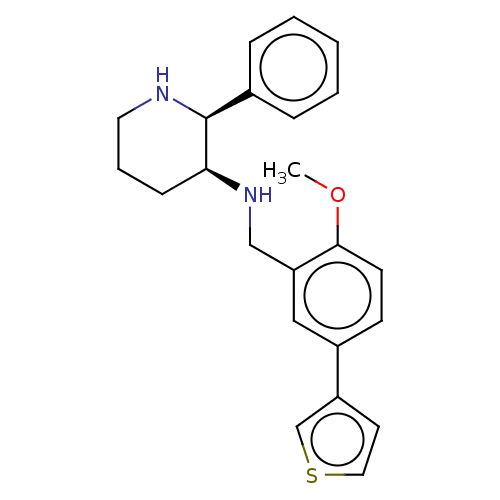
(CHEMBL149557)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccsc1 Show InChI InChI=1S/C23H26N2OS/c1-26-22-10-9-18(19-11-13-27-16-19)14-20(22)15-25-21-8-5-12-24-23(21)17-6-3-2-4-7-17/h2-4,6-7,9-11,13-14,16,21,23-25H,5,8,12,15H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of collagenase (Matrix metalloprotease-13) |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50122190
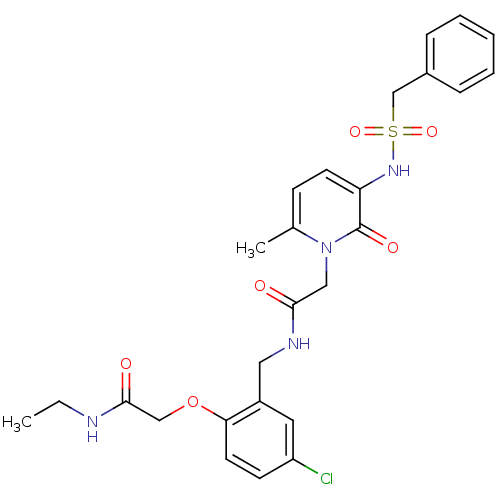
(CHEMBL296737 | N-(5-chloro-2-ethylcarbamoylmethoxy...)Show SMILES CCNC(=O)COc1ccc(Cl)cc1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C26H29ClN4O6S/c1-3-28-25(33)16-37-23-12-10-21(27)13-20(23)14-29-24(32)15-31-18(2)9-11-22(26(31)34)30-38(35,36)17-19-7-5-4-6-8-19/h4-13,30H,3,14-17H2,1-2H3,(H,28,33)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 18: 2062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.098
BindingDB Entry DOI: 10.7270/Q2P27005 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50253328
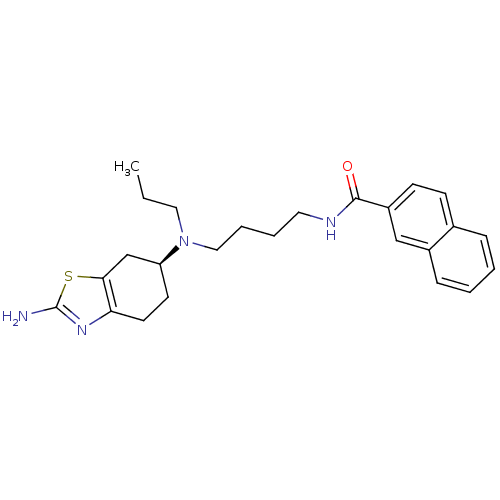
((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...)Show SMILES CCCN(CCCCNC(=O)c1ccc2ccccc2c1)[C@H]1CCc2nc(N)sc2C1 |r| Show InChI InChI=1S/C25H32N4OS/c1-2-14-29(21-11-12-22-23(17-21)31-25(26)28-22)15-6-5-13-27-24(30)20-10-9-18-7-3-4-8-19(18)16-20/h3-4,7-10,16,21H,2,5-6,11-15,17H2,1H3,(H2,26,28)(H,27,30)/t21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum |
J Med Chem 51: 5905-8 (2008)
Article DOI: 10.1021/jm800471h
BindingDB Entry DOI: 10.7270/Q2FN161H |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
Bioorg Med Chem 16: 8563-73 (2008)
Article DOI: 10.1016/j.bmc.2008.08.011
BindingDB Entry DOI: 10.7270/Q20001X4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470678
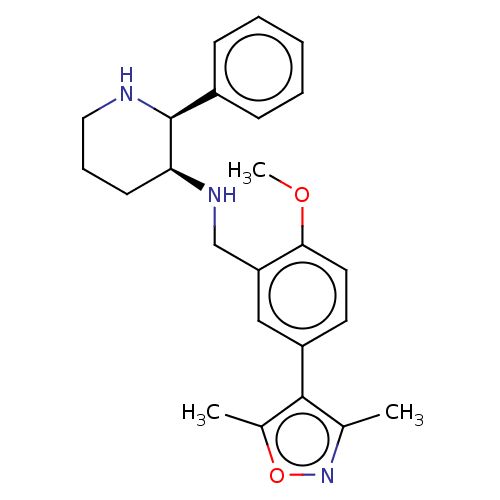
(CHEMBL146885)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1c(C)noc1C Show InChI InChI=1S/C24H29N3O2/c1-16-23(17(2)29-27-16)19-11-12-22(28-3)20(14-19)15-26-21-10-7-13-25-24(21)18-8-5-4-6-9-18/h4-6,8-9,11-12,14,21,24-26H,7,10,13,15H2,1-3H3/t21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470675
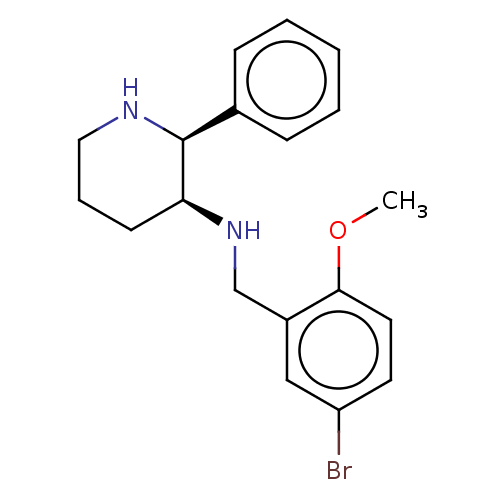
(CHEMBL344536)Show InChI InChI=1S/C19H23BrN2O/c1-23-18-10-9-16(20)12-15(18)13-22-17-8-5-11-21-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,21-22H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50410193
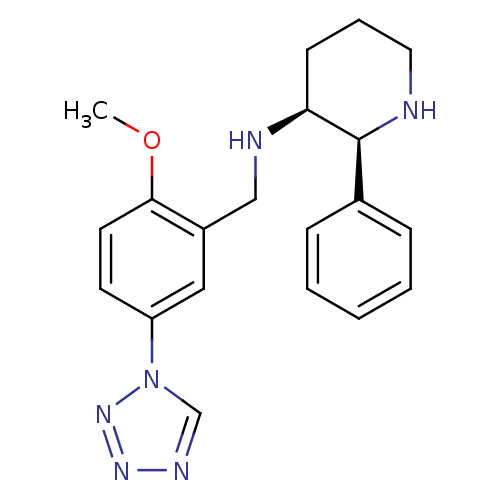
(CHEMBL356062 | GR-203040)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1cnnn1 Show InChI InChI=1S/C20H24N6O/c1-27-19-10-9-17(26-14-23-24-25-26)12-16(19)13-22-18-8-5-11-21-20(18)15-6-3-2-4-7-15/h2-4,6-7,9-10,12,14,18,20-22H,5,8,11,13H2,1H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417520

(CHEMBL1630753)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C(=C)[C@H](O)C1=C |r| Show InChI InChI=1S/C27H42O3/c1-17(8-6-9-18(2)28)23-13-14-24-21(10-7-15-27(23,24)5)11-12-22-16-25(29)20(4)26(30)19(22)3/h11-12,17-18,23-26,28-30H,3-4,6-10,13-16H2,1-2,5H3/b21-11+,22-12-/t17-,18-,23+,24-,25+,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50033533

(CHEMBL121403 | N-[(3R,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417520

(CHEMBL1630753)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C(=C)[C@H](O)C1=C |r| Show InChI InChI=1S/C27H42O3/c1-17(8-6-9-18(2)28)23-13-14-24-21(10-7-15-27(23,24)5)11-12-22-16-25(29)20(4)26(30)19(22)3/h11-12,17-18,23-26,28-30H,3-4,6-10,13-16H2,1-2,5H3/b21-11+,22-12-/t17-,18-,23+,24-,25+,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470683
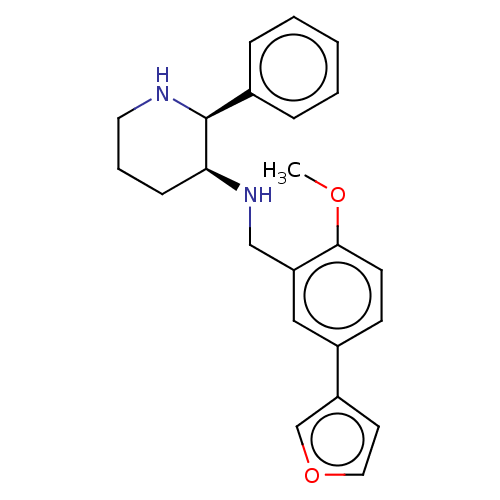
(CHEMBL356786)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccoc1 Show InChI InChI=1S/C23H26N2O2/c1-26-22-10-9-18(19-11-13-27-16-19)14-20(22)15-25-21-8-5-12-24-23(21)17-6-3-2-4-7-17/h2-4,6-7,9-11,13-14,16,21,23-25H,5,8,12,15H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470669

(CHEMBL359188)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccncc1 Show InChI InChI=1S/C24H27N3O/c1-28-23-10-9-20(18-11-14-25-15-12-18)16-21(23)17-27-22-8-5-13-26-24(22)19-6-3-2-4-7-19/h2-4,6-7,9-12,14-16,22,24,26-27H,5,8,13,17H2,1H3/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from opioid receptor kappa 1 expressed in HEK 293 cells |
J Med Chem 44: 857-62 (2001)
BindingDB Entry DOI: 10.7270/Q27H1K9D |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470672
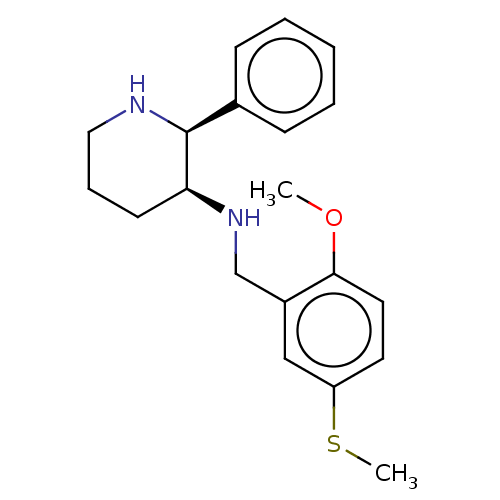
(CHEMBL434884)Show InChI InChI=1S/C20H26N2OS/c1-23-19-11-10-17(24-2)13-16(19)14-22-18-9-6-12-21-20(18)15-7-4-3-5-8-15/h3-5,7-8,10-11,13,18,20-22H,6,9,12,14H2,1-2H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417517

(CHEMBL1630757)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,wD:26.28,1.1,(14.49,-28.73,;13.13,-29.45,;13.13,-30.99,;11.83,-28.64,;10.47,-29.37,;9.16,-28.56,;7.8,-29.29,;6.46,-28.53,;7.82,-30.83,;8.71,-32.07,;7.8,-33.31,;6.35,-32.82,;5.03,-33.59,;3.68,-32.83,;3.69,-31.28,;5.03,-30.51,;6.36,-31.29,;6.35,-29.75,;5.03,-35.13,;3.69,-35.9,;3.69,-37.44,;5.02,-38.22,;5.02,-39.76,;6.36,-40.53,;3.69,-40.52,;3.69,-42.06,;2.36,-39.76,;1.03,-40.53,;2.36,-38.22,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417519

(CHEMBL1630759)Show SMILES C[C@@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,1.1,wD:26.28,(1.96,-41.79,;.6,-42.52,;.6,-44.06,;-.7,-41.71,;-2.06,-42.44,;-3.37,-41.62,;-4.73,-42.35,;-6.07,-41.6,;-4.71,-43.89,;-3.82,-45.14,;-4.73,-46.37,;-6.18,-45.89,;-7.5,-46.66,;-8.85,-45.89,;-8.84,-44.35,;-7.5,-43.58,;-6.17,-44.36,;-6.18,-42.82,;-7.5,-48.2,;-8.84,-48.97,;-8.84,-50.51,;-7.51,-51.29,;-7.51,-52.83,;-6.17,-53.6,;-8.84,-53.59,;-8.84,-55.13,;-10.17,-52.83,;-11.51,-53.6,;-10.17,-51.29,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (Matrix metalloprotease-2) |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50084768

(N-(4-Methoxymethyl-1-phenethyl-piperidin-4-yl)-N-p...)Show SMILES CCC(=O)N(c1ccccc1)[C@]1(COC)CC[NH+](CCc2ccccc2)CC1 |wU:11.11,wD:11.12,(12.88,-3.1,;11.39,-2.71,;10.3,-3.8,;10.51,-5.33,;8.97,-3.03,;8.97,-1.49,;10.3,-.72,;10.29,.82,;8.94,1.58,;7.61,.79,;7.63,-.73,;7.89,-4.13,;7.87,-5.67,;6.54,-6.44,;6.53,-7.98,;7.63,-2.6,;5.86,-2.62,;4.81,-1.8,;3.88,-3.03,;2.36,-2.85,;1.45,-4.09,;-.09,-3.93,;-1,-5.16,;-.39,-6.58,;1.15,-6.75,;2.06,-5.51,;5.07,-3.34,;6.91,-3.34,)| Show InChI InChI=1S/C24H32N2O2/c1-3-23(27)26(22-12-8-5-9-13-22)24(20-28-2)15-18-25(19-16-24)17-14-21-10-6-4-7-11-21/h4-13H,3,14-20H2,1-2H3/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417515

(CHEMBL1630755)Show SMILES [#6]-[#6@@H](-[#8])-[#6]-[#6]-[#6]-[#6@H](-[#6])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C26H42O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-18,22-25,27-29H,3,5-9,12-16H2,1-2,4H3/b21-11+/t17-,18+,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174139
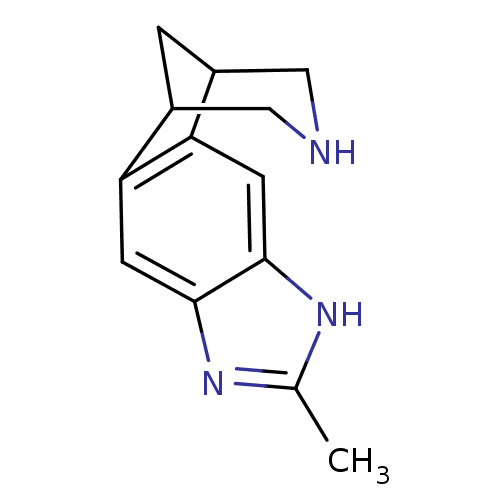
(6-methyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C13H15N3/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417517

(CHEMBL1630757)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,wD:26.28,1.1,(14.49,-28.73,;13.13,-29.45,;13.13,-30.99,;11.83,-28.64,;10.47,-29.37,;9.16,-28.56,;7.8,-29.29,;6.46,-28.53,;7.82,-30.83,;8.71,-32.07,;7.8,-33.31,;6.35,-32.82,;5.03,-33.59,;3.68,-32.83,;3.69,-31.28,;5.03,-30.51,;6.36,-31.29,;6.35,-29.75,;5.03,-35.13,;3.69,-35.9,;3.69,-37.44,;5.02,-38.22,;5.02,-39.76,;6.36,-40.53,;3.69,-40.52,;3.69,-42.06,;2.36,-39.76,;1.03,-40.53,;2.36,-38.22,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50388434

(CHEMBL605525)Show SMILES [#6]-[#6@@H](-[#6]-[#6]-[#6]C([#6])([#6])[#8])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)22-12-13-23-21(9-7-15-27(22,23)5)11-10-20-16-24(28)19(2)25(29)17-20/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b21-11+/t18-,22+,23-,24+,25+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417513
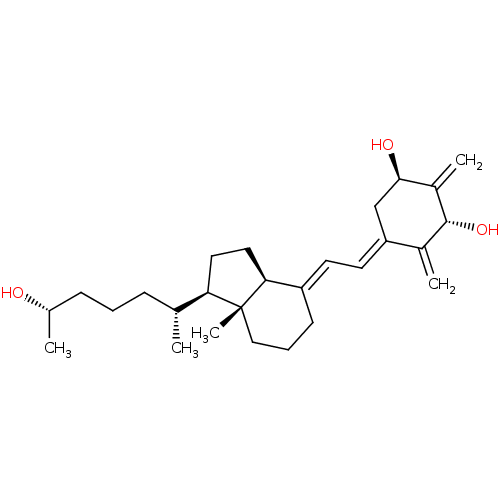
(CHEMBL1630752)Show SMILES C[C@H](O)CCC[C@@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C(=C)[C@H](O)C1=C |r| Show InChI InChI=1S/C27H42O3/c1-17(8-6-9-18(2)28)23-13-14-24-21(10-7-15-27(23,24)5)11-12-22-16-25(29)20(4)26(30)19(22)3/h11-12,17-18,23-26,28-30H,3-4,6-10,13-16H2,1-2,5H3/b21-11+,22-12-/t17-,18+,23-,24+,25-,26-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174152
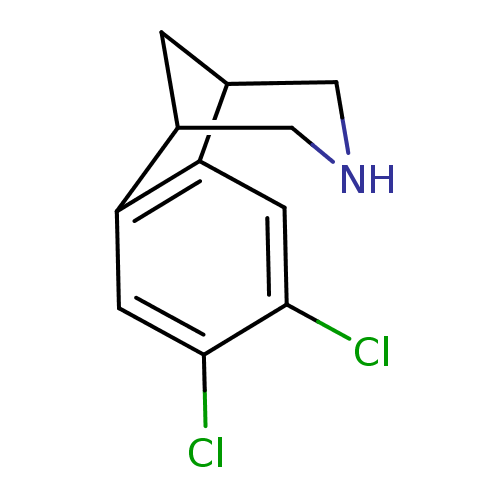
(4,5-Dichloro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2...)Show InChI InChI=1S/C11H11Cl2N/c12-10-2-8-6-1-7(5-14-4-6)9(8)3-11(10)13/h2-3,6-7,14H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417514
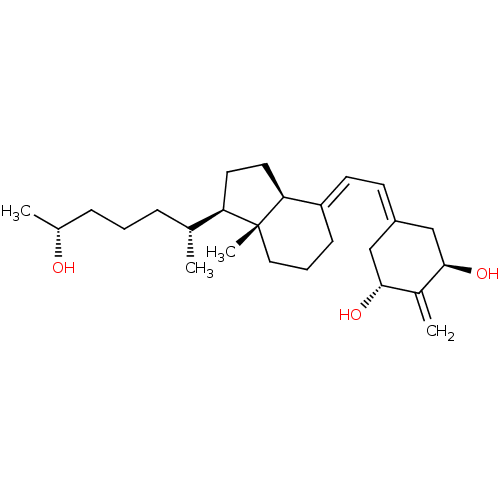
(CHEMBL1630754)Show SMILES [#6]-[#6@@H](-[#8])-[#6]-[#6]-[#6]-[#6@@H](-[#6])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C26H42O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-18,22-25,27-29H,3,5-9,12-16H2,1-2,4H3/b21-11+/t17-,18-,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50070824
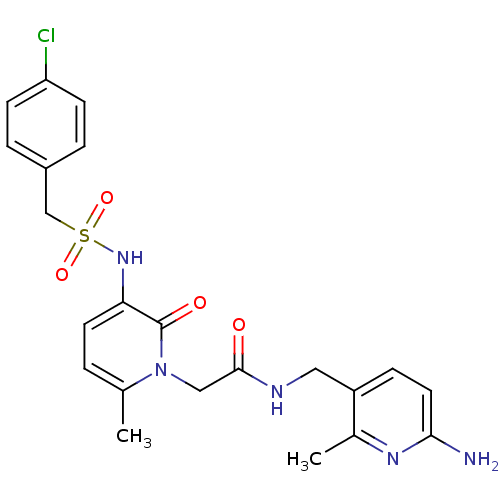
(CHEMBL47920 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccc(Cl)cc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24ClN5O4S/c1-14-3-9-19(27-33(31,32)13-16-4-7-18(23)8-5-16)22(30)28(14)12-21(29)25-11-17-6-10-20(24)26-15(17)2/h3-10,27H,11-13H2,1-2H3,(H2,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated to inhibit the thrombin enzyme |
Bioorg Med Chem Lett 8: 1719-24 (1999)
BindingDB Entry DOI: 10.7270/Q2319V13 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174145
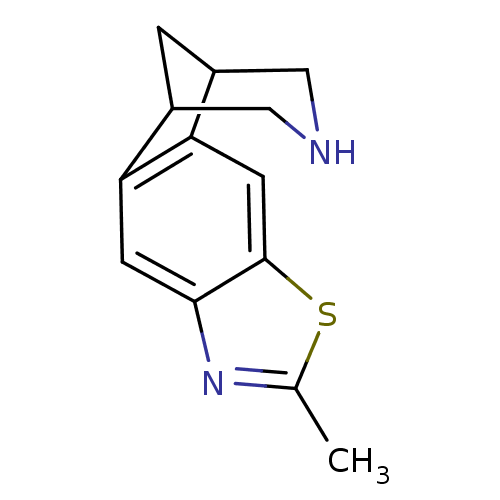
(6-methyl-7-thia-5,13-diazatetracyclo[9.3.1.02,10.0...)Show InChI InChI=1S/C13H14N2S/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470667
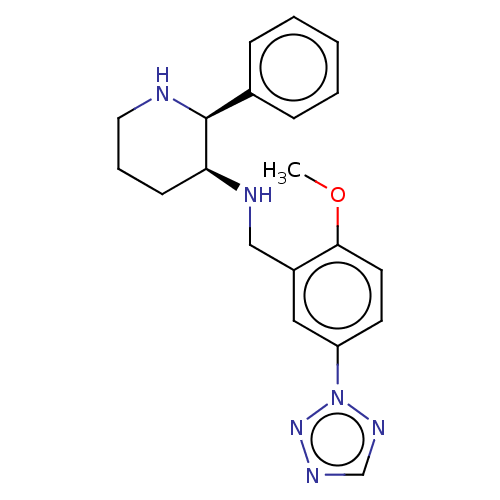
(CHEMBL424301)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1ncnn1 Show InChI InChI=1S/C20H24N6O/c1-27-19-10-9-17(26-24-14-23-25-26)12-16(19)13-22-18-8-5-11-21-20(18)15-6-3-2-4-7-15/h2-4,6-7,9-10,12,14,18,20-22H,5,8,11,13H2,1H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174148
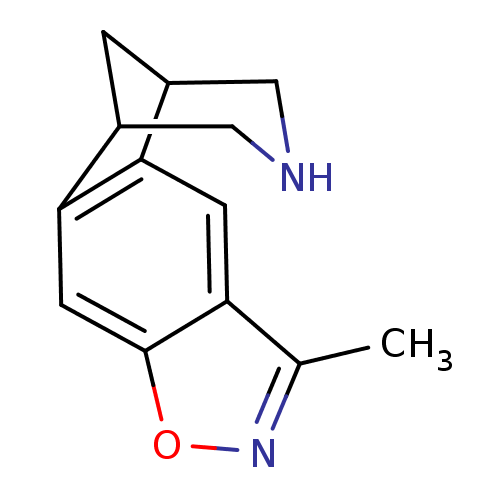
(5-methyl-7-oxa-6,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-10-3-11-8-2-9(6-14-5-8)12(11)4-13(10)16-15-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174154
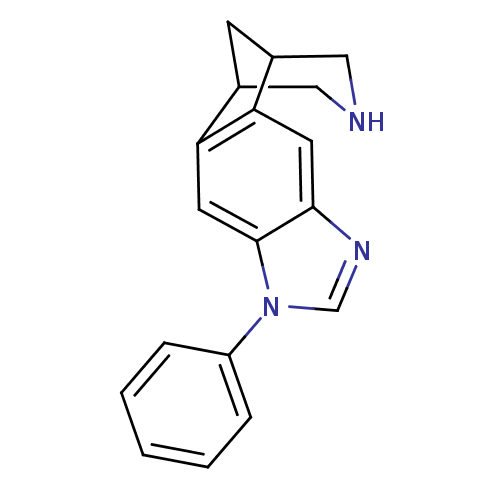
(7-phenyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C18H17N3/c1-2-4-14(5-3-1)21-11-20-17-7-15-12-6-13(10-19-9-12)16(15)8-18(17)21/h1-5,7-8,11-13,19H,6,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174156
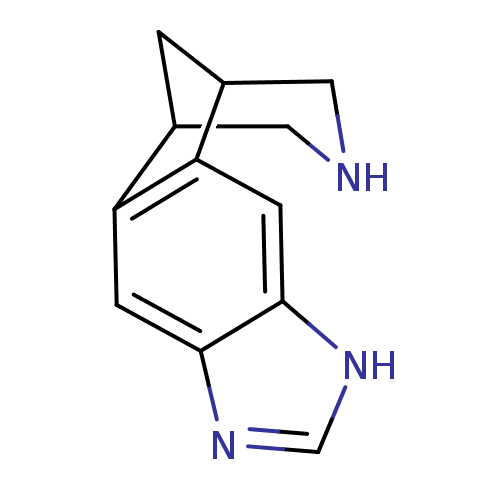
(5,7,13-triazatetracyclo[9.3.1.02,10.04,8]pentadeca...)Show InChI InChI=1S/C12H13N3/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
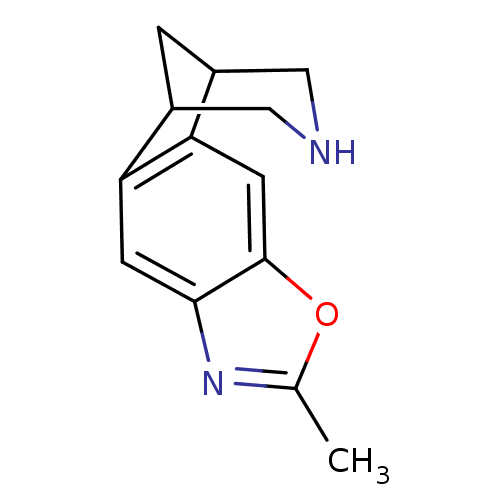
(Homo sapiens (Human)) | BDBM50174190
(6-methyl-7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
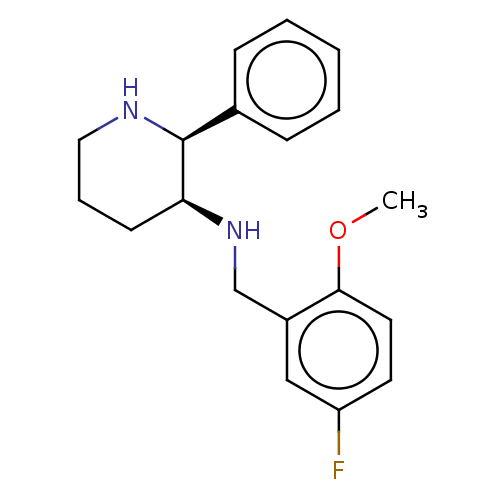
(Homo sapiens (Human)) | BDBM50470677
(CHEMBL147776)Show InChI InChI=1S/C19H23FN2O/c1-23-18-10-9-16(20)12-15(18)13-22-17-8-5-11-21-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,21-22H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
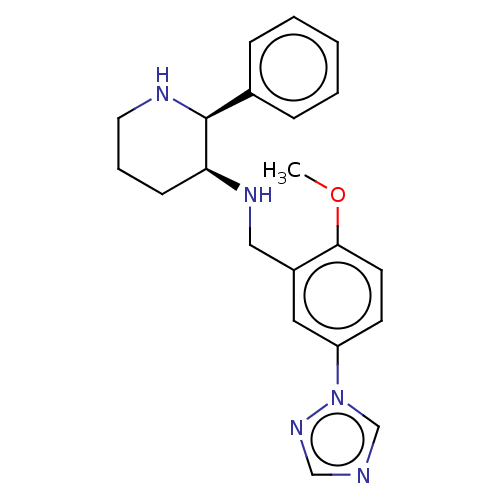
(Homo sapiens (Human)) | BDBM50470676
(CHEMBL359255)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1cncn1 Show InChI InChI=1S/C21H25N5O/c1-27-20-10-9-18(26-15-22-14-25-26)12-17(20)13-24-19-8-5-11-23-21(19)16-6-3-2-4-7-16/h2-4,6-7,9-10,12,14-15,19,21,23-24H,5,8,11,13H2,1H3/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470671
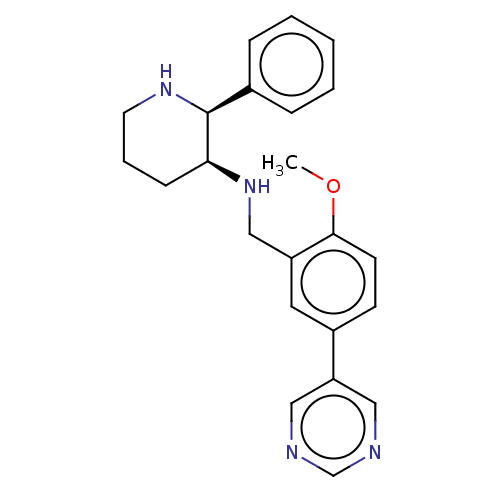
(CHEMBL358575)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1cncnc1 Show InChI InChI=1S/C23H26N4O/c1-28-22-10-9-18(20-13-24-16-25-14-20)12-19(22)15-27-21-8-5-11-26-23(21)17-6-3-2-4-7-17/h2-4,6-7,9-10,12-14,16,21,23,26-27H,5,8,11,15H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174170
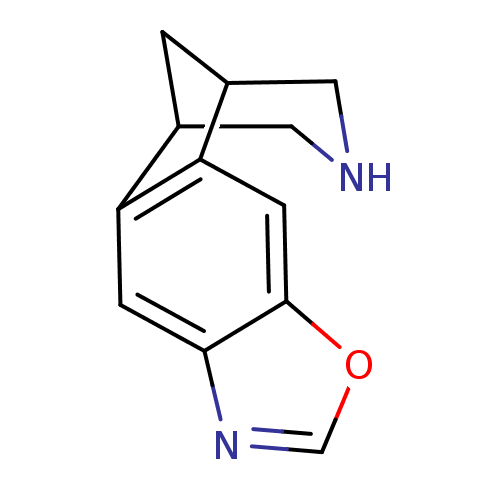
(7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04,8]pentad...)Show InChI InChI=1S/C12H12N2O/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50370684
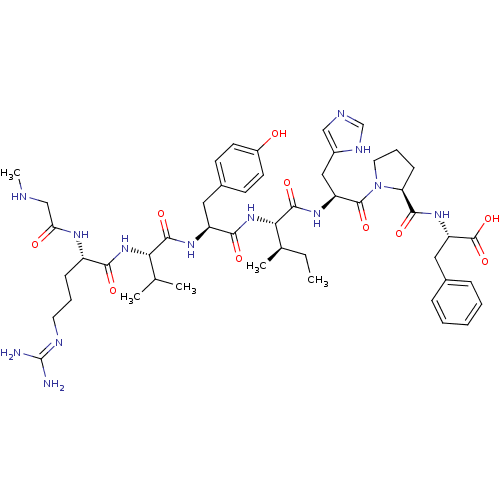
(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 1 in rat liver membrane using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 2 hr at ... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50236864
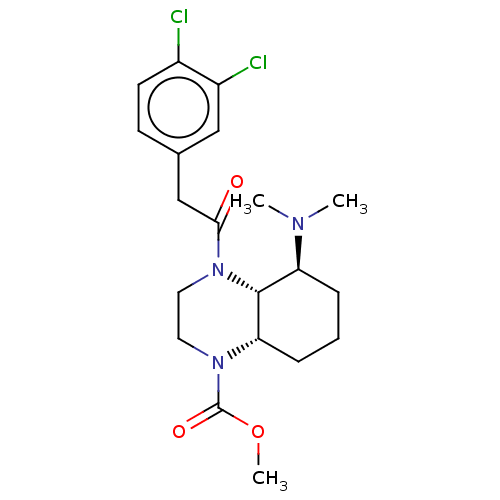
(CHEMBL4096473)Show SMILES [H][C@]12CCC[C@H](N(C)C)[C@@]1([H])N(CCN2C(=O)OC)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H27Cl2N3O3/c1-23(2)16-5-4-6-17-19(16)25(10-9-24(17)20(27)28-3)18(26)12-13-7-8-14(21)15(22)11-13/h7-8,11,16-17,19H,4-6,9-10,12H2,1-3H3/t16-,17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. August Wolff GmbH& Co. KG Arzneimittel
Curated by ChEMBL
| Assay Description
Inhibition of high affinity uptake of [3H]DA using rat nerve endings obtained from brain regions enriched in DAT. |
J Med Chem 60: 2526-2551 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01868
BindingDB Entry DOI: 10.7270/Q20C4Z1Z |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174141
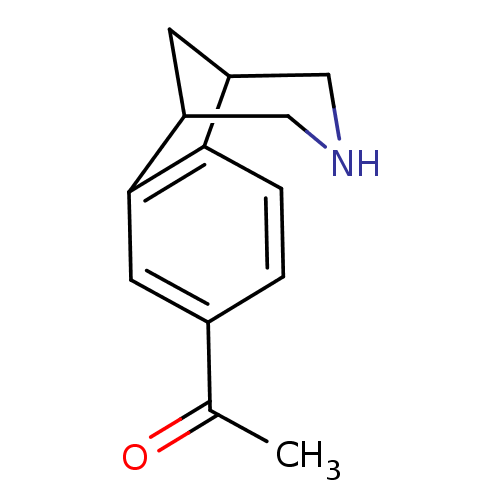
(1-(10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-tr...)Show InChI InChI=1S/C13H15NO/c1-8(15)9-2-3-12-10-4-11(7-14-6-10)13(12)5-9/h2-3,5,10-11,14H,4,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370684

(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data