

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427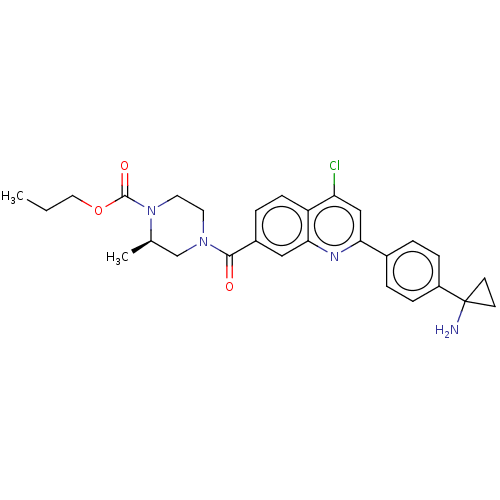 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433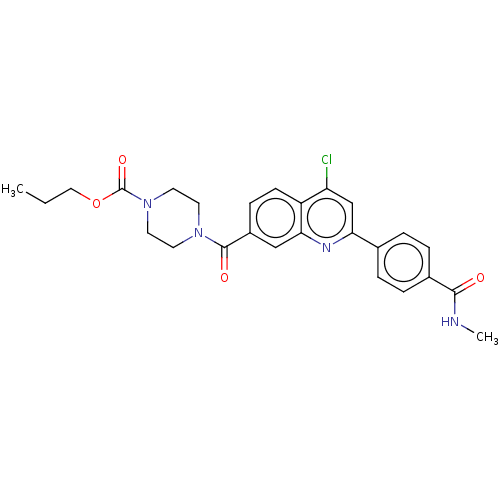 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434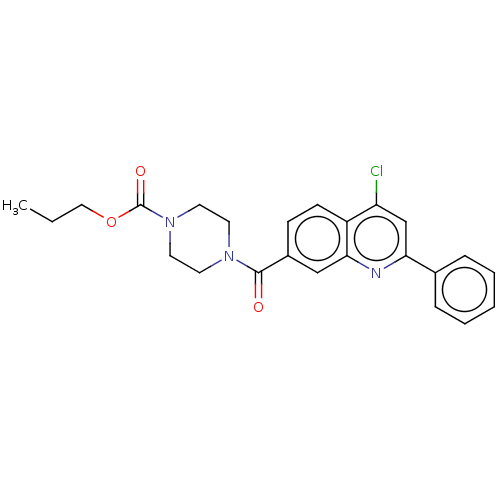 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50133870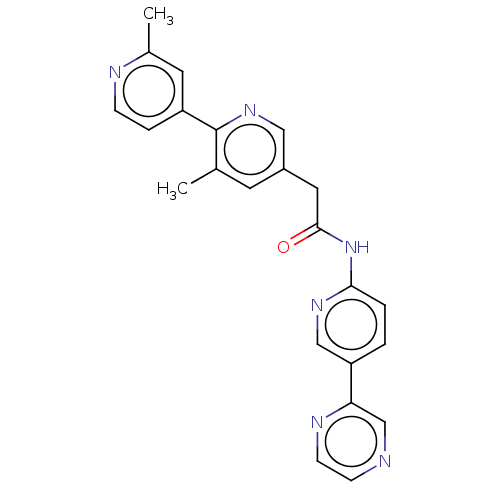 (CHEBI:78030 | CHEMBL3188386 | US10251893, Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257135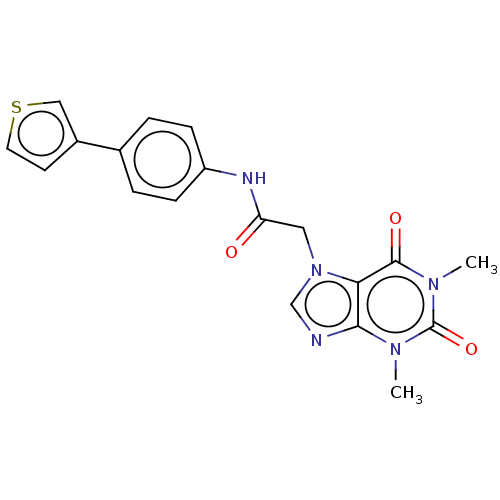 (CHEMBL4080208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257160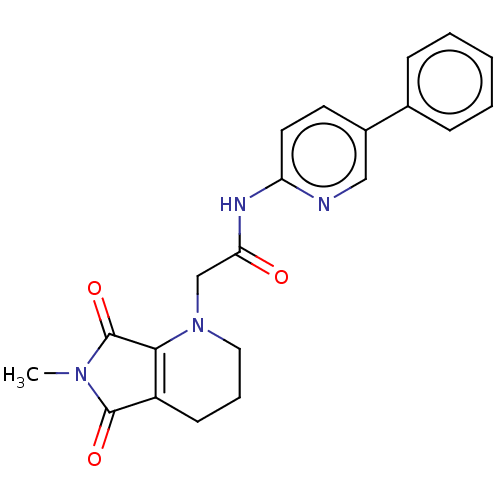 (CHEMBL4096728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257143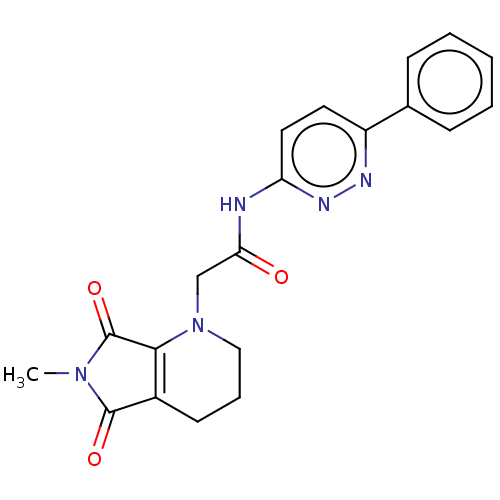 (CHEMBL4080021) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50133866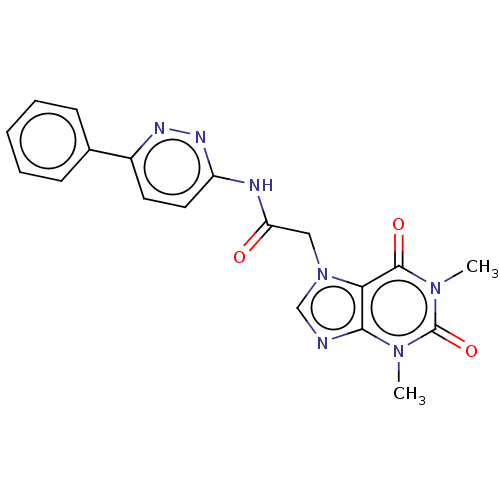 (CHEMBL3633802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257141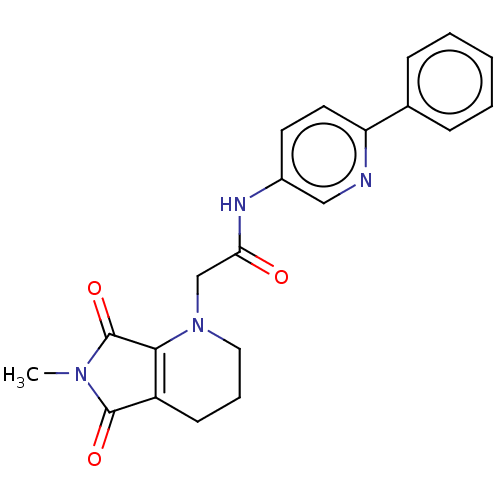 (CHEMBL4069295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257139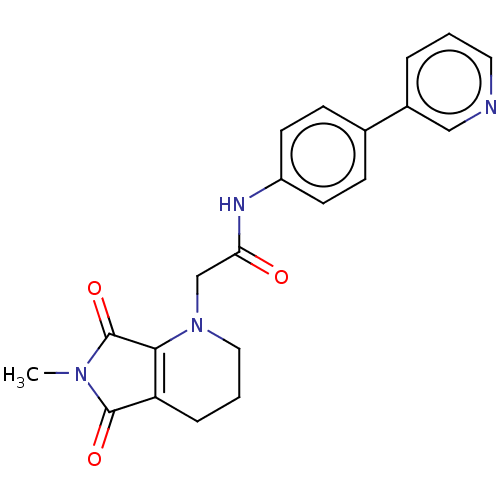 (CHEMBL4104447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257144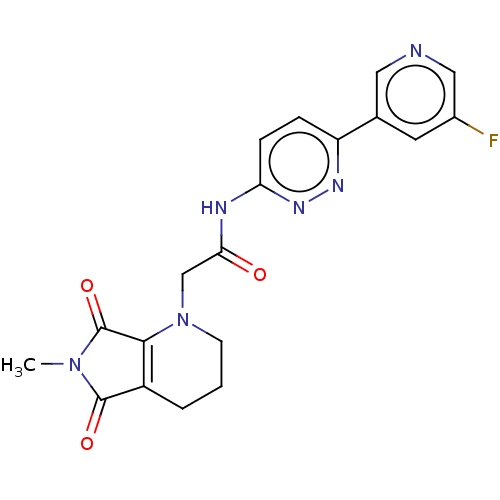 (CHEMBL4066589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257145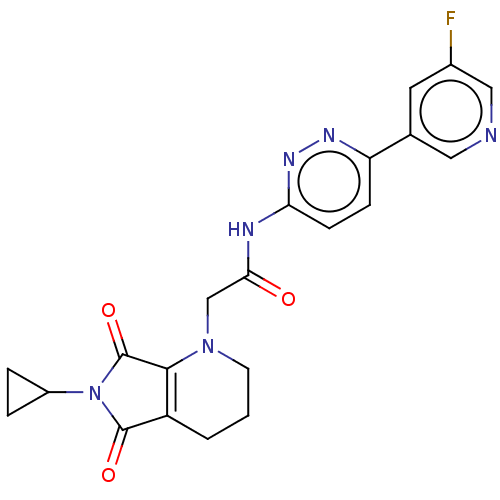 (CHEMBL4095609) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257142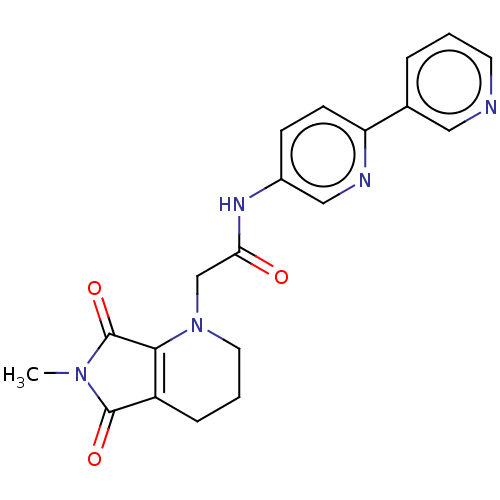 (CHEMBL4096529) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257138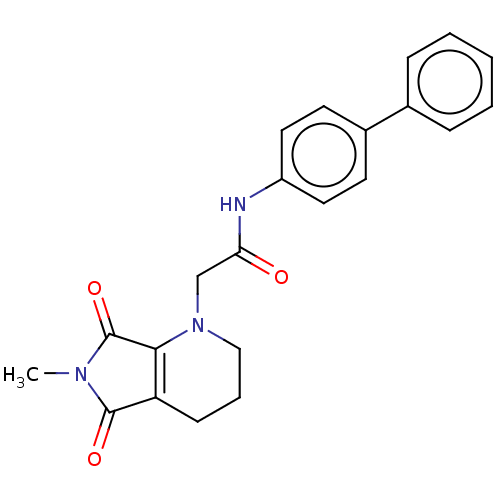 (CHEMBL4066796) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257157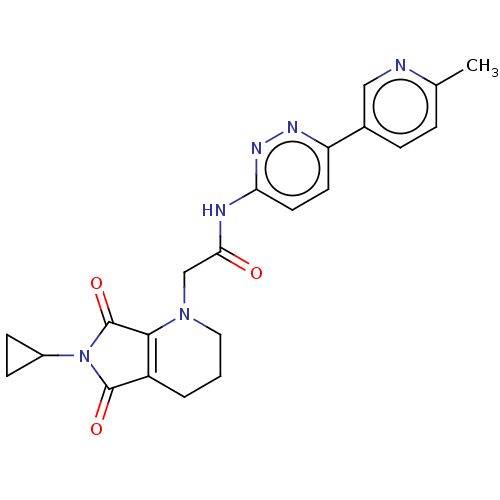 (CHEMBL4103356) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257137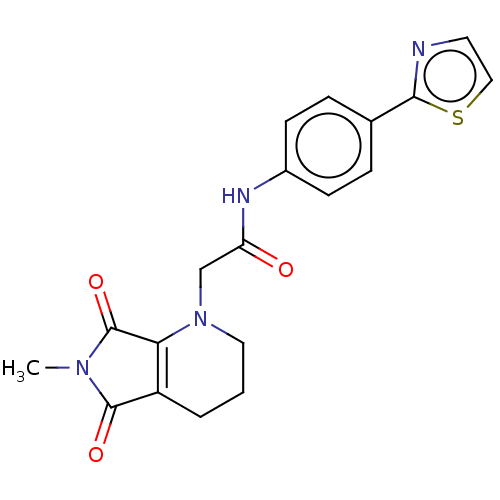 (CHEMBL4094309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257136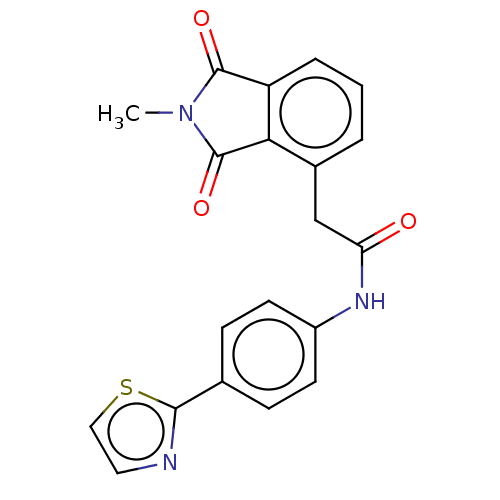 (CHEMBL4061069) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257134 (CHEMBL4066798) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257161 (CHEMBL4075204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257158 (CHEMBL4104257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257162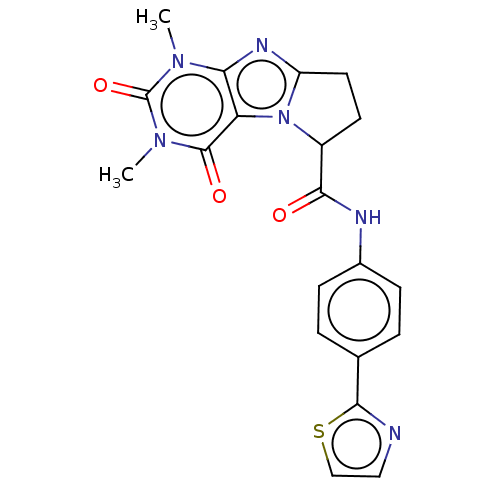 (CHEMBL4102043) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50318567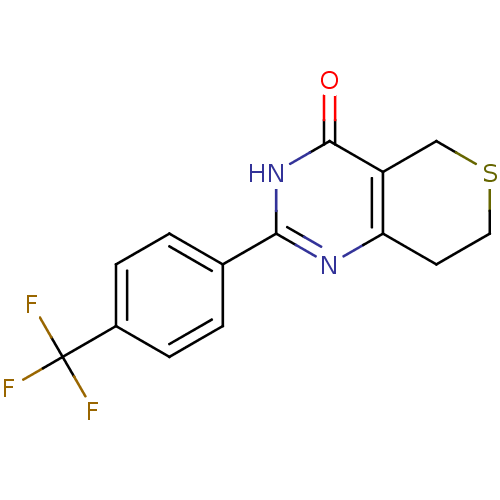 (2-(4-(Trifluoromethyl)phenyl)-7,8-dihydro-5H-thiop...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of PARP2 (unknown origin) | J Med Chem 56: 4497-508 (2013) Article DOI: 10.1021/jm400211f BindingDB Entry DOI: 10.7270/Q23R0V82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257129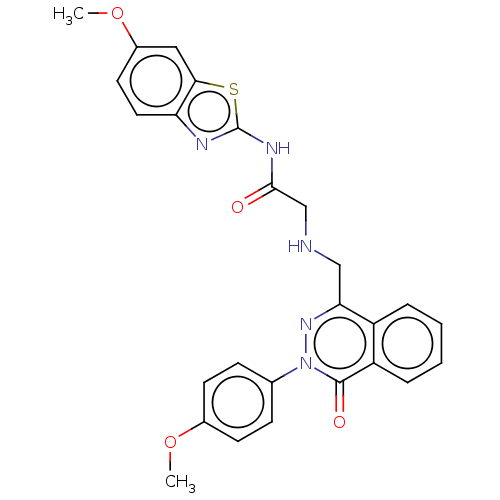 (CHEMBL4088085) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257159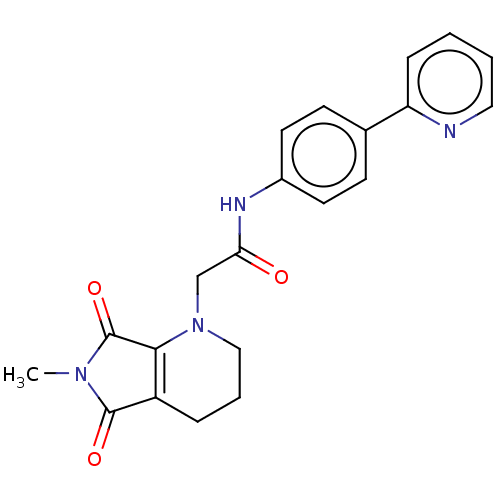 (CHEMBL4086549) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502413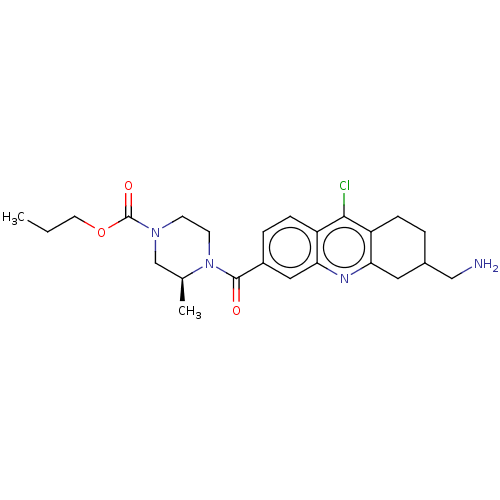 (CHEMBL4521324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257140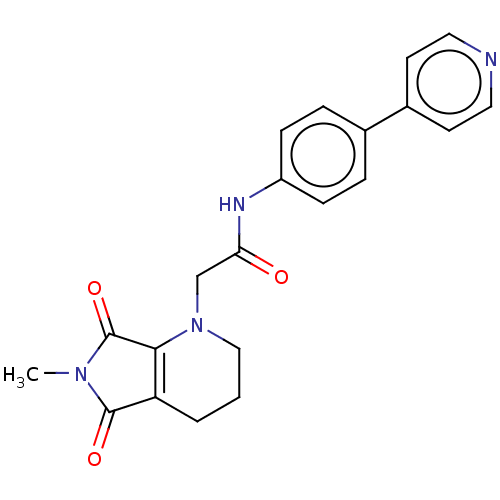 (CHEMBL4078705) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM430624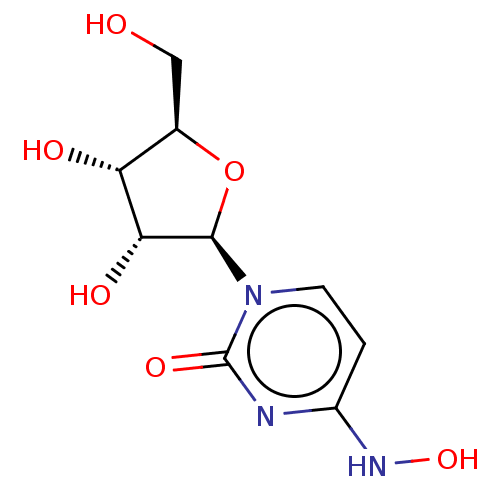 (Beta-D-N4-hydroxycytidine | EIDD-1931 | N(4)-Hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill | Assay Description Calu-3 2B4 cells were adsorbed with MOI 0.1 PFU/cell of SARS-CoV-2 (2019-nCoV/USA-WA1/2020 strain) at 37°C. Plates were manually rocked every 10 min ... | Sci Transl Med 12: 1-15 (2020) Article DOI: 10.1126/scitranslmed.abb5883 BindingDB Entry DOI: 10.7270/Q26D5WDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM430624 (Beta-D-N4-hydroxycytidine | EIDD-1931 | N(4)-Hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill | Assay Description Calu-3 2B4 cells were adsorbed with MOI 0.1 PFU/cell of SARS-CoV-2 (2019-nCoV/USA-WA1/2020 strain) at 37°C. Plates were manually rocked every 10 min ... | Sci Transl Med 12: 1-15 (2020) Article DOI: 10.1126/scitranslmed.abb5883 BindingDB Entry DOI: 10.7270/Q26D5WDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50318567 (2-(4-(Trifluoromethyl)phenyl)-7,8-dihydro-5H-thiop...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of PARP1 (unknown origin) | J Med Chem 56: 4497-508 (2013) Article DOI: 10.1021/jm400211f BindingDB Entry DOI: 10.7270/Q23R0V82 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502429 (CHEMBL4475972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orf1a protein (MERS-CoV) | BDBM430624 (Beta-D-N4-hydroxycytidine | EIDD-1931 | N(4)-Hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill | Assay Description At 48 hours prior to infection, Calu-3 2B4 cells were plated in a 96-well black-walled clear bottom plate at 5x104 cells/well. A 10 mM stock of NHC w... | Sci Transl Med 12: 1-15 (2020) Article DOI: 10.1126/scitranslmed.abb5883 BindingDB Entry DOI: 10.7270/Q26D5WDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502416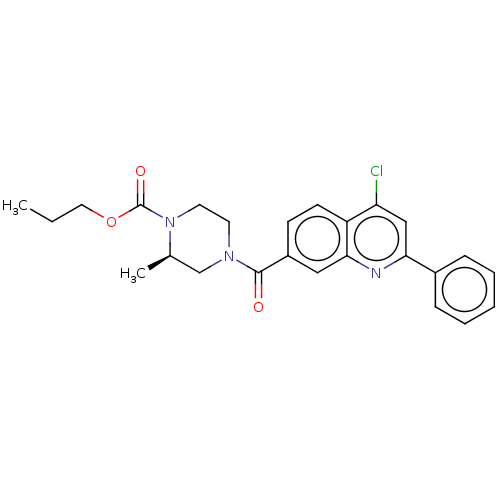 (CHEMBL4472455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502436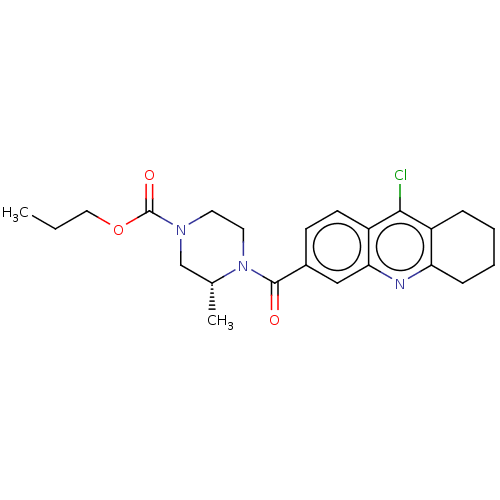 (CHEMBL4450314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502414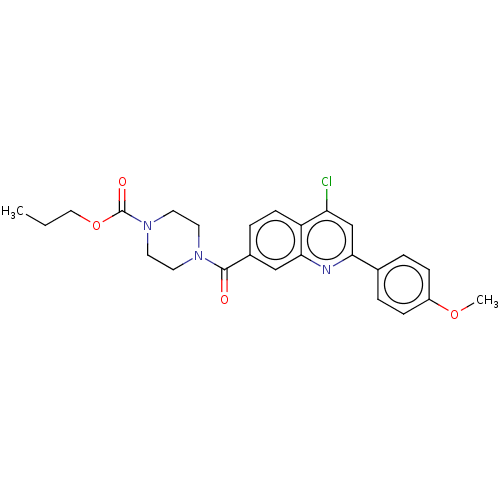 (CHEMBL4440399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM430624 (Beta-D-N4-hydroxycytidine | EIDD-1931 | N(4)-Hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill | Assay Description Vero E6 cells were plated at 20,000 cells/well in a 96-well plate. 24hr later, medium containing a dose response of NHC was added concurrent with SAR... | Sci Transl Med 12: 1-15 (2020) Article DOI: 10.1126/scitranslmed.abb5883 BindingDB Entry DOI: 10.7270/Q26D5WDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502426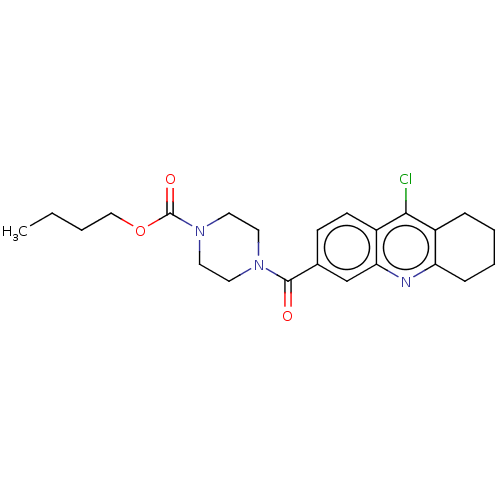 (CHEMBL4448008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502415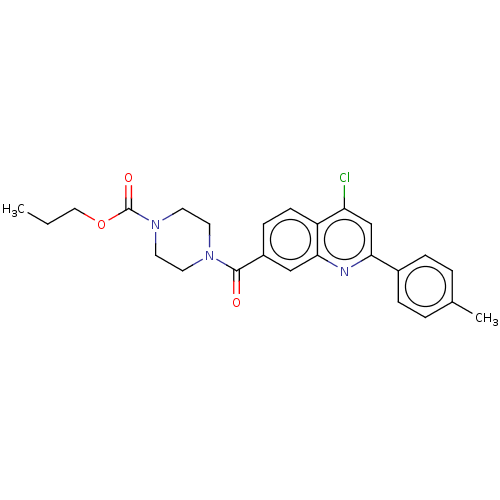 (CHEMBL4579512) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502438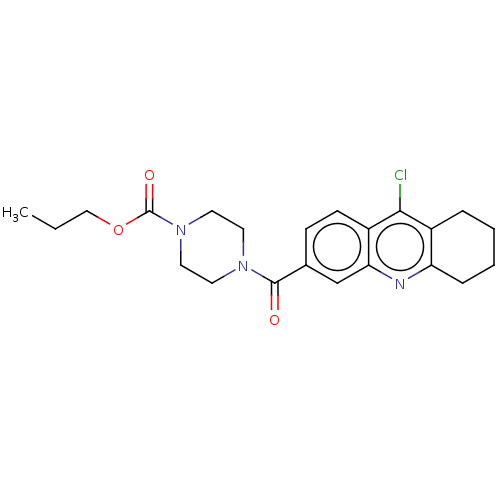 (CHEMBL4530816) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502428 (CHEMBL4584168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM642792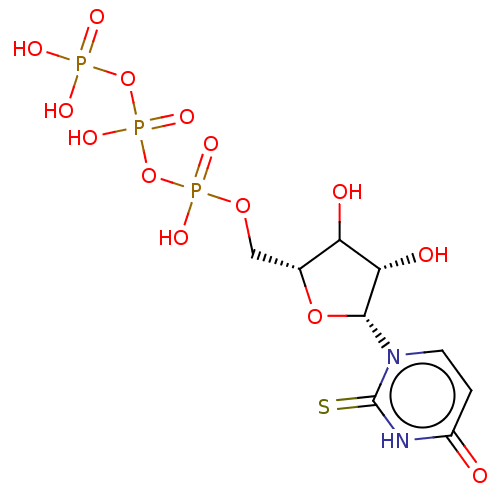 (US11857560, I.D. ENUC-01829) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM642796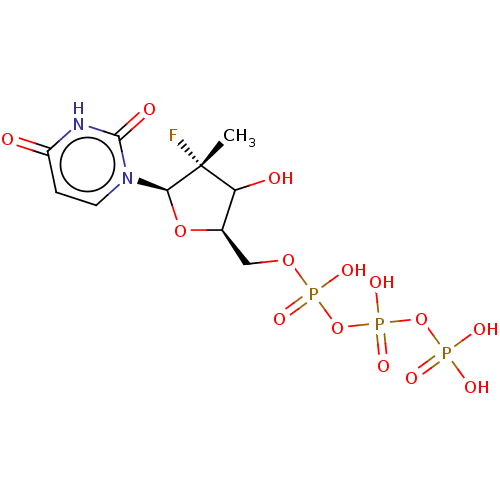 (US11857560, I.D. ENUC-01842) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM642799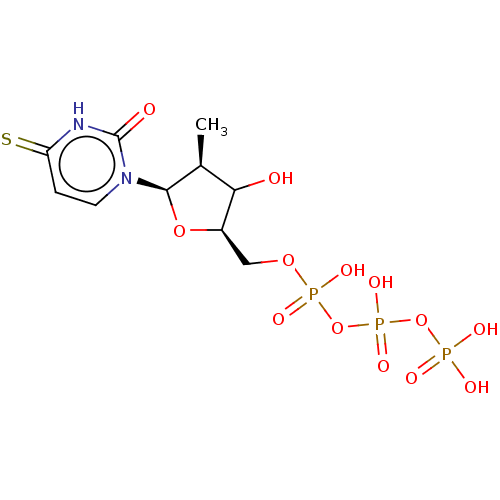 (US11857560, I.D. ENUC-01952) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM642799 (US11857560, I.D. ENUC-01952) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502418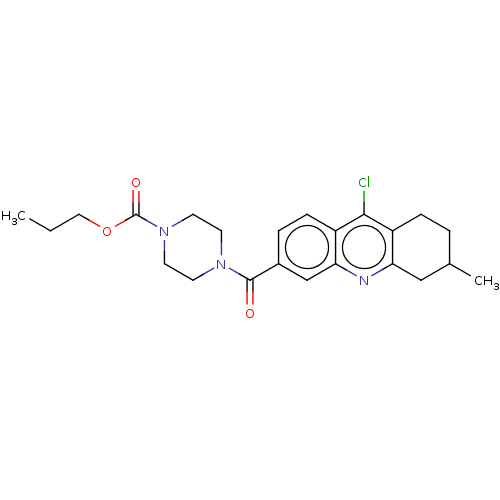 (CHEMBL4532072) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502419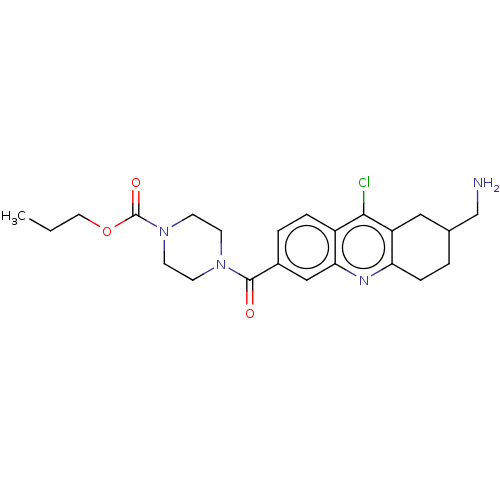 (CHEMBL4462956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502435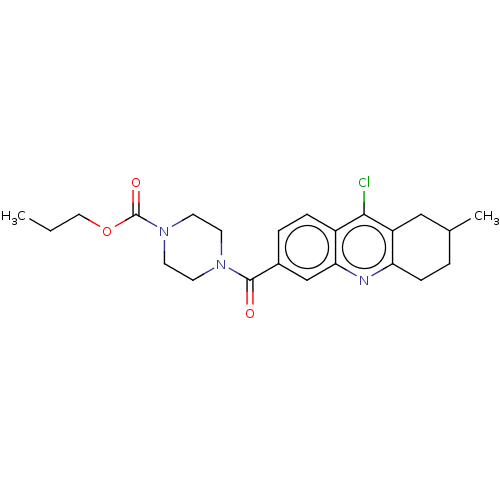 (CHEMBL4541197) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |