 Found 575 hits with Last Name = 'pasternak' and Initial = 'gw'
Found 575 hits with Last Name = 'pasternak' and Initial = 'gw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(MOUSE) | BDBM85821
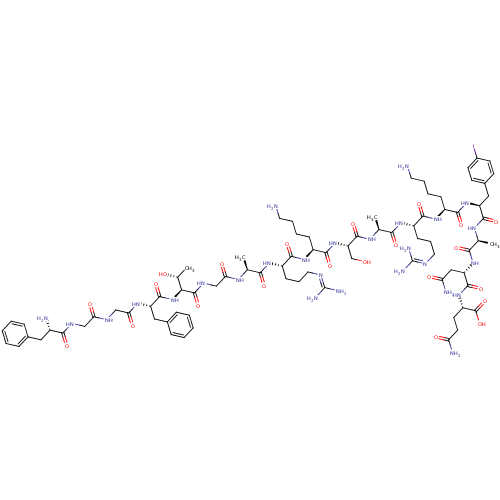
(OFQ/N,Iodo[Tyr14])Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(I)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C82H126IN27O22/c1-43(98-64(116)41-97-79(130)66(46(4)112)110-77(128)57(36-48-19-9-6-10-20-48)101-65(117)40-95-63(115)39-96-70(121)51(86)35-47-17-7-5-8-18-47)67(118)102-54(23-15-33-93-81(89)90)71(122)105-53(22-12-14-32-85)74(125)109-60(42-111)78(129)100-44(2)68(119)103-55(24-16-34-94-82(91)92)72(123)104-52(21-11-13-31-84)73(124)108-58(37-49-25-27-50(83)28-26-49)75(126)99-45(3)69(120)107-59(38-62(88)114)76(127)106-56(80(131)132)29-30-61(87)113/h5-10,17-20,25-28,43-46,51-60,66,111-112H,11-16,21-24,29-42,84-86H2,1-4H3,(H2,87,113)(H2,88,114)(H,95,115)(H,96,121)(H,97,130)(H,98,116)(H,99,126)(H,100,129)(H,101,117)(H,102,118)(H,103,119)(H,104,123)(H,105,122)(H,106,127)(H,107,120)(H,108,124)(H,109,125)(H,110,128)(H,131,132)(H4,89,90,93)(H4,91,92,94)/t43-,44-,45-,46+,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 381-415 (2001)
BindingDB Entry DOI: 10.7270/Q2QJ7FWB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50274460
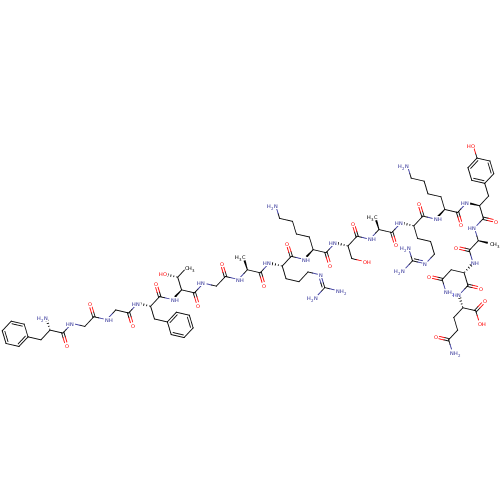
(CHEMBL504540 | FGGFTGARKSARKYANQ | OFQ/N,[Tyr14])Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H127N27O23/c1-43(97-64(116)41-96-79(130)66(46(4)111)109-77(128)57(36-48-19-9-6-10-20-48)100-65(117)40-94-63(115)39-95-70(121)51(85)35-47-17-7-5-8-18-47)67(118)101-54(23-15-33-92-81(88)89)71(122)104-53(22-12-14-32-84)74(125)108-60(42-110)78(129)99-44(2)68(119)102-55(24-16-34-93-82(90)91)72(123)103-52(21-11-13-31-83)73(124)107-58(37-49-25-27-50(112)28-26-49)75(126)98-45(3)69(120)106-59(38-62(87)114)76(127)105-56(80(131)132)29-30-61(86)113/h5-10,17-20,25-28,43-46,51-60,66,110-112H,11-16,21-24,29-42,83-85H2,1-4H3,(H2,86,113)(H2,87,114)(H,94,115)(H,95,121)(H,96,130)(H,97,116)(H,98,126)(H,99,129)(H,100,117)(H,101,118)(H,102,119)(H,103,123)(H,104,122)(H,105,127)(H,106,120)(H,107,124)(H,108,125)(H,109,128)(H,131,132)(H4,88,89,92)(H4,90,91,93)/t43-,44-,45-,46+,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 381-415 (2001)
BindingDB Entry DOI: 10.7270/Q2QJ7FWB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM21842
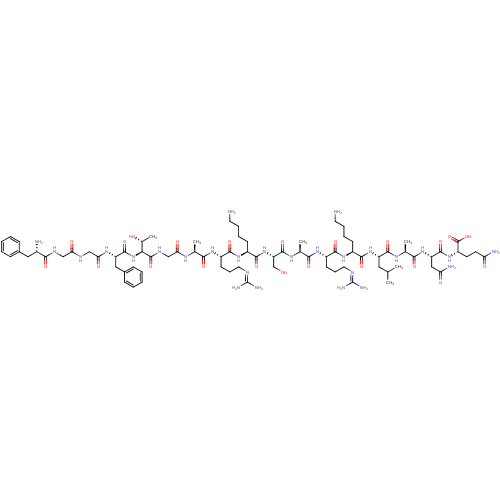
((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 381-415 (2001)
BindingDB Entry DOI: 10.7270/Q2QJ7FWB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21865
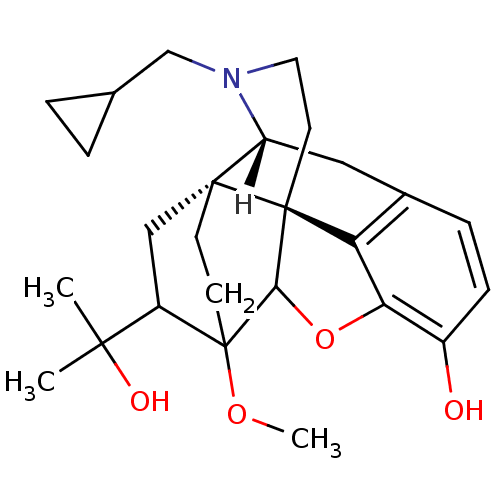
((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50346951
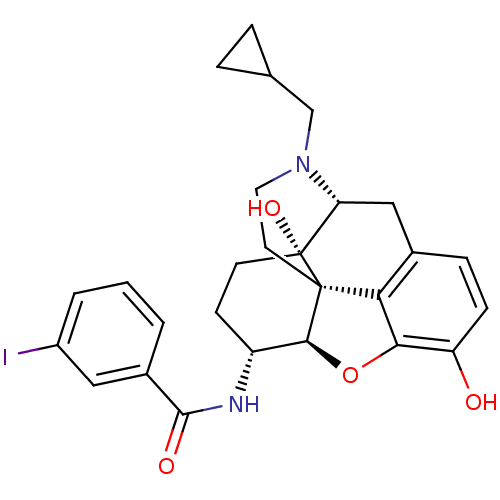
(CHEMBL1795711 | CHEMBL1795714)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C27H29IN2O4/c28-18-3-1-2-17(12-18)25(32)29-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-30(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned KOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBOxyA from MOR-1 expressed in CHO cells |
Bioorg Med Chem Lett 21: 4001-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.008
BindingDB Entry DOI: 10.7270/Q29C6ZDF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50346952
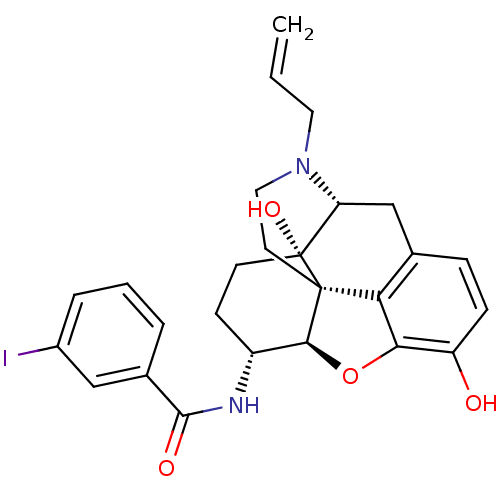
(CHEMBL1795712 | CHEMBL1795715)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C26H27IN2O4/c1-2-11-29-12-10-25-21-15-6-7-19(30)22(21)33-23(25)18(8-9-26(25,32)20(29)14-15)28-24(31)16-4-3-5-17(27)13-16/h2-7,13,18,20,23,30,32H,1,8-12,14H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-U69593 from KOR-1 expressed in CHO cells after 60 mins |
Bioorg Med Chem Lett 21: 4001-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.008
BindingDB Entry DOI: 10.7270/Q29C6ZDF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50346952

(CHEMBL1795712 | CHEMBL1795715)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C26H27IN2O4/c1-2-11-29-12-10-25-21-15-6-7-19(30)22(21)33-23(25)18(8-9-26(25,32)20(29)14-15)28-24(31)16-4-3-5-17(27)13-16/h2-7,13,18,20,23,30,32H,1,8-12,14H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned KOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50493749

(CHEMBL2437063)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]5([H])C=C[C@H]2NC(=O)\C=C\c1ccccc1)ccc3O |r,c:19,THB:10:9:6.4.5:14| Show InChI InChI=1S/C26H26N2O3/c1-28-14-13-26-18-9-10-19(27-22(30)12-7-16-5-3-2-4-6-16)25(26)31-24-21(29)11-8-17(23(24)26)15-20(18)28/h2-12,18-20,25,29H,13-15H2,1H3,(H,27,30)/b12-7+/t18-,19+,20+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned mu opioid receptor expressed in CHO cells after 90 mins |
Eur J Med Chem 69: 786-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.031
BindingDB Entry DOI: 10.7270/Q2MK6GTW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50346951

(CHEMBL1795711 | CHEMBL1795714)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C27H29IN2O4/c28-18-3-1-2-17(12-18)25(32)29-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-30(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50395117
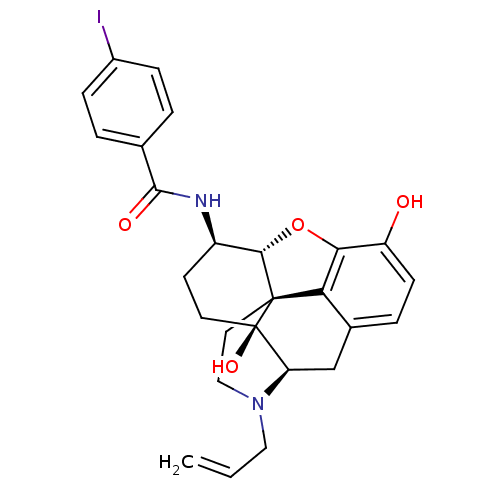
(CHEMBL2163536)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(I)cc1 |r| Show InChI InChI=1S/C26H27IN2O4/c1-2-12-29-13-11-25-21-16-5-8-19(30)22(21)33-23(25)18(9-10-26(25,32)20(29)14-16)28-24(31)15-3-6-17(27)7-4-15/h2-8,18,20,23,30,32H,1,9-14H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50493758
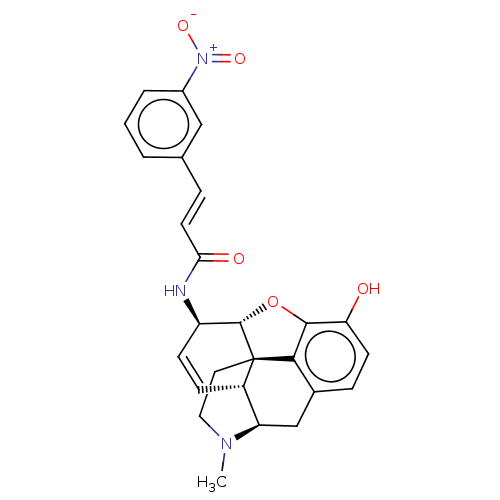
(CHEMBL2437067)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]5([H])C=C[C@H]2NC(=O)\C=C\c1cccc(c1)[N+]([O-])=O)ccc3O |r,c:19,THB:10:9:6.4.5:14| Show InChI InChI=1S/C26H25N3O5/c1-28-12-11-26-18-7-8-19(27-22(31)10-5-15-3-2-4-17(13-15)29(32)33)25(26)34-24-21(30)9-6-16(23(24)26)14-20(18)28/h2-10,13,18-20,25,30H,11-12,14H2,1H3,(H,27,31)/b10-5+/t18-,19+,20+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned mu opioid receptor expressed in CHO cells after 90 mins |
Eur J Med Chem 69: 786-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.031
BindingDB Entry DOI: 10.7270/Q2MK6GTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM85821

(OFQ/N,Iodo[Tyr14])Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(I)cc1)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C82H126IN27O22/c1-43(98-64(116)41-97-79(130)66(46(4)112)110-77(128)57(36-48-19-9-6-10-20-48)101-65(117)40-95-63(115)39-96-70(121)51(86)35-47-17-7-5-8-18-47)67(118)102-54(23-15-33-93-81(89)90)71(122)105-53(22-12-14-32-85)74(125)109-60(42-111)78(129)100-44(2)68(119)103-55(24-16-34-94-82(91)92)72(123)104-52(21-11-13-31-84)73(124)108-58(37-49-25-27-50(83)28-26-49)75(126)99-45(3)69(120)107-59(38-62(88)114)76(127)106-56(80(131)132)29-30-61(87)113/h5-10,17-20,25-28,43-46,51-60,66,111-112H,11-16,21-24,29-42,84-86H2,1-4H3,(H2,87,113)(H2,88,114)(H,95,115)(H,96,121)(H,97,130)(H,98,116)(H,99,126)(H,100,129)(H,101,117)(H,102,118)(H,103,119)(H,104,123)(H,105,122)(H,106,127)(H,107,120)(H,108,124)(H,109,125)(H,110,128)(H,131,132)(H4,89,90,93)(H4,91,92,94)/t43-,44-,45-,46+,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 381-415 (2001)
BindingDB Entry DOI: 10.7270/Q2QJ7FWB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50189257
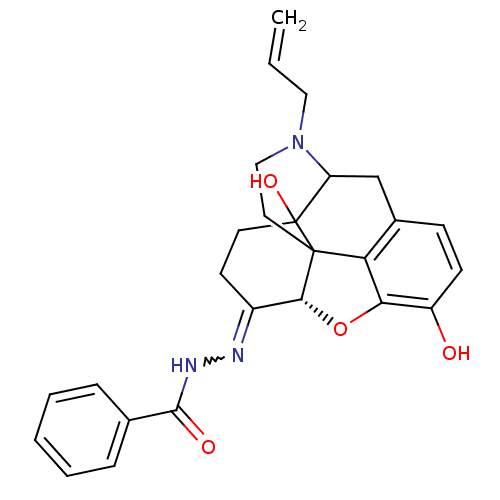
(CHEMBL378753 | NalBzOH)Show SMILES Oc1ccc2CC3N(CC=C)CCC45[C@H](Oc1c24)C(CCC35O)=NNC(=O)c1ccccc1 |w:23.28,TLB:22:21:7.11.12:17.5.4,THB:8:7:21:17.5.4,16:17:21:7.11.12| Show InChI InChI=1S/C26H27N3O4/c1-2-13-29-14-12-25-21-17-8-9-19(30)22(21)33-23(25)18(10-11-26(25,32)20(29)15-17)27-28-24(31)16-6-4-3-5-7-16/h2-9,20,23,30,32H,1,10-15H2,(H,28,31)/t20?,23-,25?,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21865

((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50346950
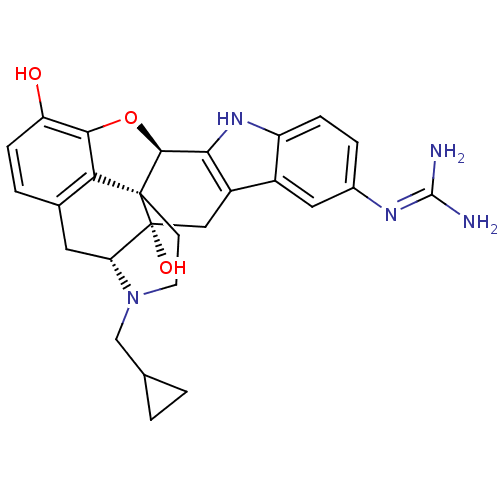
(CHEMBL485832)Show SMILES NC(N)=Nc1ccc2[nH]c3[C@@H]4Oc5c6c(C[C@H]7N(CC8CC8)CC[C@@]46[C@@]7(O)Cc3c2c1)ccc5O |r,wU:25.29,16.16,wD:24.26,10.10,(29.24,2.21,;28.18,1.09,;28.63,-.39,;26.68,1.44,;25.63,.32,;26.07,-1.15,;25.02,-2.26,;23.54,-1.9,;22.28,-2.76,;21.07,-1.84,;19.56,-2.11,;18.6,-3.32,;16.7,-2.72,;17.22,-1.24,;16.07,-.08,;16.57,1.37,;18.08,1.66,;17.24,3.55,;17.28,5.09,;18.63,5.82,;20.18,5.78,;19.45,7.14,;16.72,1.91,;17.37,-.05,;18.71,-.83,;19.2,.35,;19.7,1.82,;20.59,.77,;21.58,-.4,;23.11,-.44,;24.15,.67,;14.56,-.37,;14.05,-1.82,;15.05,-2.99,;14.52,-4.44,)| Show InChI InChI=1S/C27H29N5O3/c28-25(29)30-15-4-5-18-16(10-15)17-11-27(34)20-9-14-3-6-19(33)23-21(14)26(27,24(35-23)22(17)31-18)7-8-32(20)12-13-1-2-13/h3-6,10,13,20,24,31,33-34H,1-2,7-9,11-12H2,(H4,28,29,30)/t20-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from KOR-1 expressed in CHO cells |
Bioorg Med Chem Lett 21: 4001-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.008
BindingDB Entry DOI: 10.7270/Q29C6ZDF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50493755

(CHEMBL2437064)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]5([H])C=C[C@H]2NC(=O)\C=C\c1ccc(Cl)cc1)ccc3O |r,c:19,THB:10:9:6.4.5:14| Show InChI InChI=1S/C26H25ClN2O3/c1-29-13-12-26-18-8-9-19(28-22(31)11-4-15-2-6-17(27)7-3-15)25(26)32-24-21(30)10-5-16(23(24)26)14-20(18)29/h2-11,18-20,25,30H,12-14H2,1H3,(H,28,31)/b11-4+/t18-,19+,20+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned mu opioid receptor expressed in CHO cells after 90 mins |
Eur J Med Chem 69: 786-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.031
BindingDB Entry DOI: 10.7270/Q2MK6GTW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123752
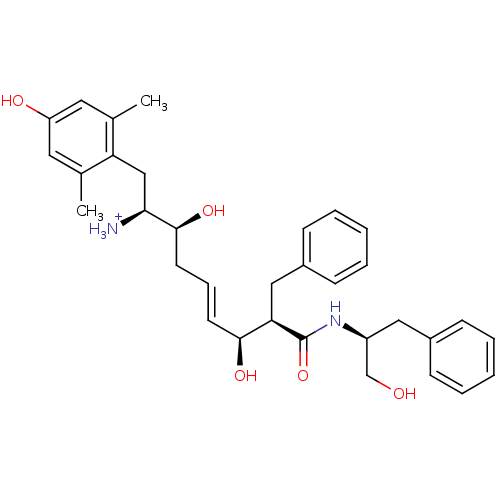
((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)N[C@H](CO)Cc1ccccc1 Show InChI InChI=1S/C33H42N2O5/c1-22-16-27(37)17-23(2)28(22)20-30(34)32(39)15-9-14-31(38)29(19-25-12-7-4-8-13-25)33(40)35-26(21-36)18-24-10-5-3-6-11-24/h3-14,16-17,26,29-32,36-39H,15,18-21,34H2,1-2H3,(H,35,40)/p+1/b14-9+/t26-,29+,30-,31-,32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123763
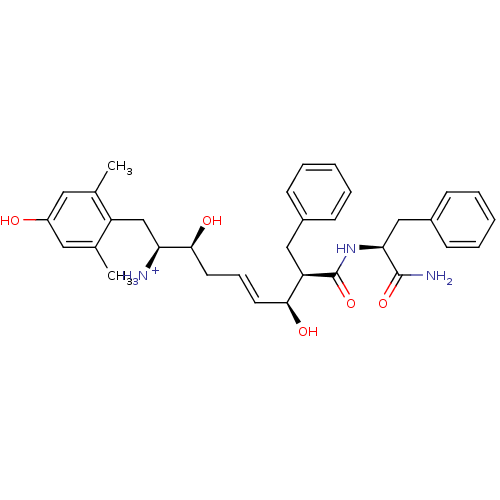
((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C33H41N3O5/c1-21-16-25(37)17-22(2)26(21)20-28(34)31(39)15-9-14-30(38)27(18-23-10-5-3-6-11-23)33(41)36-29(32(35)40)19-24-12-7-4-8-13-24/h3-14,16-17,27-31,37-39H,15,18-20,34H2,1-2H3,(H2,35,40)(H,36,41)/p+1/b14-9+/t27-,28+,29+,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM21842

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 381-415 (2001)
BindingDB Entry DOI: 10.7270/Q2QJ7FWB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21025

((2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydro...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C29H39N5O7/c1-17(2)13-24(29(40)41)34-28(39)23(15-19-7-5-4-6-8-19)33-25(36)16-31-26(37)18(3)32-27(38)22(30)14-20-9-11-21(35)12-10-20/h4-12,17-18,22-24,35H,13-16,30H2,1-3H3,(H,31,37)(H,32,38)(H,33,36)(H,34,39)(H,40,41)/t18-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1246-55 (1994)
BindingDB Entry DOI: 10.7270/Q2CF9NN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50395110
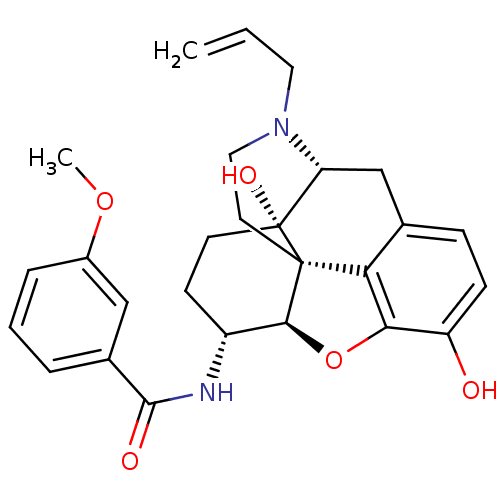
(CHEMBL2163543)Show SMILES COc1cccc(c1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC=C)c45 |r| Show InChI InChI=1S/C27H30N2O5/c1-3-12-29-13-11-26-22-16-7-8-20(30)23(22)34-24(26)19(9-10-27(26,32)21(29)15-16)28-25(31)17-5-4-6-18(14-17)33-2/h3-8,14,19,21,24,30,32H,1,9-13,15H2,2H3,(H,28,31)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50493757

(CHEMBL2437065)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]5([H])C=C[C@H]2NC(=O)\C=C\c1ccc(cc1)C(F)(F)F)ccc3O |r,c:19,THB:10:9:6.4.5:14| Show InChI InChI=1S/C27H25F3N2O3/c1-32-13-12-26-18-8-9-19(25(26)35-24-21(33)10-5-16(23(24)26)14-20(18)32)31-22(34)11-4-15-2-6-17(7-3-15)27(28,29)30/h2-11,18-20,25,33H,12-14H2,1H3,(H,31,34)/b11-4+/t18-,19+,20+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned mu opioid receptor expressed in CHO cells after 90 mins |
Eur J Med Chem 69: 786-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.031
BindingDB Entry DOI: 10.7270/Q2MK6GTW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50346950

(CHEMBL485832)Show SMILES NC(N)=Nc1ccc2[nH]c3[C@@H]4Oc5c6c(C[C@H]7N(CC8CC8)CC[C@@]46[C@@]7(O)Cc3c2c1)ccc5O |r,wU:25.29,16.16,wD:24.26,10.10,(29.24,2.21,;28.18,1.09,;28.63,-.39,;26.68,1.44,;25.63,.32,;26.07,-1.15,;25.02,-2.26,;23.54,-1.9,;22.28,-2.76,;21.07,-1.84,;19.56,-2.11,;18.6,-3.32,;16.7,-2.72,;17.22,-1.24,;16.07,-.08,;16.57,1.37,;18.08,1.66,;17.24,3.55,;17.28,5.09,;18.63,5.82,;20.18,5.78,;19.45,7.14,;16.72,1.91,;17.37,-.05,;18.71,-.83,;19.2,.35,;19.7,1.82,;20.59,.77,;21.58,-.4,;23.11,-.44,;24.15,.67,;14.56,-.37,;14.05,-1.82,;15.05,-2.99,;14.52,-4.44,)| Show InChI InChI=1S/C27H29N5O3/c28-25(29)30-15-4-5-18-16(10-15)17-11-27(34)20-9-14-3-6-19(33)23-21(14)26(27,24(35-23)22(17)31-18)7-8-32(20)12-13-1-2-13/h3-6,10,13,20,24,31,33-34H,1-2,7-9,11-12H2,(H4,28,29,30)/t20-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNalA from KOR-1 expressed in CHO cells |
Bioorg Med Chem Lett 21: 4001-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.008
BindingDB Entry DOI: 10.7270/Q29C6ZDF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNalA from KOR-1 expressed in CHO cells |
Bioorg Med Chem Lett 21: 4001-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.008
BindingDB Entry DOI: 10.7270/Q29C6ZDF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50189257

(CHEMBL378753 | NalBzOH)Show SMILES Oc1ccc2CC3N(CC=C)CCC45[C@H](Oc1c24)C(CCC35O)=NNC(=O)c1ccccc1 |w:23.28,TLB:22:21:7.11.12:17.5.4,THB:8:7:21:17.5.4,16:17:21:7.11.12| Show InChI InChI=1S/C26H27N3O4/c1-2-13-29-14-12-25-21-17-8-9-19(30)22(21)33-23(25)18(10-11-26(25,32)20(29)15-17)27-28-24(31)16-6-4-3-5-7-16/h2-9,20,23,30,32H,1,10-15H2,(H,28,31)/t20?,23-,25?,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50346952

(CHEMBL1795712 | CHEMBL1795715)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C26H27IN2O4/c1-2-11-29-12-10-25-21-15-6-7-19(30)22(21)33-23(25)18(8-9-26(25,32)20(29)14-15)28-24(31)16-4-3-5-17(27)13-16/h2-7,13,18,20,23,30,32H,1,8-12,14H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50395111
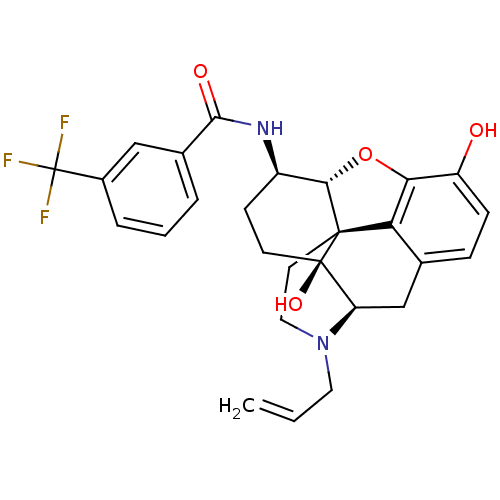
(CHEMBL2163542)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H27F3N2O4/c1-2-11-32-12-10-25-21-15-6-7-19(33)22(21)36-23(25)18(8-9-26(25,35)20(32)14-15)31-24(34)16-4-3-5-17(13-16)27(28,29)30/h2-7,13,18,20,23,33,35H,1,8-12,14H2,(H,31,34)/t18-,20-,23+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned KOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50395107
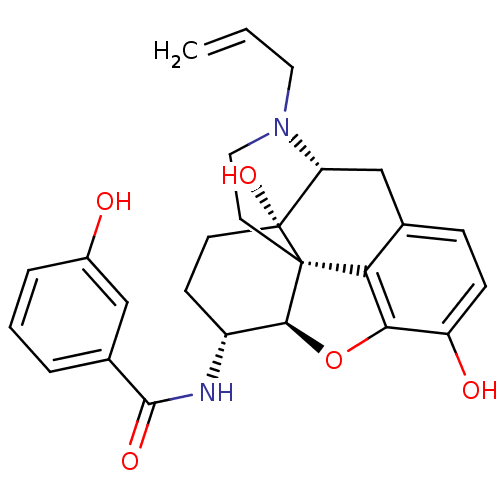
(CHEMBL2163546)Show SMILES Oc1cccc(c1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC=C)c45 |r| Show InChI InChI=1S/C26H28N2O5/c1-2-11-28-12-10-25-21-15-6-7-19(30)22(21)33-23(25)18(8-9-26(25,32)20(28)14-15)27-24(31)16-4-3-5-17(29)13-16/h2-7,13,18,20,23,29-30,32H,1,8-12,14H2,(H,27,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from KOR-1 expressed in CHO cells |
Bioorg Med Chem Lett 21: 4001-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.008
BindingDB Entry DOI: 10.7270/Q29C6ZDF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50346951

(CHEMBL1795711 | CHEMBL1795714)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C27H29IN2O4/c28-18-3-1-2-17(12-18)25(32)29-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-30(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-U69593 from KOR-1 expressed in CHO cells after 60 mins |
Bioorg Med Chem Lett 21: 4001-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.008
BindingDB Entry DOI: 10.7270/Q29C6ZDF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM86518
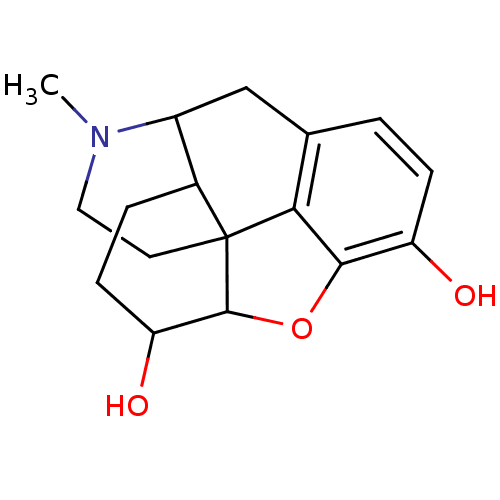
(CAS_509-60-4 | DIHYDROMORPHINE | NSC_273032)Show SMILES CN1CCC23C4Oc5c2c(CC1C3CCC4O)ccc5O |TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H21NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2,4,10-11,13,16,19-20H,3,5-8H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 492: 123-30 (2004)
Article DOI: 10.1016/j.ejphar.2004.03.050
BindingDB Entry DOI: 10.7270/Q2416VMW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50346951

(CHEMBL1795711 | CHEMBL1795714)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C27H29IN2O4/c28-18-3-1-2-17(12-18)25(32)29-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-30(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned DOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123754
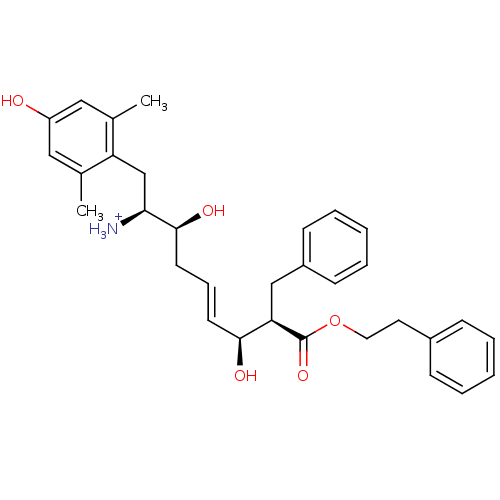
((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)OCCc1ccccc1 Show InChI InChI=1S/C32H39NO5/c1-22-18-26(34)19-23(2)27(22)21-29(33)31(36)15-9-14-30(35)28(20-25-12-7-4-8-13-25)32(37)38-17-16-24-10-5-3-6-11-24/h3-14,18-19,28-31,34-36H,15-17,20-21,33H2,1-2H3/p+1/b14-9+/t28-,29+,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123756
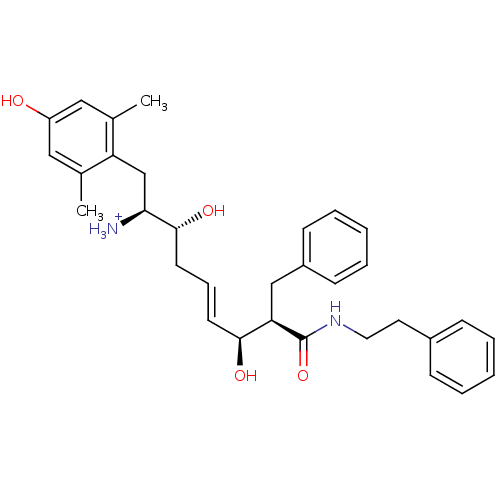
((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C32H40N2O4/c1-22-18-26(35)19-23(2)27(22)21-29(33)31(37)15-9-14-30(36)28(20-25-12-7-4-8-13-25)32(38)34-17-16-24-10-5-3-6-11-24/h3-14,18-19,28-31,35-37H,15-17,20-21,33H2,1-2H3,(H,34,38)/p+1/b14-9+/t28-,29+,30+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095150
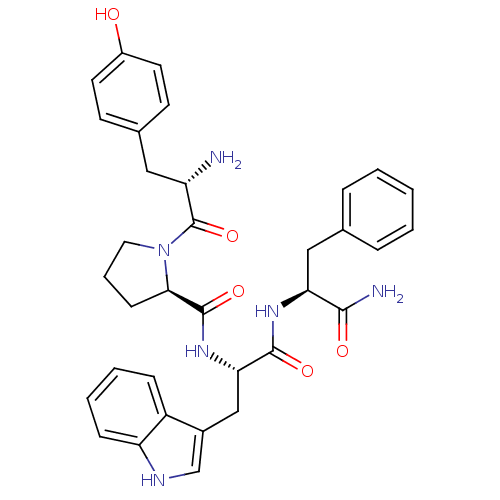
((R)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21865

((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1246-55 (1994)
BindingDB Entry DOI: 10.7270/Q2CF9NN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50395117

(CHEMBL2163536)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(I)cc1 |r| Show InChI InChI=1S/C26H27IN2O4/c1-2-12-29-13-11-25-21-16-5-8-19(30)22(21)33-23(25)18(9-10-26(25,32)20(29)14-16)28-24(31)15-3-6-17(27)7-4-15/h2-8,18,20,23,30,32H,1,9-14H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned KOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50395112
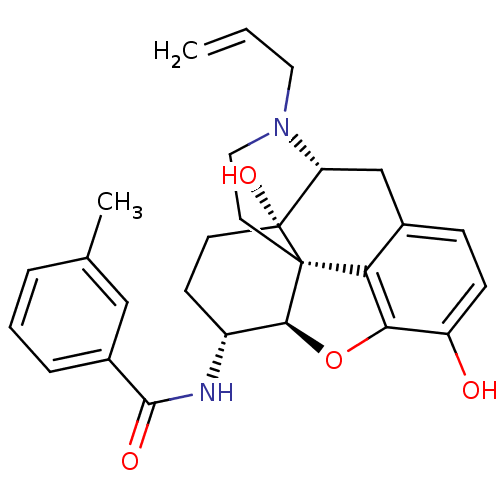
(CHEMBL2163541)Show SMILES Cc1cccc(c1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC=C)c45 |r| Show InChI InChI=1S/C27H30N2O4/c1-3-12-29-13-11-26-22-17-7-8-20(30)23(22)33-24(26)19(9-10-27(26,32)21(29)15-17)28-25(31)18-6-4-5-16(2)14-18/h3-8,14,19,21,24,30,32H,1,9-13,15H2,2H3,(H,28,31)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123750
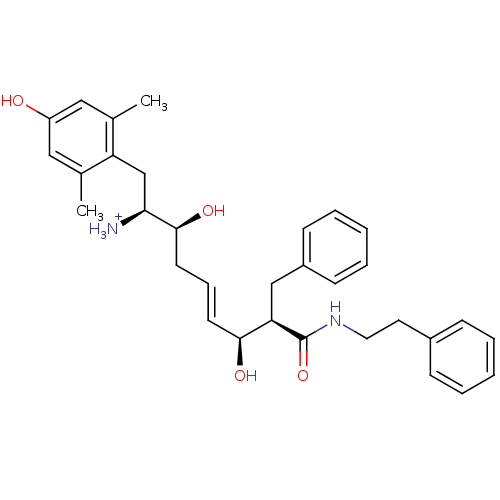
((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C32H40N2O4/c1-22-18-26(35)19-23(2)27(22)21-29(33)31(37)15-9-14-30(36)28(20-25-12-7-4-8-13-25)32(38)34-17-16-24-10-5-3-6-11-24/h3-14,18-19,28-31,35-37H,15-17,20-21,33H2,1-2H3,(H,34,38)/p+1/b14-9+/t28-,29+,30+,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50189257

(CHEMBL378753 | NalBzOH)Show SMILES Oc1ccc2CC3N(CC=C)CCC45[C@H](Oc1c24)C(CCC35O)=NNC(=O)c1ccccc1 |w:23.28,TLB:22:21:7.11.12:17.5.4,THB:8:7:21:17.5.4,16:17:21:7.11.12| Show InChI InChI=1S/C26H27N3O4/c1-2-13-29-14-12-25-21-17-8-9-19(30)22(21)33-23(25)18(10-11-26(25,32)20(29)15-17)27-28-24(31)16-6-4-3-5-7-16/h2-9,20,23,30,32H,1,10-15H2,(H,28,31)/t20?,23-,25?,26?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1246-55 (1994)
BindingDB Entry DOI: 10.7270/Q2CF9NN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21865

((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1246-55 (1994)
BindingDB Entry DOI: 10.7270/Q2CF9NN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000507
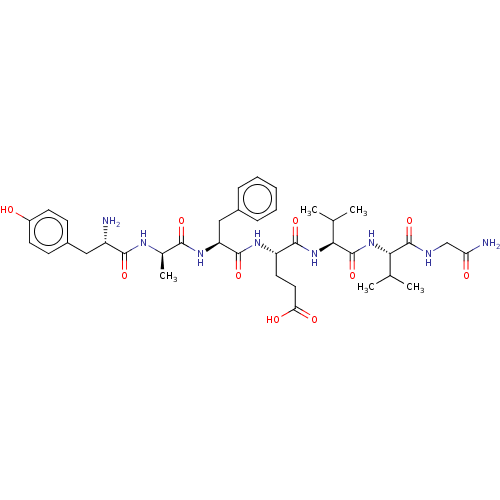
((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C38H54N8O10/c1-20(2)31(37(55)41-19-29(40)48)46-38(56)32(21(3)4)45-35(53)27(15-16-30(49)50)43-36(54)28(18-23-9-7-6-8-10-23)44-33(51)22(5)42-34(52)26(39)17-24-11-13-25(47)14-12-24/h6-14,20-22,26-28,31-32,47H,15-19,39H2,1-5H3,(H2,40,48)(H,41,55)(H,42,52)(H,43,54)(H,44,51)(H,45,53)(H,46,56)(H,49,50)/t22-,26+,27+,28+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1246-55 (1994)
BindingDB Entry DOI: 10.7270/Q2CF9NN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123751
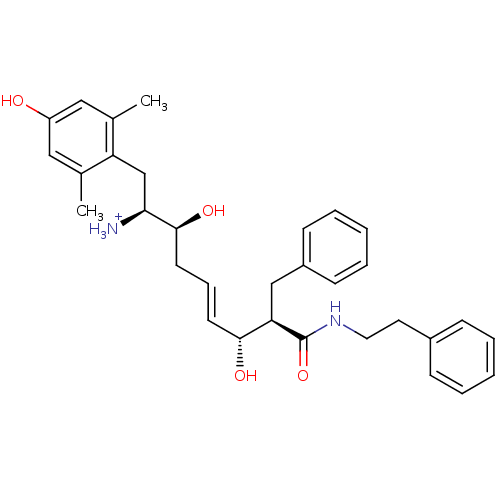
((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@@H](O)[C@@H](Cc1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C32H40N2O4/c1-22-18-26(35)19-23(2)27(22)21-29(33)31(37)15-9-14-30(36)28(20-25-12-7-4-8-13-25)32(38)34-17-16-24-10-5-3-6-11-24/h3-14,18-19,28-31,35-37H,15-17,20-21,33H2,1-2H3,(H,34,38)/p+1/b14-9+/t28-,29+,30-,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50395109
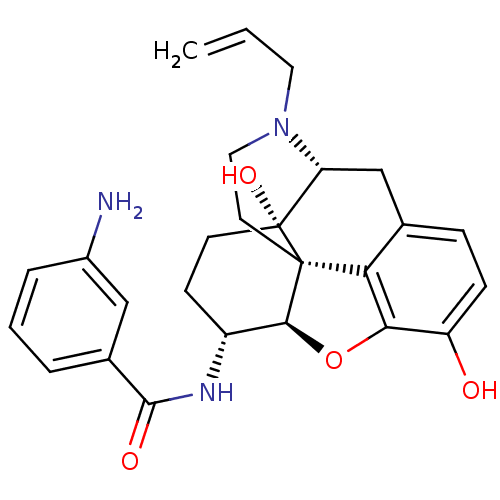
(CHEMBL2163544)Show SMILES Nc1cccc(c1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC=C)c45 |r| Show InChI InChI=1S/C26H29N3O4/c1-2-11-29-12-10-25-21-15-6-7-19(30)22(21)33-23(25)18(8-9-26(25,32)20(29)14-15)28-24(31)16-4-3-5-17(27)13-16/h2-7,13,18,20,23,30,32H,1,8-12,14,27H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned KOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123759
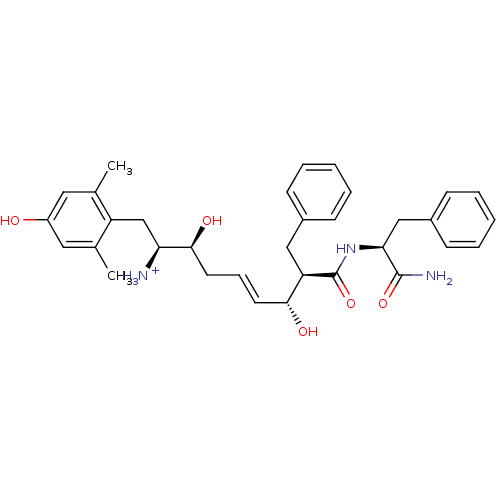
((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@@H](O)[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C33H41N3O5/c1-21-16-25(37)17-22(2)26(21)20-28(34)31(39)15-9-14-30(38)27(18-23-10-5-3-6-11-23)33(41)36-29(32(35)40)19-24-12-7-4-8-13-24/h3-14,16-17,27-31,37-39H,15,18-20,34H2,1-2H3,(H2,35,40)(H,36,41)/p+1/b14-9+/t27-,28+,29+,30-,31+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM50106276
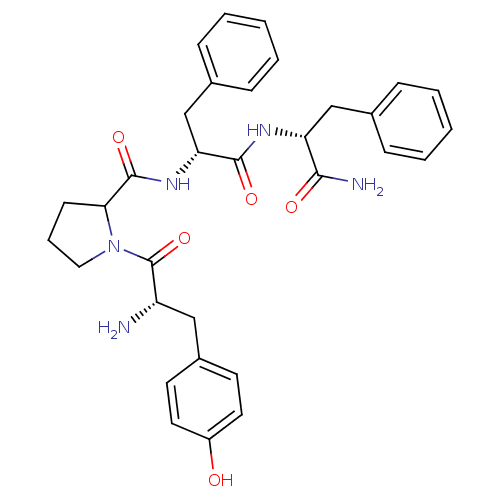
(1-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-pyrroli...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCCC1C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26+,27+,28?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50395109

(CHEMBL2163544)Show SMILES Nc1cccc(c1)C(=O)N[C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC=C)c45 |r| Show InChI InChI=1S/C26H29N3O4/c1-2-11-29-12-10-25-21-15-6-7-19(30)22(21)33-23(25)18(8-9-26(25,32)20(29)14-15)28-24(31)16-4-3-5-17(27)13-16/h2-7,13,18,20,23,30,32H,1,8-12,14,27H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50123762
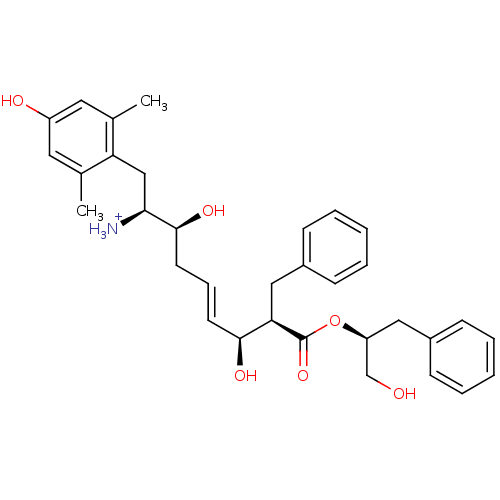
((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1C[C@H]([NH3+])[C@@H](O)C\C=C\[C@H](O)[C@@H](Cc1ccccc1)C(=O)O[C@H](CO)Cc1ccccc1 Show InChI InChI=1S/C33H41NO6/c1-22-16-26(36)17-23(2)28(22)20-30(34)32(38)15-9-14-31(37)29(19-25-12-7-4-8-13-25)33(39)40-27(21-35)18-24-10-5-3-6-11-24/h3-14,16-17,27,29-32,35-38H,15,18-21,34H2,1-2H3/p+1/b14-9+/t27-,29+,30-,31-,32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells |
J Med Chem 46: 677-80 (2003)
Article DOI: 10.1021/jm025608s
BindingDB Entry DOI: 10.7270/Q2959J96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data