

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400125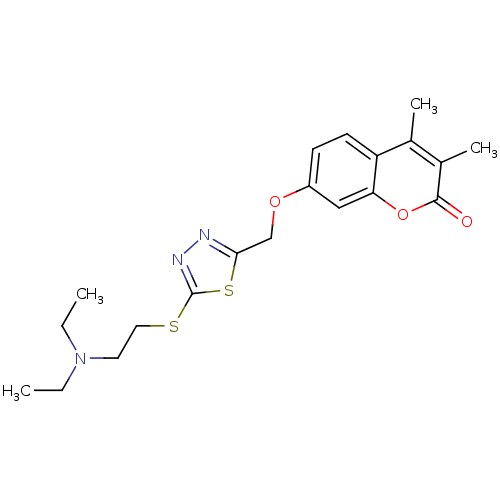 (CHEMBL2178426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400133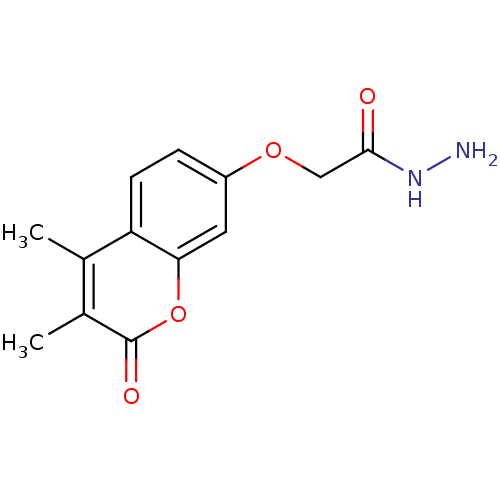 (CHEMBL2178987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400111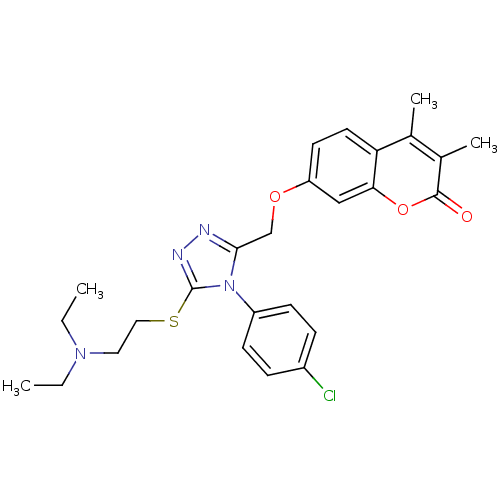 (CHEMBL2178440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400112 (CHEMBL2178439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400114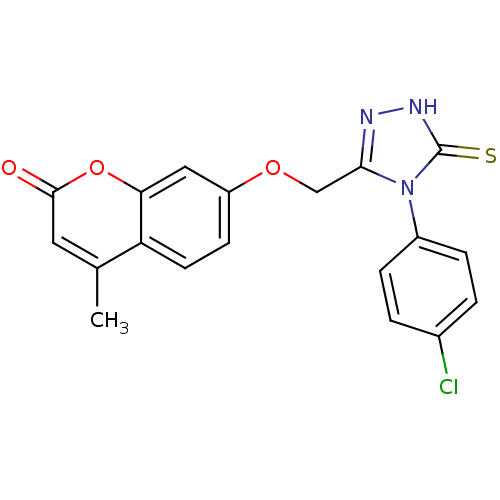 (CHEMBL2178437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400115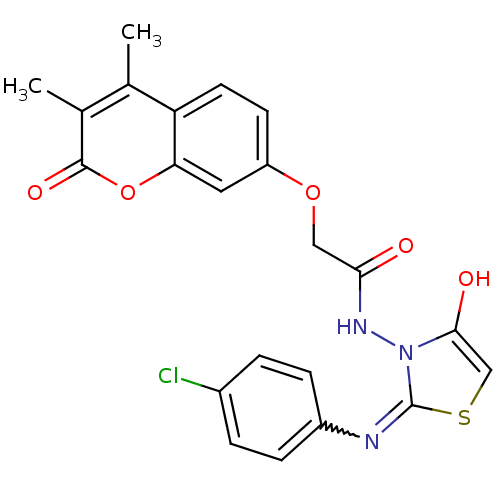 (CHEMBL2178436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400131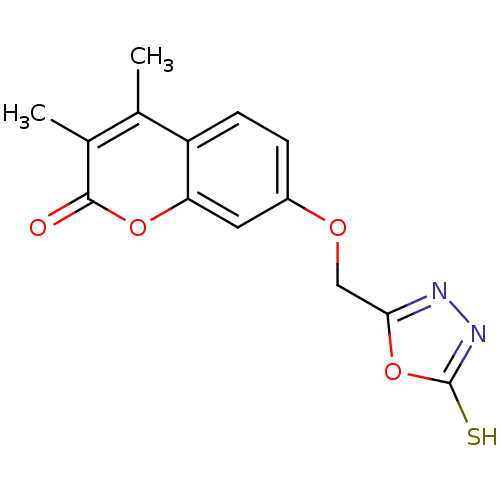 (CHEMBL2178989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400109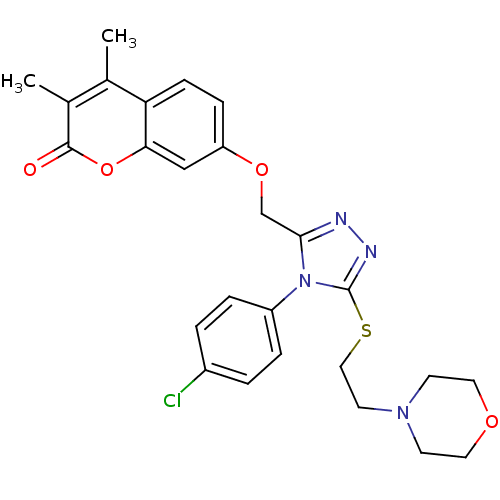 (CHEMBL2178442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400110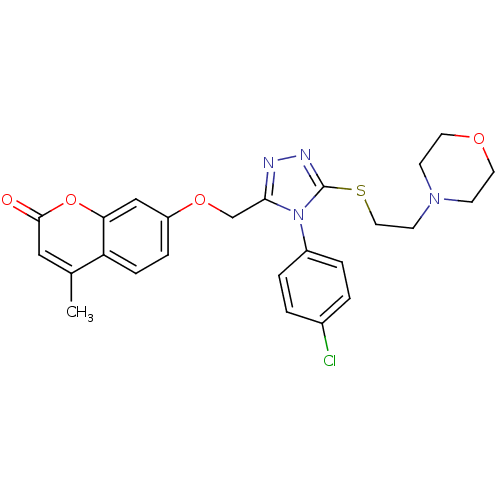 (CHEMBL2178441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400107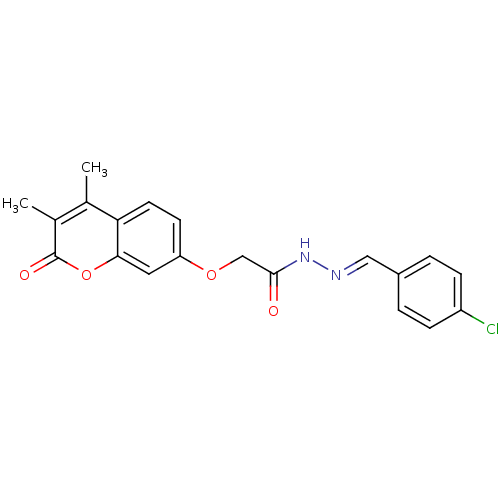 (CHEMBL2178981) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400105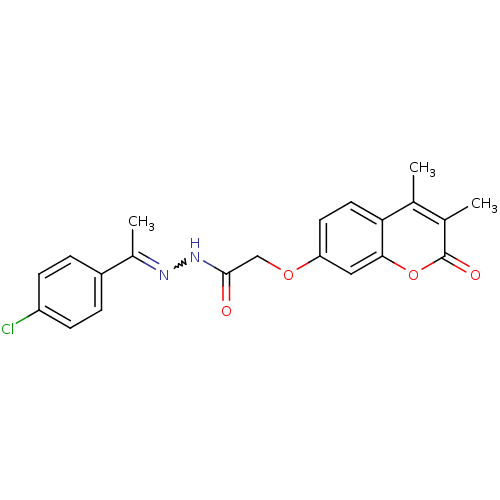 (CHEMBL2178983) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400103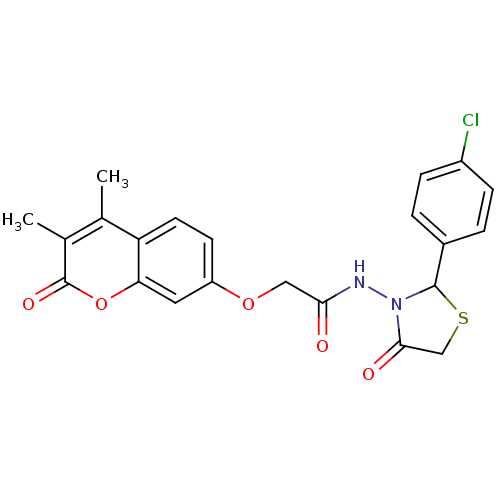 (CHEMBL2178985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400129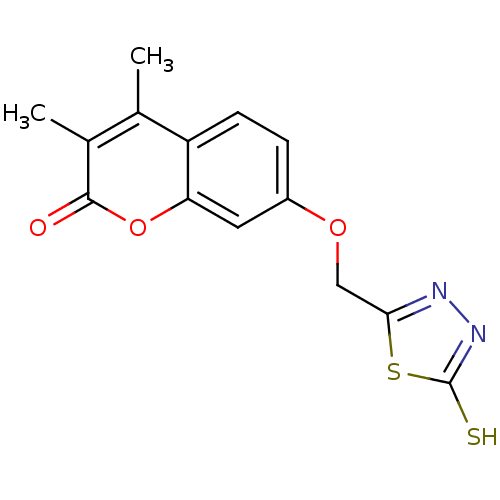 (CHEMBL2178991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400123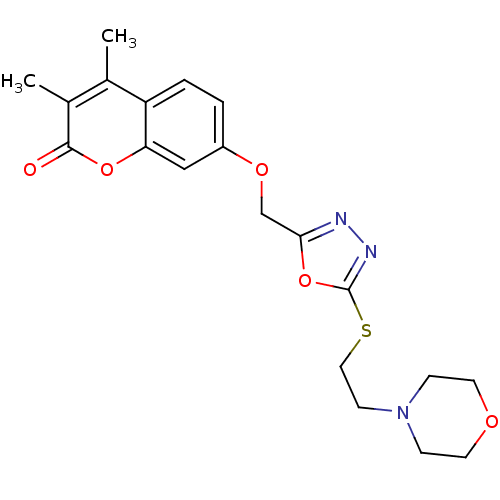 (CHEMBL2178428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400121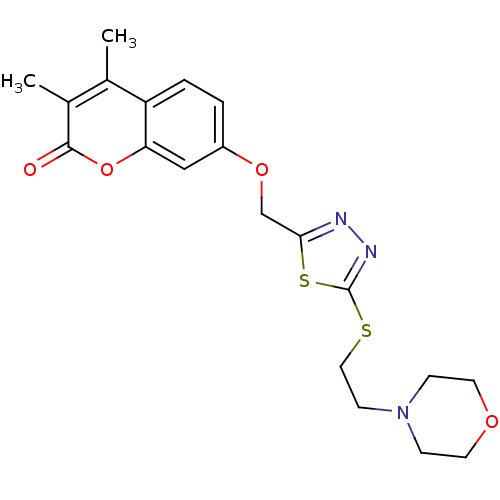 (CHEMBL2178430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400118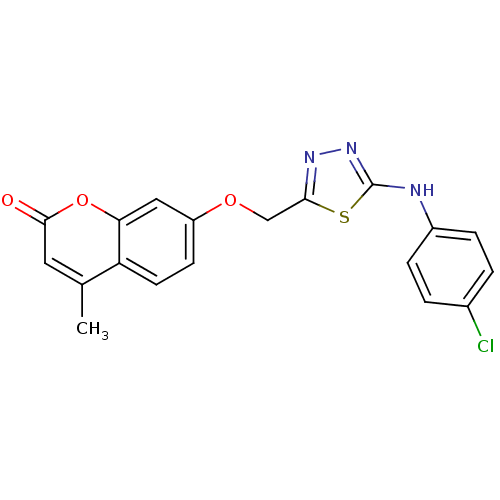 (CHEMBL2178433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400127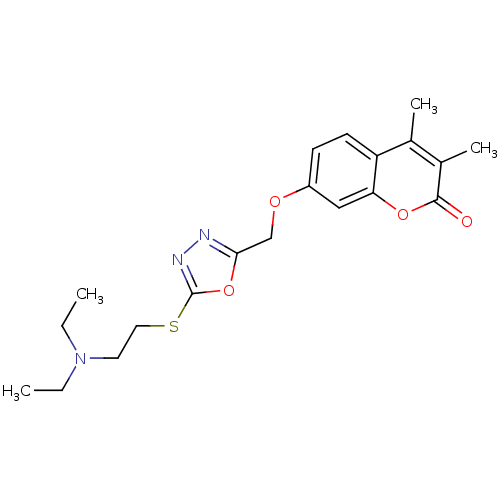 (CHEMBL2178993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400128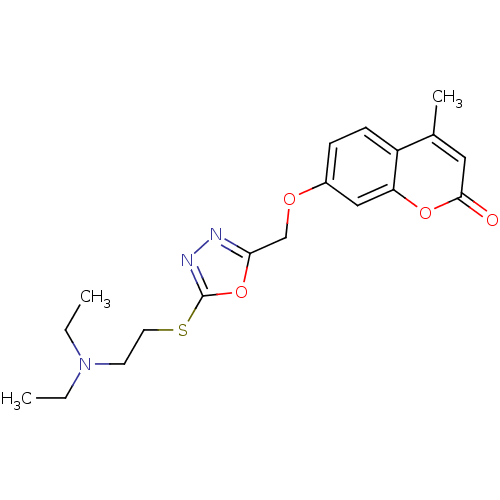 (CHEMBL2178992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400116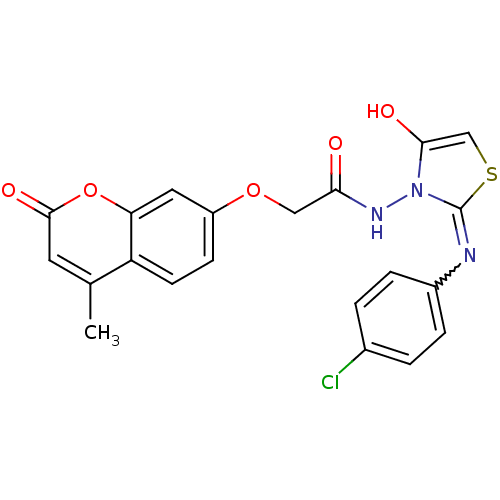 (CHEMBL2178435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400124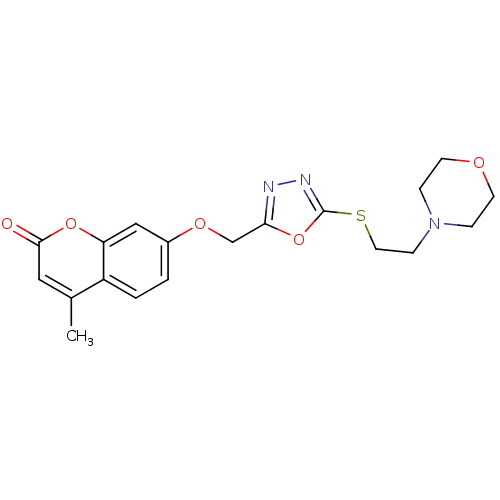 (CHEMBL2178427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400117 (CHEMBL2178434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400113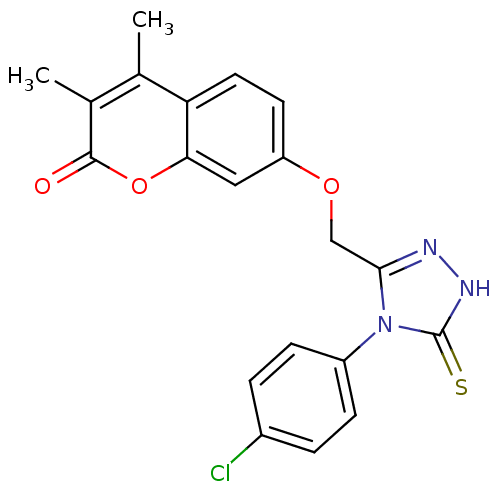 (CHEMBL2178438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400120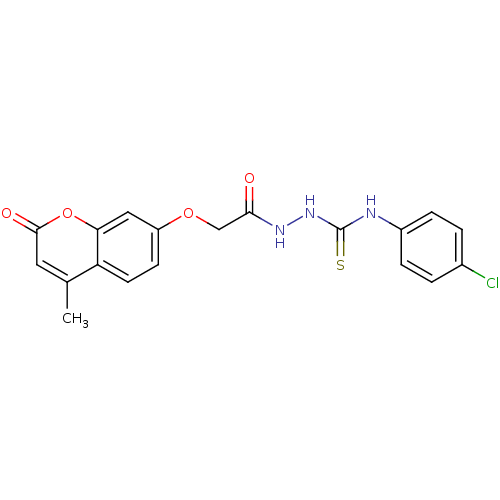 (CHEMBL2178431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400119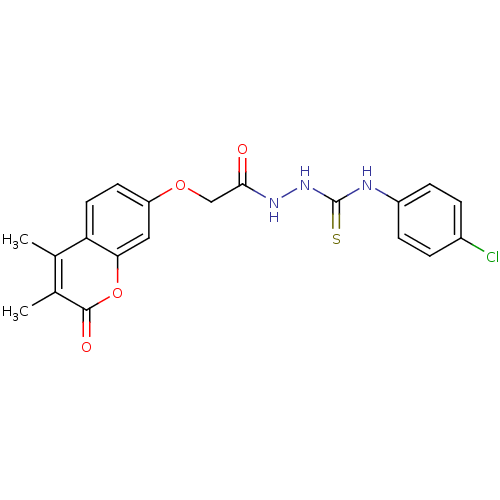 (CHEMBL2178432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400106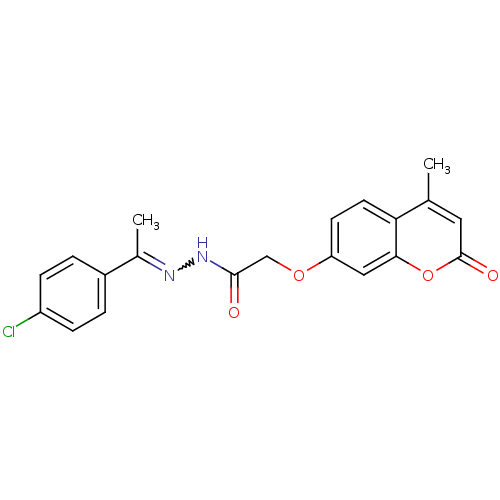 (CHEMBL2178982) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400108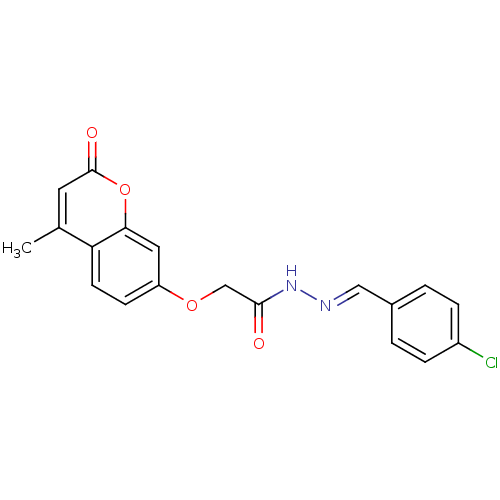 (CHEMBL2178980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400126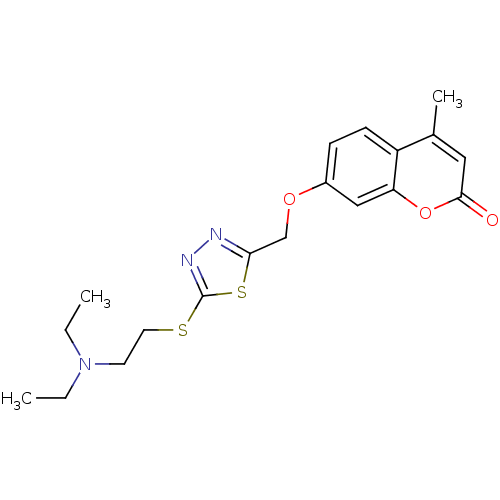 (CHEMBL2178425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400122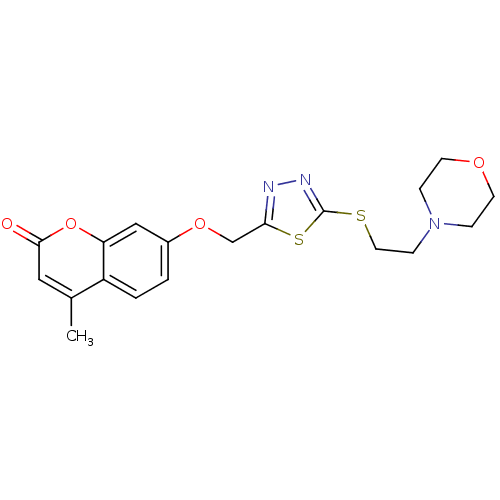 (CHEMBL2178429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400134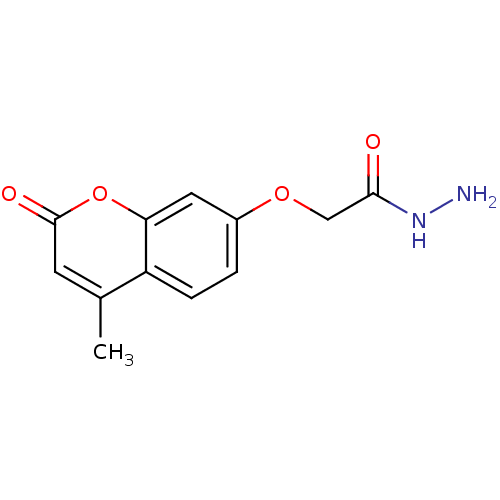 (CHEMBL2178986) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400130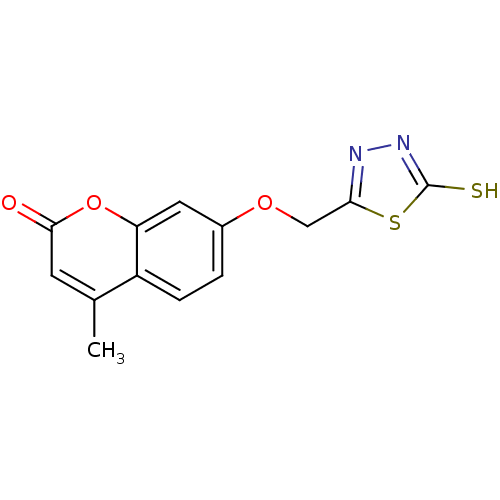 (CHEMBL2178990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400104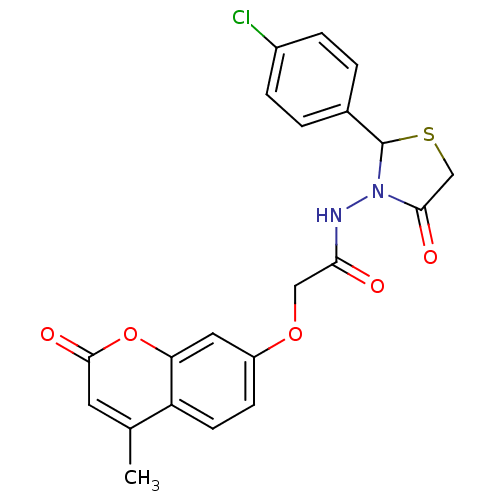 (CHEMBL2178984) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM50400132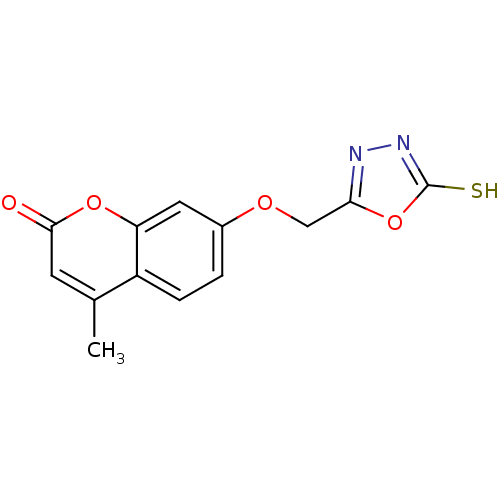 (CHEMBL2178988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center Curated by ChEMBL | Assay Description Inhibition of bovine brain MAOA using kinuramine as substrate preincubated for 30 mins prior to substrate addition measured after 30 mins by fluorome... | J Med Chem 55: 10424-36 (2012) Article DOI: 10.1021/jm301014y BindingDB Entry DOI: 10.7270/Q2G73FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50352114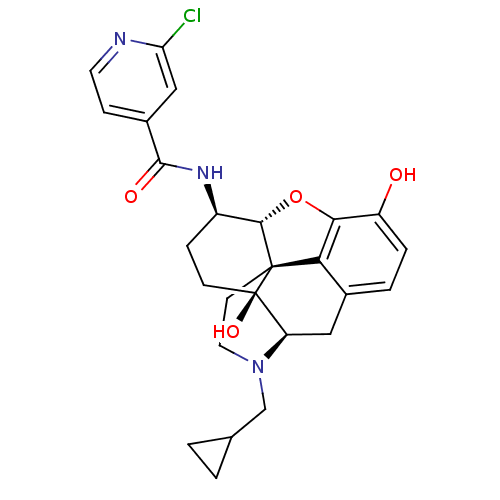 (CHEMBL1824509 | CHEMBL1852788) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50434787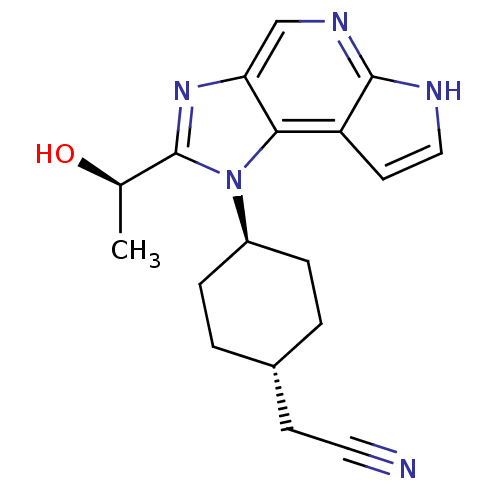 (CHEMBL2386635 | US10487083, Example C | US10703751...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant JAK1 (unknown origin) using Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 mins in presence of ATP | J Med Chem 56: 4764-85 (2013) Article DOI: 10.1021/jm4004895 BindingDB Entry DOI: 10.7270/Q2CV4K40 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50399663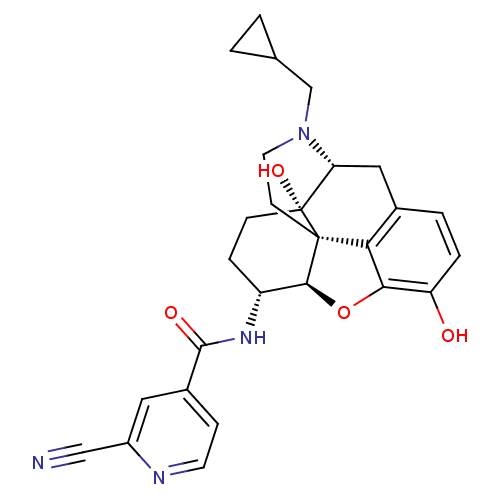 (CHEMBL2177697) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50352114 (CHEMBL1824509 | CHEMBL1852788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50508336 (CHEMBL4530500) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cell membranes by competitive radioligand binding assay | J Med Chem 62: 561-574 (2019) Article DOI: 10.1021/acs.jmedchem.8b01158 BindingDB Entry DOI: 10.7270/Q2CN776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50352115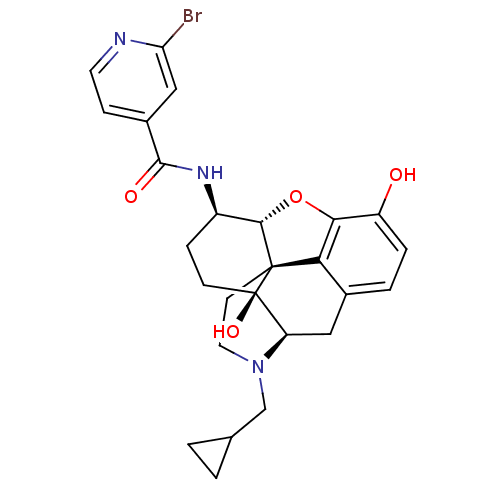 (CHEMBL1824510 | CHEMBL1852385) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50434786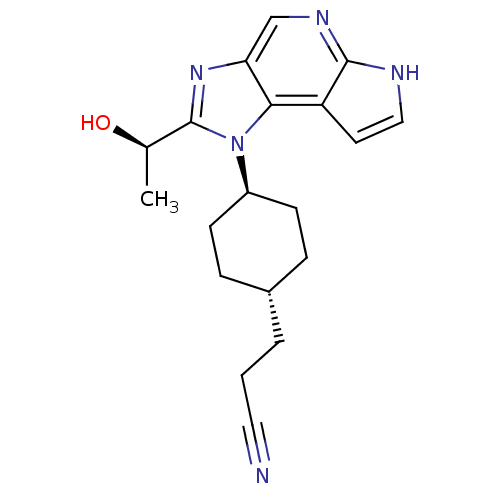 (CHEMBL2386636) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant JAK1 (unknown origin) using Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 mins in presence of ATP | J Med Chem 56: 4764-85 (2013) Article DOI: 10.1021/jm4004895 BindingDB Entry DOI: 10.7270/Q2CV4K40 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50508338 (CHEMBL4569785) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cell membranes by competitive radioligand binding assay | J Med Chem 62: 561-574 (2019) Article DOI: 10.1021/acs.jmedchem.8b01158 BindingDB Entry DOI: 10.7270/Q2CN776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50508331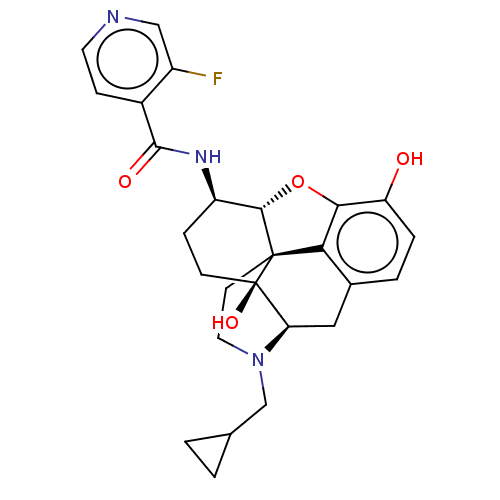 (CHEMBL4538841) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cell membranes by competitive radioligand binding assay | J Med Chem 62: 561-574 (2019) Article DOI: 10.1021/acs.jmedchem.8b01158 BindingDB Entry DOI: 10.7270/Q2CN776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50352116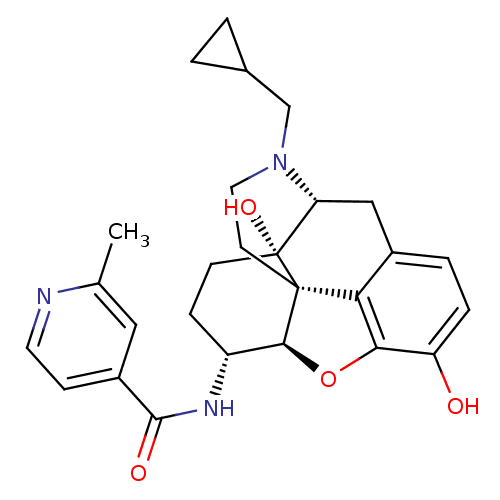 (CHEMBL1824512 | CHEMBL1852558) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using Leu-Pro-Leu-Asp-Lys-Asp-Tyr-Tyr-Val-Val-Arg as substrate after 30 mins in presence of ATP | J Med Chem 56: 4764-85 (2013) Article DOI: 10.1021/jm4004895 BindingDB Entry DOI: 10.7270/Q2CV4K40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50399661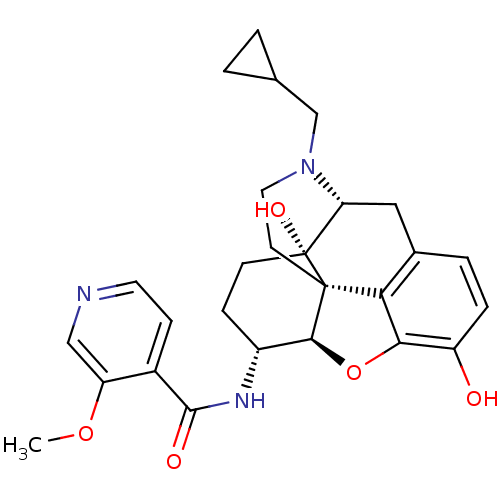 (CHEMBL2178339) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50508340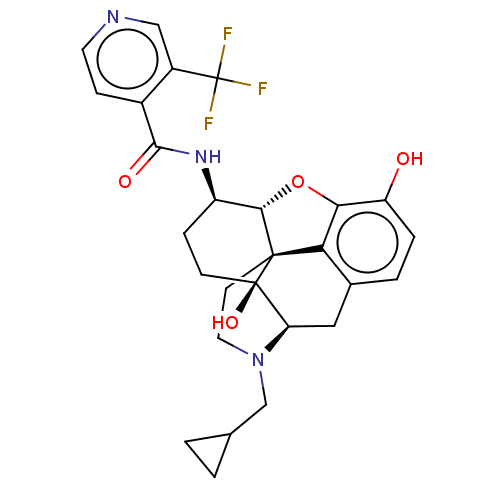 (CHEMBL4469986) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cell membranes by competitive radioligand binding assay | J Med Chem 62: 561-574 (2019) Article DOI: 10.1021/acs.jmedchem.8b01158 BindingDB Entry DOI: 10.7270/Q2CN776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50352118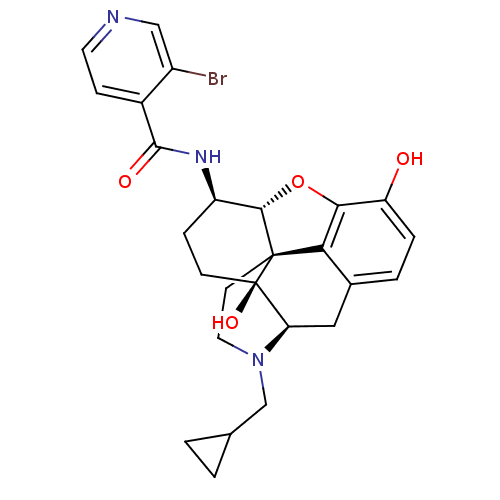 (CHEMBL1824513 | CHEMBL1852602) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50399662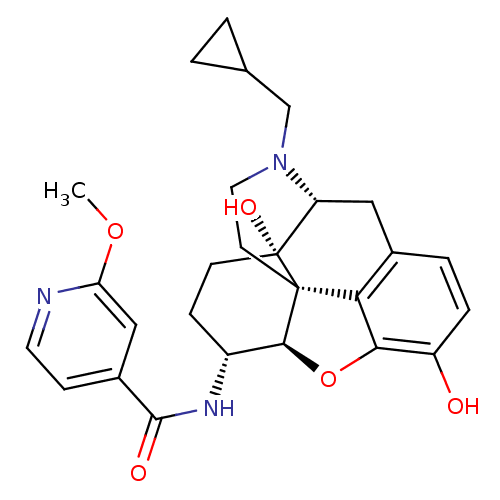 (CHEMBL2178338) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50352121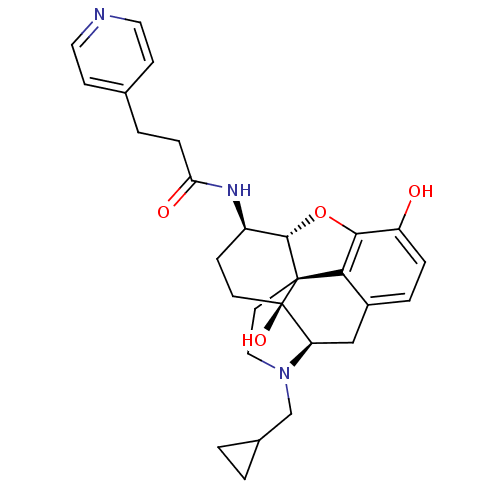 (CHEMBL1824511 | CHEMBL1852555) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50352116 (CHEMBL1824512 | CHEMBL1852558) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]DPN from kappa opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50352119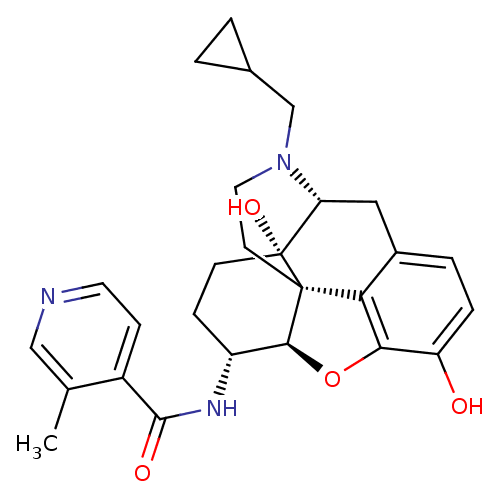 (CHEMBL1824515 | CHEMBL1852393) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from mu opioid receptor expressed in CHO cells after 1 hr | J Med Chem 55: 10118-29 (2012) Article DOI: 10.1021/jm301247n BindingDB Entry DOI: 10.7270/Q29P32SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1837 total ) | Next | Last >> |