

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50532207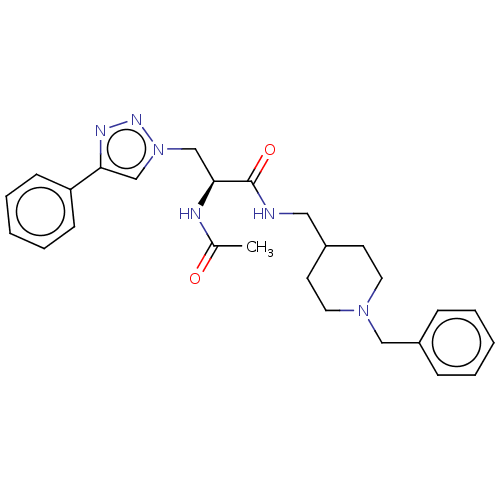 (CHEMBL4464964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50464942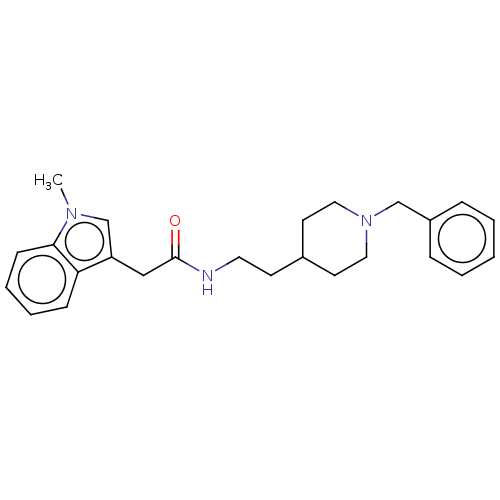 (CHEMBL4293015) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271142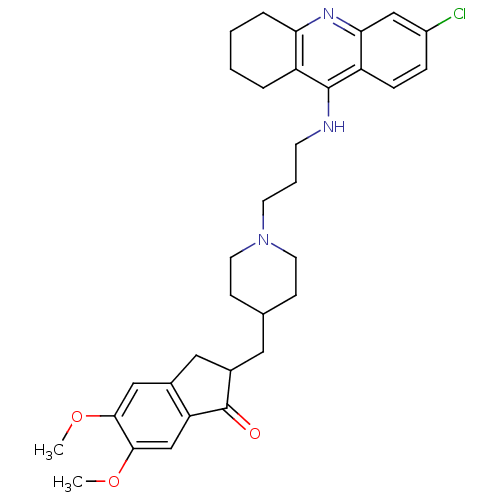 (6-Chloro-9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50464944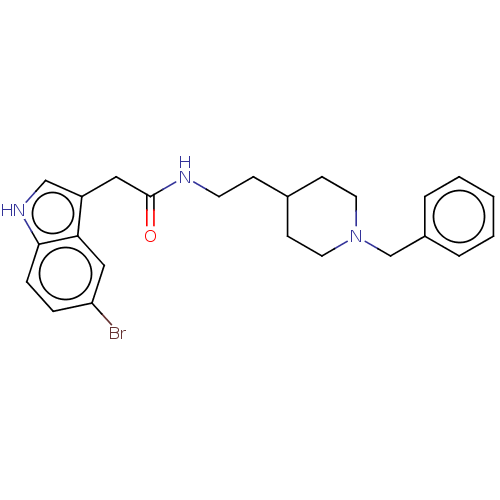 (CHEMBL4285818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50464946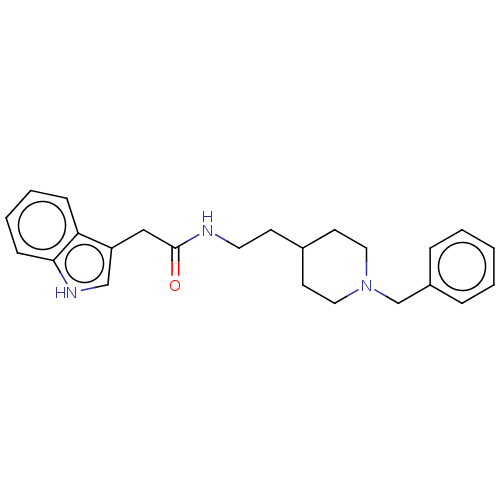 (CHEMBL4283651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50187201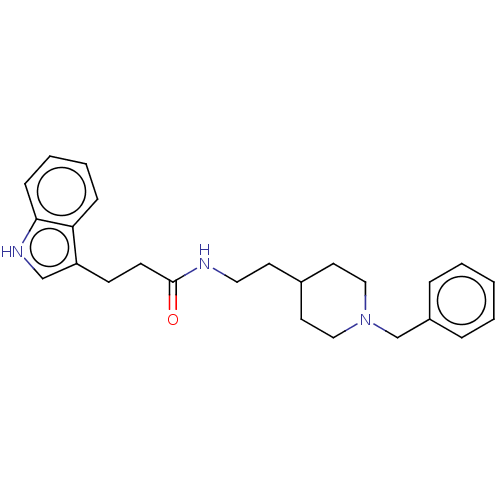 (CHEMBL3824248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50464947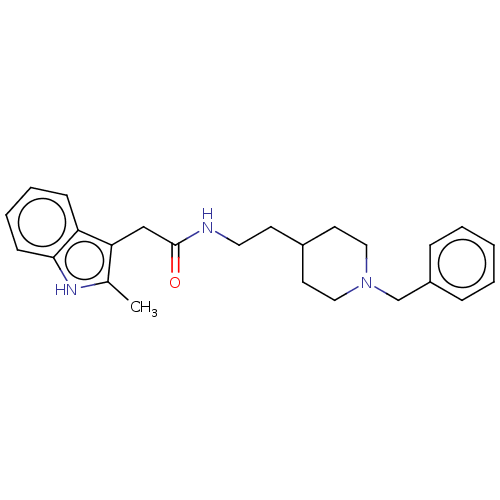 (CHEMBL4277879) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532198 (CHEMBL4520568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50464943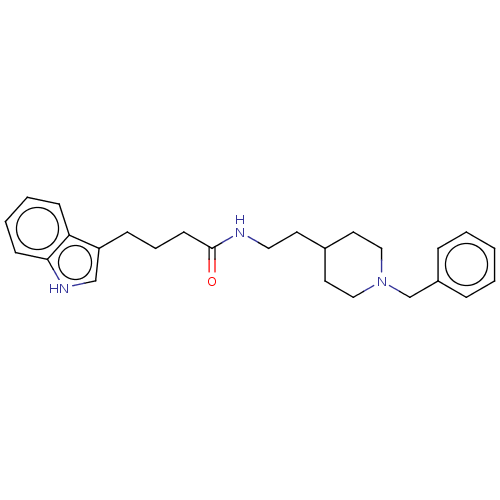 (CHEMBL4294109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277655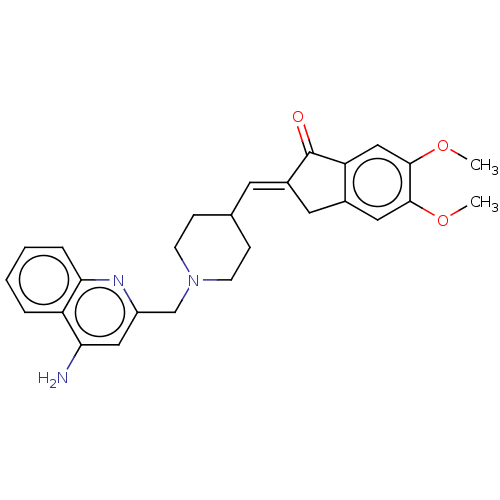 (CHEMBL4166300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532202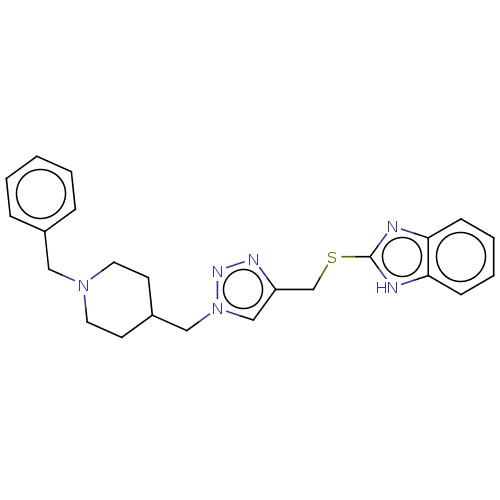 (CHEMBL4446441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trans-sialidase (Trypanosoma cruzi) | BDBM84698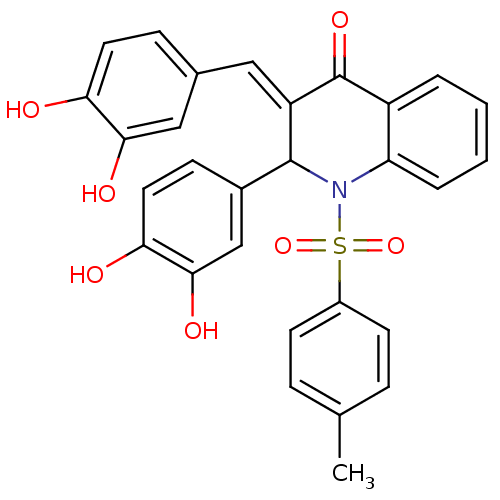 (Quinolinone derivative, 9) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sao Paulo Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trans-sialidase using Trifluoromethylumbelliferyl alpha-sialoside as substrate by UV/visible spectrophotometer | Bioorg Med Chem 25: 6049-6059 (2017) Article DOI: 10.1016/j.bmc.2017.09.042 BindingDB Entry DOI: 10.7270/Q21Z4700 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50464945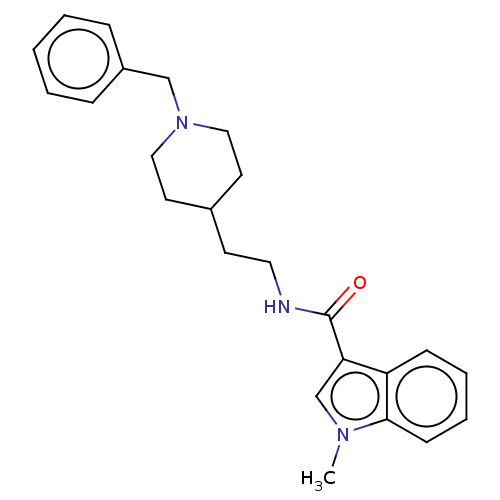 (CHEMBL4282414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277652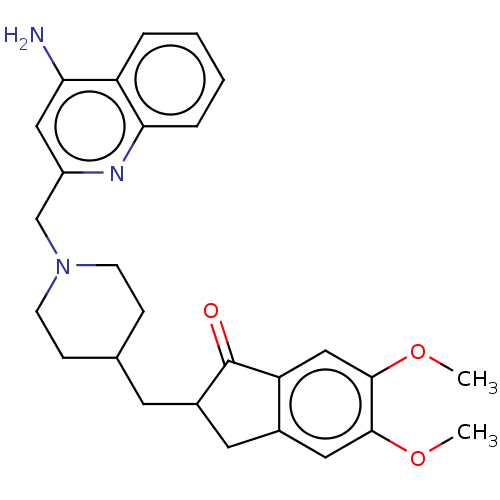 (CHEMBL4174269) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532208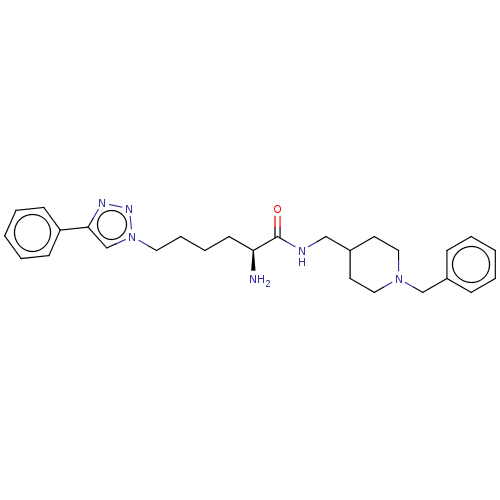 (CHEMBL4467345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532206 (CHEMBL4445099) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464943 (CHEMBL4294109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464944 (CHEMBL4285818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464942 (CHEMBL4293015) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532201 (CHEMBL4557745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532200 (CHEMBL4440392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464946 (CHEMBL4283651) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277655 (CHEMBL4166300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50532209 (CHEMBL4534345) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50532200 (CHEMBL4440392) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50277650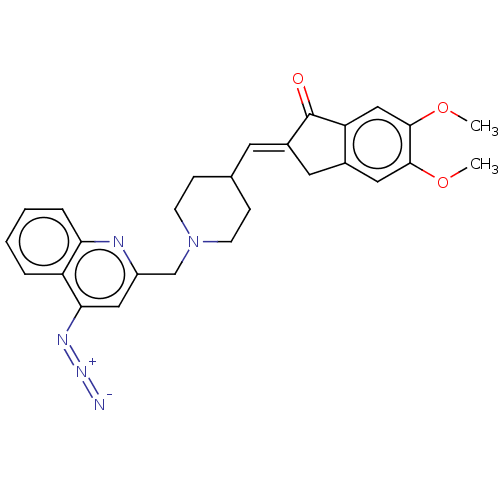 (CHEMBL4172587) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by U... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50532205 (CHEMBL4574766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50532199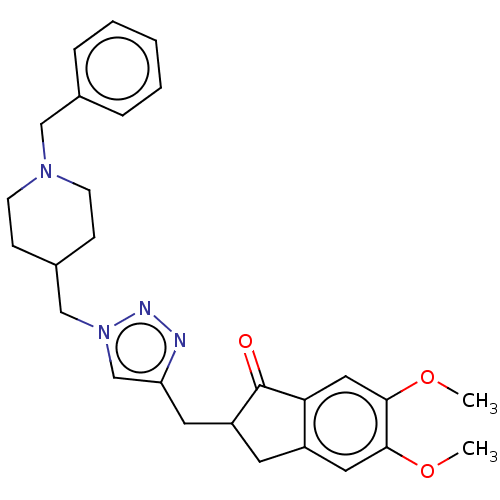 (CHEMBL4438642) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532204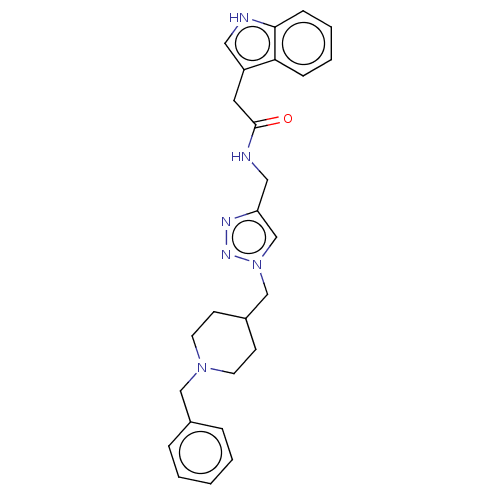 (CHEMBL4448634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187201 (CHEMBL3824248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277651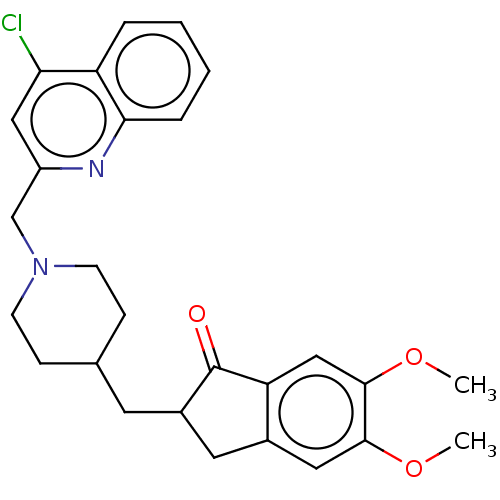 (CHEMBL4174514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464945 (CHEMBL4282414) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277652 (CHEMBL4174269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50532201 (CHEMBL4557745) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532203 (CHEMBL4437391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464947 (CHEMBL4277879) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50532202 (CHEMBL4446441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50457242 (CHEMBL4217654) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase expressed in Escherichia coli BL21 (DE3) pre-incubated for 30 mins using TS2 substrate in pre... | Eur J Med Chem 140: 187-199 (2017) Article DOI: 10.1016/j.ejmech.2017.08.064 BindingDB Entry DOI: 10.7270/Q2R2141M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50457241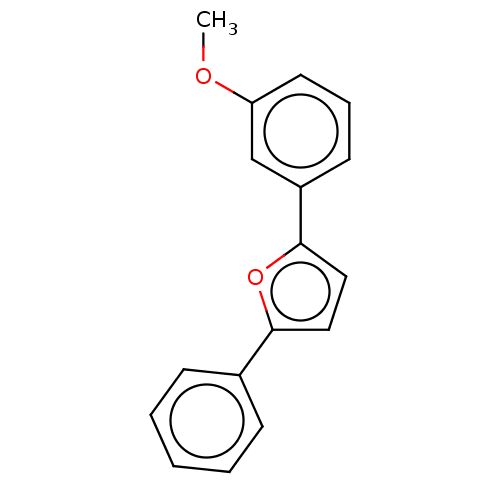 (CHEMBL4212705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase expressed in Escherichia coli BL21 (DE3) pre-incubated for 30 mins using TS2 substrate in pre... | Eur J Med Chem 140: 187-199 (2017) Article DOI: 10.1016/j.ejmech.2017.08.064 BindingDB Entry DOI: 10.7270/Q2R2141M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50277664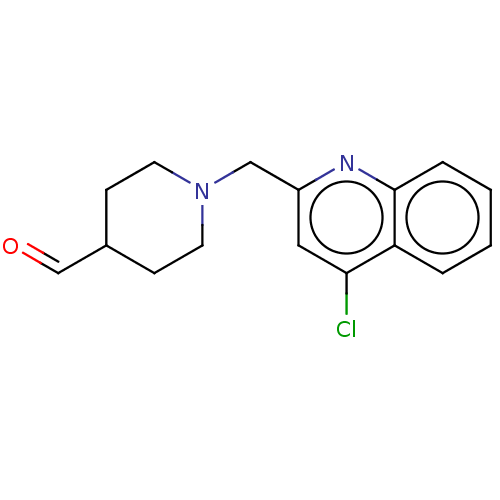 (CHEMBL4164010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured over 5 m... | Eur J Med Chem 139: 773-791 (2017) Article DOI: 10.1016/j.ejmech.2017.08.051 BindingDB Entry DOI: 10.7270/Q2XS5XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532205 (CHEMBL4574766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50532209 (CHEMBL4534345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... | Bioorg Med Chem 27: 931-943 (2019) Article DOI: 10.1016/j.bmc.2018.12.030 BindingDB Entry DOI: 10.7270/Q2XP78D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |