

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149327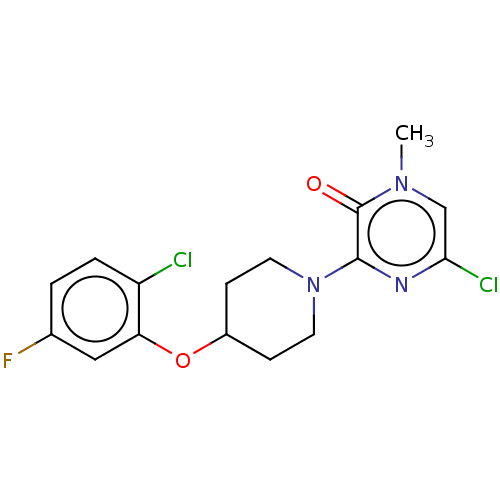 (US8962837, 12) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149331 (US8962837, 18) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149340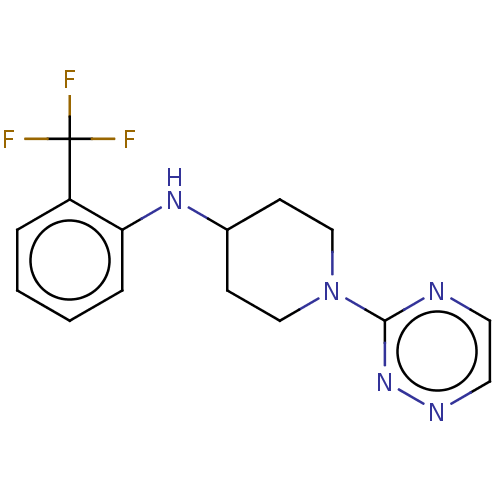 (US8962837, 36) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149338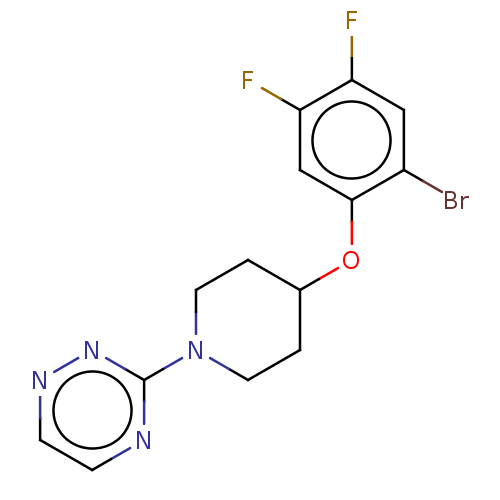 (US8962837, 33) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149337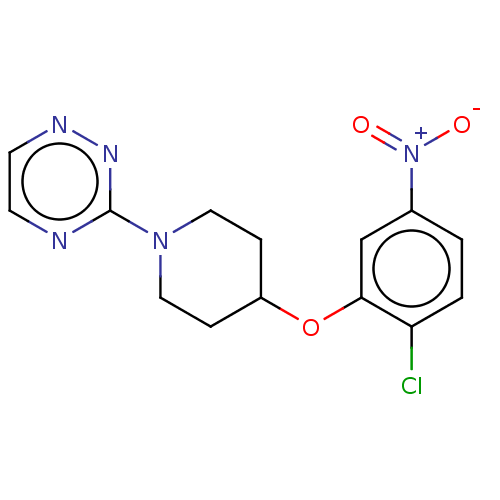 (US8962837, 32) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149333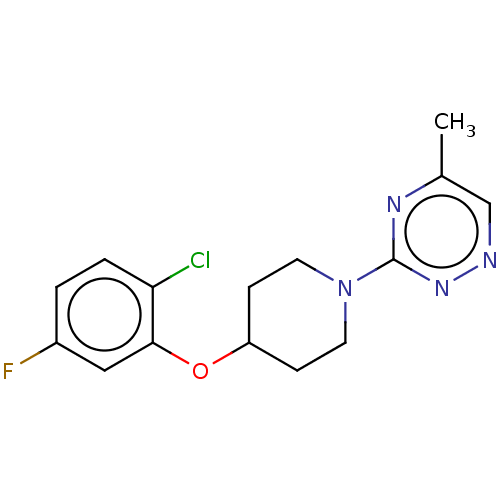 (US8962837, 25) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149330 (US8962837, 17) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149336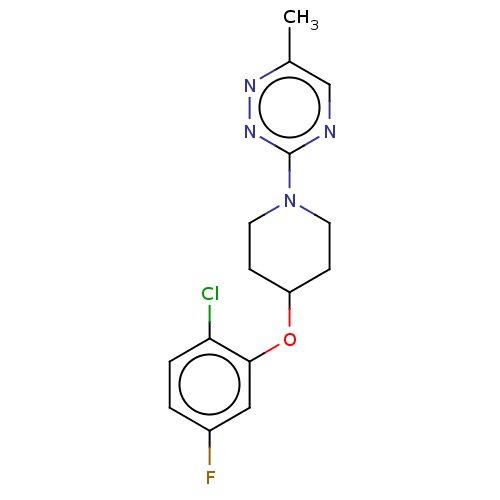 (US8962837, 30) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149326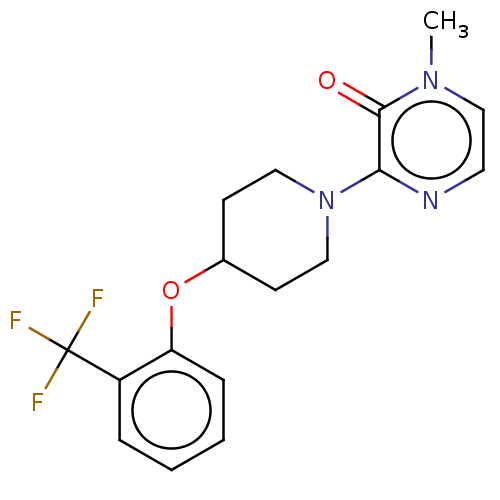 (US8962837, 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149323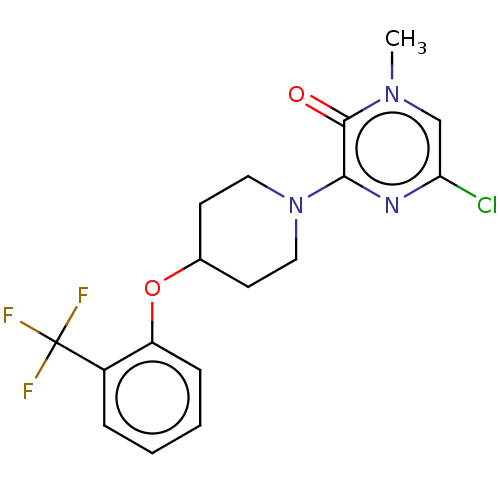 (US8962837, 7) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149343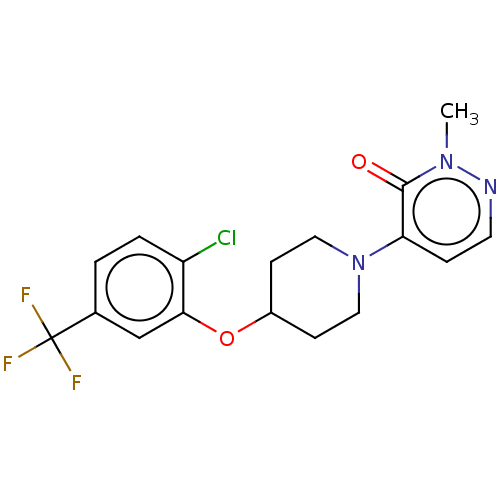 (US8962837, 40) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149324 (US8962837, 8) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150667 ((S)-2-({(2S,3S,6R)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149334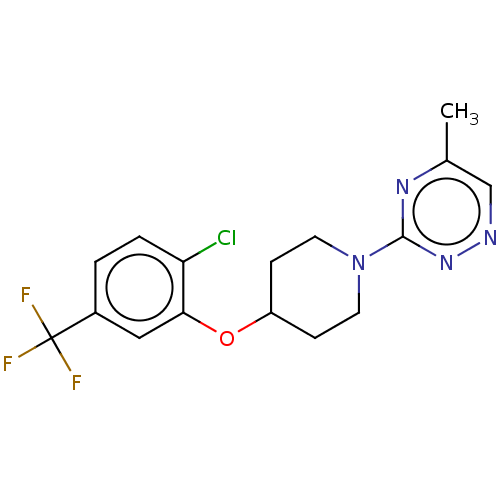 (US8962837, 27) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149345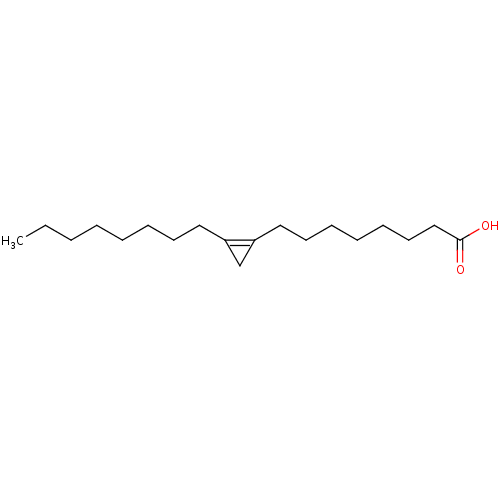 (US8962837, Sterculic acid) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50366346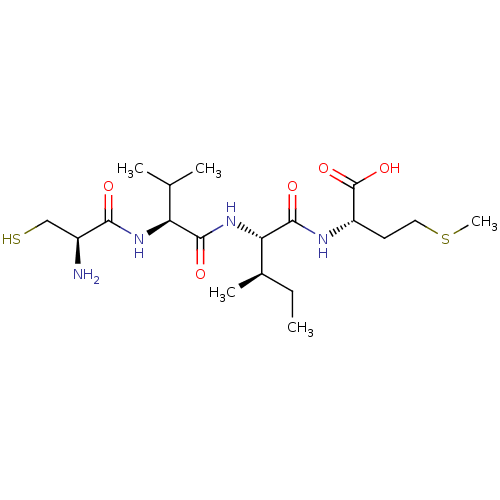 (CHEMBL1790477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149341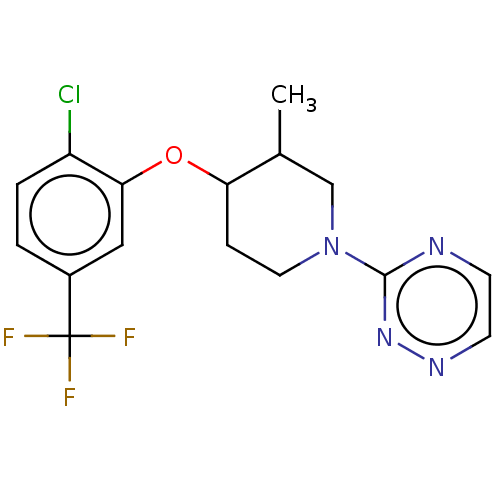 (US8962837, 37) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149339 (US8962837, 35) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149332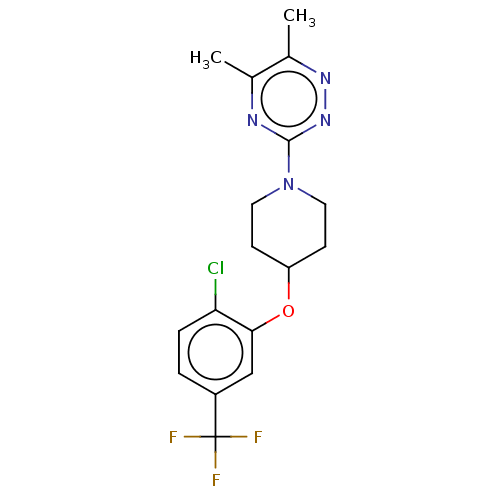 (US8962837, 19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149329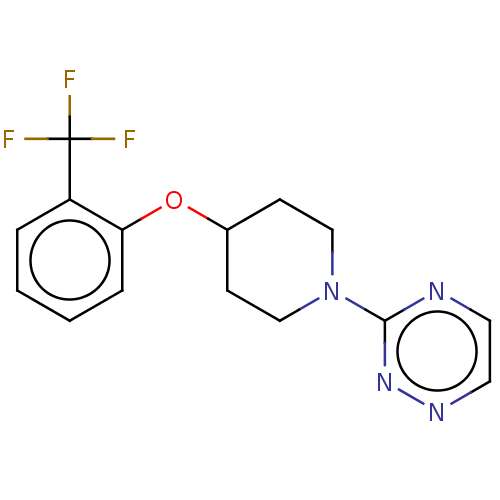 (US8962837, 14) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149328 (US8962837, 13) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149325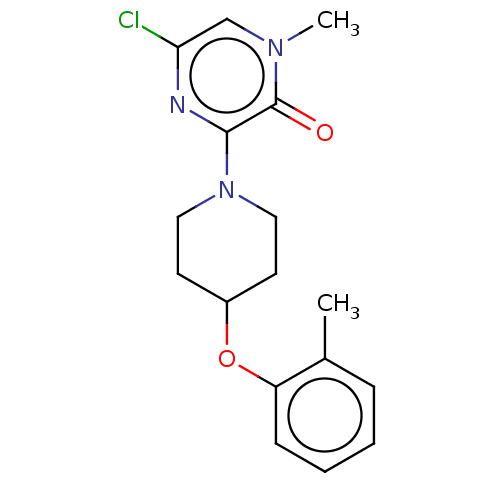 (US8962837, 9) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149322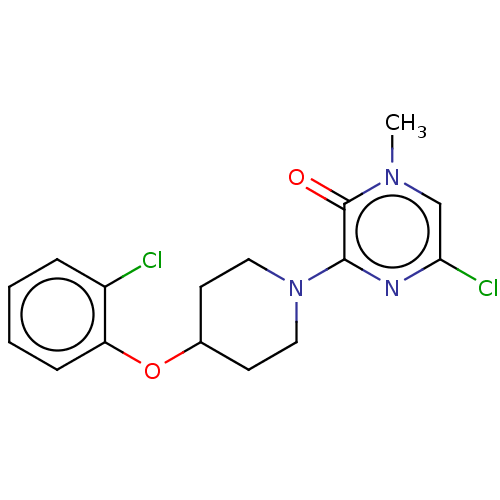 (US8962837, 6) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149344 (US8962837, 42) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149335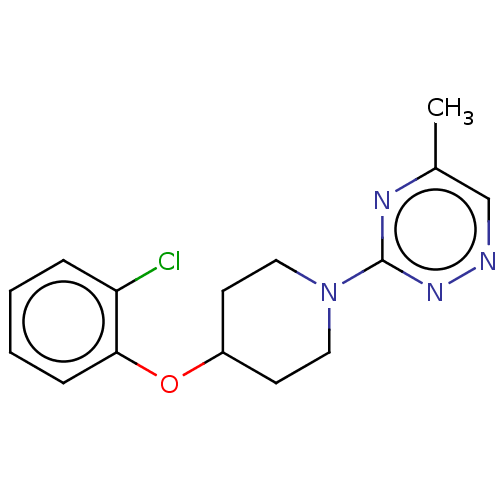 (US8962837, 28) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM149342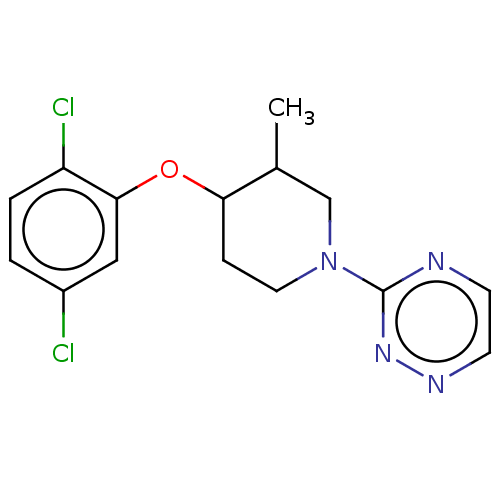 (US8962837, 38) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Pierre Fabre Medicament US Patent | Assay Description Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (AT... | US Patent US8962837 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150666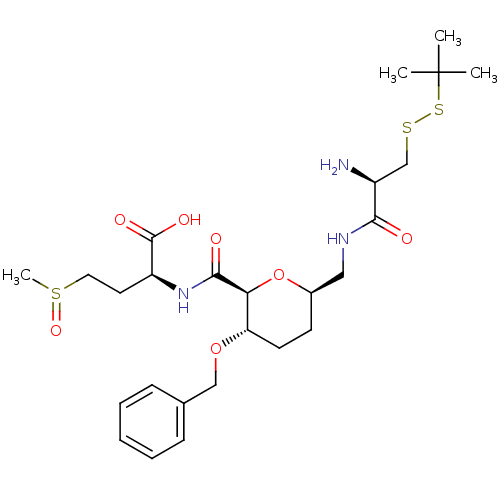 ((S)-2-({(2S,3S,6R)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150662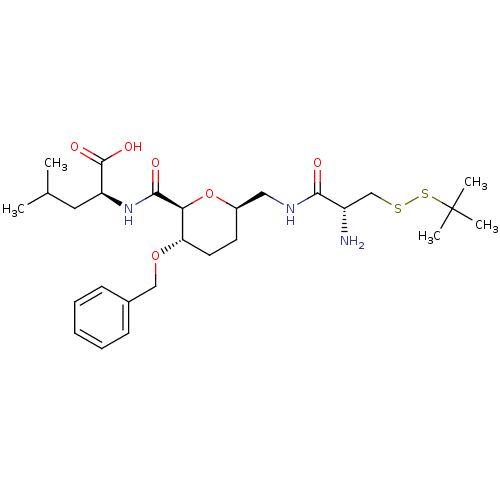 ((S)-2-({(2S,3S,6R)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150665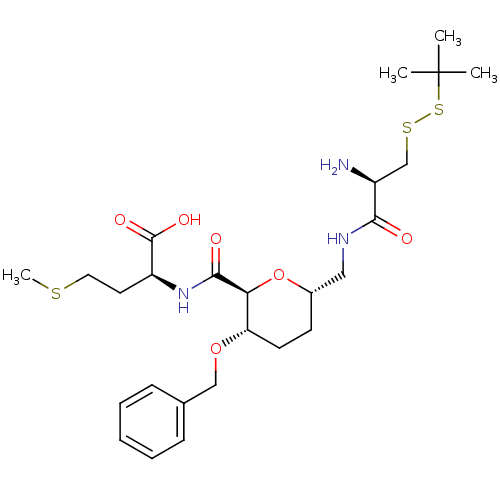 ((S)-2-({(2S,3S,6S)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150665 ((S)-2-({(2S,3S,6S)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150662 ((S)-2-({(2S,3S,6R)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150664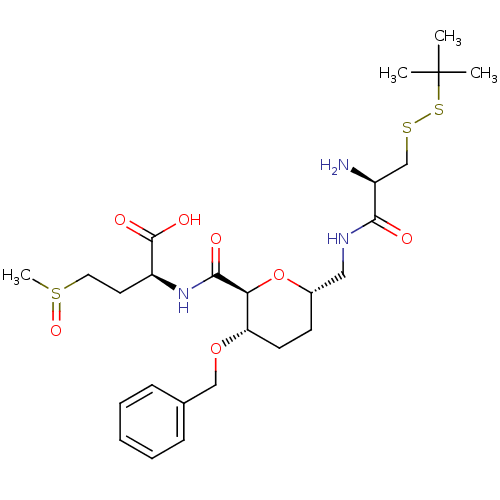 ((S)-2-({(2S,3S,6S)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150663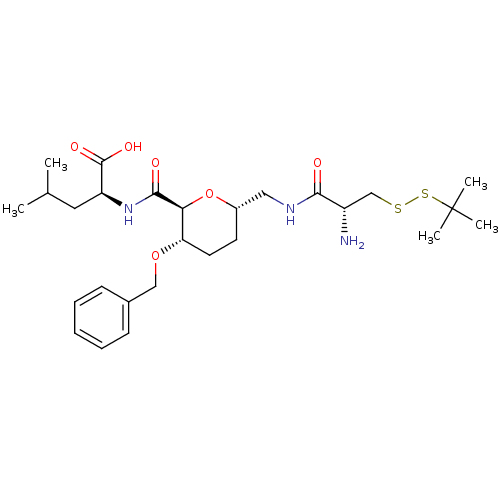 ((S)-2-({(2S,3S,6S)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150671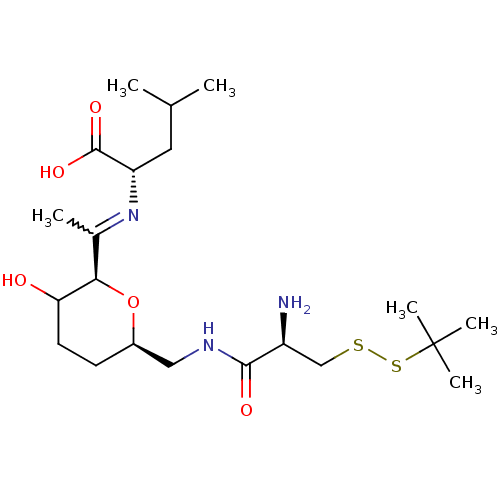 ((S)-2-(1-{(2R,6R)-6-[((R)-2-Amino-3-tert-butyldisu...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150669 ((S)-2-(1-{(2R,3S,6S)-6-[((R)-2-Amino-3-tert-butyld...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150668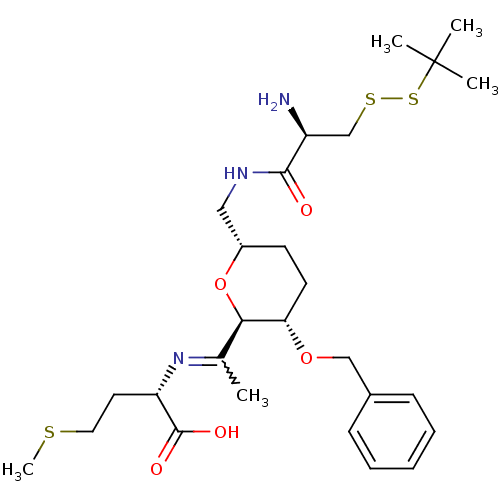 ((S)-2-(1-{(2R,3S,6S)-6-[((R)-2-Amino-3-tert-butyld...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150663 ((S)-2-({(2S,3S,6S)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150670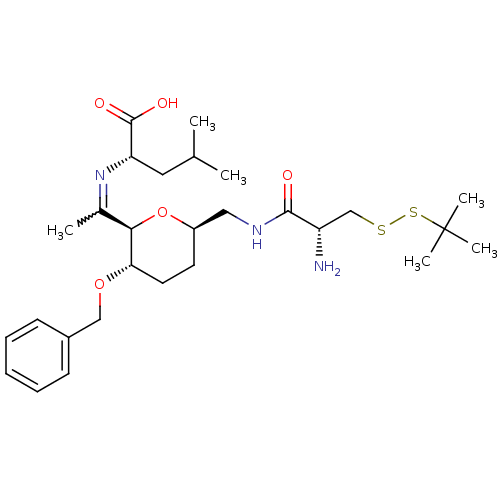 ((S)-2-(1-{(2R,3S,6R)-6-[((R)-2-Amino-3-tert-butyld...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150672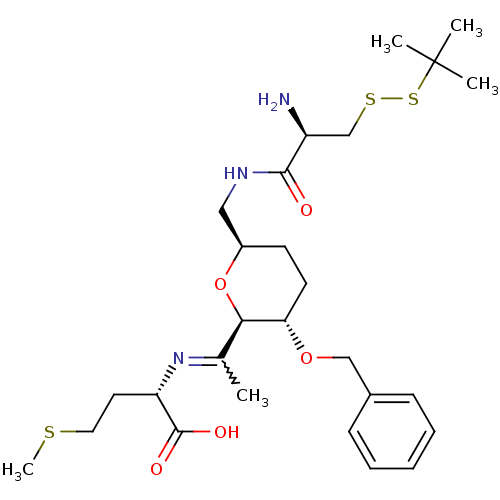 ((S)-2-(1-{(2R,3S,6R)-6-[((R)-2-Amino-3-tert-butyld...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150664 ((S)-2-({(2S,3S,6S)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150671 ((S)-2-(1-{(2R,6R)-6-[((R)-2-Amino-3-tert-butyldisu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150672 ((S)-2-(1-{(2R,3S,6R)-6-[((R)-2-Amino-3-tert-butyld...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]FPP incorporation by Protein Farnesyltransferase | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150666 ((S)-2-({(2S,3S,6R)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50150667 ((S)-2-({(2S,3S,6R)-6-[((R)-2-Amino-3-tert-butyldis...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of [3H]GGPP incorporation by Protein Geranylgeranyl transferase type I | J Med Chem 47: 3920-3 (2004) Article DOI: 10.1021/jm049927q BindingDB Entry DOI: 10.7270/Q2FT8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||