 Found 3768 hits with Last Name = 'dam' and Initial = 'j'
Found 3768 hits with Last Name = 'dam' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G |
Bioorg Med Chem Lett 20: 7222-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.109
BindingDB Entry DOI: 10.7270/Q2GB24B9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091753
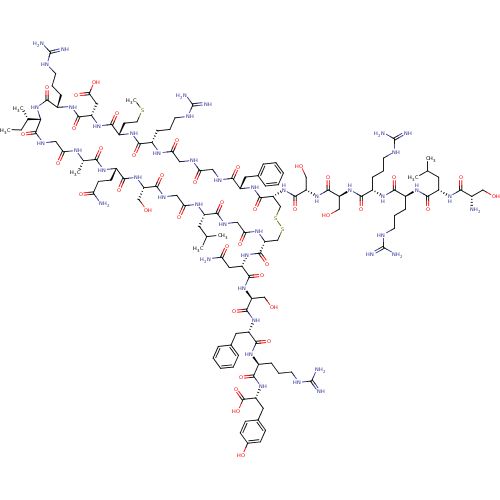
(CHEMBL405854 | H-Ser-Leu-Arg-Arg-Ser-Ser-cyclic(Cy...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CO Show InChI InChI=1S/C127H203N45O39S3/c1-9-64(6)99-121(209)150-52-94(182)151-65(7)100(188)155-76(34-35-91(129)179)109(197)167-85(56-174)104(192)149-53-96(184)153-78(43-62(2)3)102(190)148-54-97(185)154-89(119(207)164-82(48-92(130)180)114(202)169-86(57-175)116(204)163-81(46-67-23-14-11-15-24-67)113(201)158-73(27-18-39-143-125(135)136)107(195)166-84(122(210)211)47-68-30-32-69(178)33-31-68)60-213-214-61-90(171-118(206)88(59-177)170-117(205)87(58-176)168-108(196)74(28-19-40-144-126(137)138)156-106(194)72(26-17-38-142-124(133)134)157-112(200)79(44-63(4)5)161-101(189)70(128)55-173)120(208)162-80(45-66-21-12-10-13-22-66)103(191)147-50-93(181)146-51-95(183)152-71(25-16-37-141-123(131)132)105(193)160-77(36-42-212-8)110(198)165-83(49-98(186)187)115(203)159-75(111(199)172-99)29-20-41-145-127(139)140/h10-15,21-24,30-33,62-65,70-90,99,173-178H,9,16-20,25-29,34-61,128H2,1-8H3,(H2,129,179)(H2,130,180)(H,146,181)(H,147,191)(H,148,190)(H,149,192)(H,150,209)(H,151,182)(H,152,183)(H,153,184)(H,154,185)(H,155,188)(H,156,194)(H,157,200)(H,158,201)(H,159,203)(H,160,193)(H,161,189)(H,162,208)(H,163,204)(H,164,207)(H,165,198)(H,166,195)(H,167,197)(H,168,196)(H,169,202)(H,170,205)(H,171,206)(H,172,199)(H,186,187)(H,210,211)(H4,131,132,141)(H4,133,134,142)(H4,135,136,143)(H4,137,138,144)(H4,139,140,145)/t64-,65-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84+,85-,86-,87-,88-,89+,90+,99-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
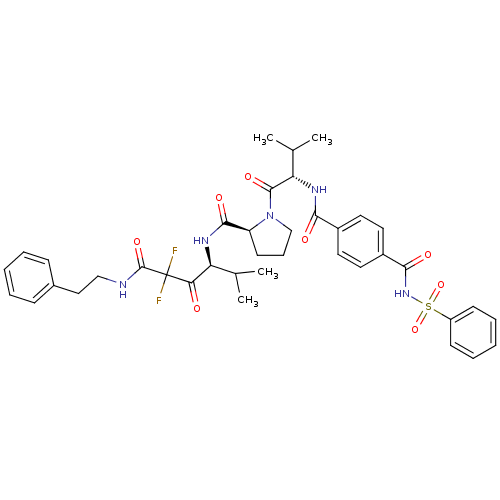
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 1
(Homo sapiens (Human)) | BDBM50091751
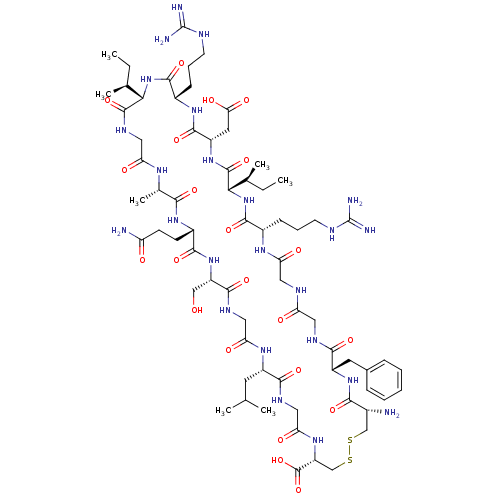
(CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(O)=O)[C@@H](C)CC Show InChI InChI=1S/C70H114N24O22S2/c1-8-35(5)55-66(113)83-28-50(98)84-37(7)57(104)88-42(19-20-48(72)96)62(109)92-46(31-95)61(108)82-29-52(100)86-43(23-34(3)4)59(106)81-30-53(101)87-47(68(115)116)33-118-117-32-39(71)58(105)90-44(24-38-15-11-10-12-16-38)60(107)80-26-49(97)79-27-51(99)85-40(17-13-21-77-69(73)74)63(110)94-56(36(6)9-2)67(114)91-45(25-54(102)103)65(112)89-41(64(111)93-55)18-14-22-78-70(75)76/h10-12,15-16,34-37,39-47,55-56,95H,8-9,13-14,17-33,71H2,1-7H3,(H2,72,96)(H,79,97)(H,80,107)(H,81,106)(H,82,108)(H,83,113)(H,84,98)(H,85,99)(H,86,100)(H,87,101)(H,88,104)(H,89,112)(H,90,105)(H,91,114)(H,92,109)(H,93,111)(H,94,110)(H,102,103)(H,115,116)(H4,73,74,77)(H4,75,76,78)/t35-,36-,37-,39+,40-,41-,42-,43-,44-,45-,46-,47+,55-,56-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091751

(CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(O)=O)[C@@H](C)CC Show InChI InChI=1S/C70H114N24O22S2/c1-8-35(5)55-66(113)83-28-50(98)84-37(7)57(104)88-42(19-20-48(72)96)62(109)92-46(31-95)61(108)82-29-52(100)86-43(23-34(3)4)59(106)81-30-53(101)87-47(68(115)116)33-118-117-32-39(71)58(105)90-44(24-38-15-11-10-12-16-38)60(107)80-26-49(97)79-27-51(99)85-40(17-13-21-77-69(73)74)63(110)94-56(36(6)9-2)67(114)91-45(25-54(102)103)65(112)89-41(64(111)93-55)18-14-22-78-70(75)76/h10-12,15-16,34-37,39-47,55-56,95H,8-9,13-14,17-33,71H2,1-7H3,(H2,72,96)(H,79,97)(H,80,107)(H,81,106)(H,82,108)(H,83,113)(H,84,98)(H,85,99)(H,86,100)(H,87,101)(H,88,104)(H,89,112)(H,90,105)(H,91,114)(H,92,109)(H,93,111)(H,94,110)(H,102,103)(H,115,116)(H4,73,74,77)(H4,75,76,78)/t35-,36-,37-,39+,40-,41-,42-,43-,44-,45-,46-,47+,55-,56-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301481
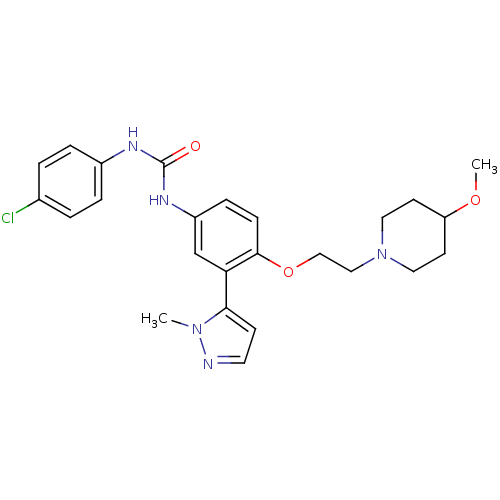
(1-(4-chlorophenyl)-3-(4-(2-(4-methoxypiperidin-1-y...)Show SMILES COC1CCN(CCOc2ccc(NC(=O)Nc3ccc(Cl)cc3)cc2-c2ccnn2C)CC1 Show InChI InChI=1S/C25H30ClN5O3/c1-30-23(9-12-27-30)22-17-20(29-25(32)28-19-5-3-18(26)4-6-19)7-8-24(22)34-16-15-31-13-10-21(33-2)11-14-31/h3-9,12,17,21H,10-11,13-16H2,1-2H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301511
(1-(4-chlorophenyl)-3-(3-(1-methyl-1H-pyrazol-5-yl)...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCCC1 Show InChI InChI=1S/C23H26ClN5O2/c1-28-21(10-11-25-28)20-16-19(27-23(30)26-18-6-4-17(24)5-7-18)8-9-22(20)31-15-14-29-12-2-3-13-29/h4-11,16H,2-3,12-15H2,1H3,(H2,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301506
(1-(3-(4-chloro-1-methyl-1H-pyrazol-5-yl)-4-(2-morp...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)Nc2ccc(F)cc2F)ccc1OCCN1CCOCC1 |(27.36,-35.94,;27.33,-37.48,;28.56,-38.41,;28.05,-39.87,;26.51,-39.83,;25.57,-41.06,;26.07,-38.36,;24.62,-37.84,;23.29,-38.61,;21.95,-37.84,;20.62,-38.61,;19.29,-37.85,;19.29,-36.31,;17.96,-38.62,;16.62,-37.85,;16.61,-36.3,;15.28,-35.54,;13.95,-36.31,;12.62,-35.54,;13.95,-37.85,;15.28,-38.62,;15.28,-40.16,;21.95,-36.31,;23.27,-35.53,;24.62,-36.3,;25.95,-35.53,;25.94,-33.99,;27.28,-33.21,;28.61,-33.97,;29.37,-35.3,;30.89,-35.31,;31.67,-33.99,;30.9,-32.66,;29.37,-32.65,)| Show InChI InChI=1S/C23H24ClF2N5O3/c1-30-22(18(24)14-27-30)17-13-16(28-23(32)29-20-4-2-15(25)12-19(20)26)3-5-21(17)34-11-8-31-6-9-33-10-7-31/h2-5,12-14H,6-11H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301502
(1-(3-(4-bromo-1-methyl-1H-pyrazol-5-yl)-4-(2-(pyrr...)Show SMILES Cn1ncc(Br)c1-c1cc(NC(=O)Nc2ccc(F)cc2F)ccc1OCCN1CCCC1 |(28.77,-15.18,;28.73,-16.71,;29.96,-17.65,;29.45,-19.1,;27.91,-19.07,;26.98,-20.29,;27.47,-17.59,;26.02,-17.07,;24.69,-17.84,;23.36,-17.07,;22.03,-17.85,;20.69,-17.08,;20.69,-15.54,;19.36,-17.85,;18.03,-17.08,;18.02,-15.53,;16.69,-14.77,;15.36,-15.54,;14.02,-14.77,;15.35,-17.08,;16.69,-17.85,;16.69,-19.39,;23.35,-15.54,;24.68,-14.76,;26.02,-15.53,;27.35,-14.76,;27.35,-13.22,;28.68,-12.44,;30.02,-13.21,;30.19,-14.73,;31.69,-15.05,;32.46,-13.71,;31.42,-12.57,)| Show InChI InChI=1S/C23H24BrF2N5O2/c1-30-22(18(24)14-27-30)17-13-16(5-7-21(17)33-11-10-31-8-2-3-9-31)28-23(32)29-20-6-4-15(25)12-19(20)26/h4-7,12-14H,2-3,8-11H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301517
(1-(4-chlorophenyl)-3-(3-(1-methyl-1H-pyrazol-5-yl)...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCOCC1 Show InChI InChI=1S/C23H26ClN5O3/c1-28-21(8-9-25-28)20-16-19(27-23(30)26-18-4-2-17(24)3-5-18)6-7-22(20)32-15-12-29-10-13-31-14-11-29/h2-9,16H,10-15H2,1H3,(H2,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036095
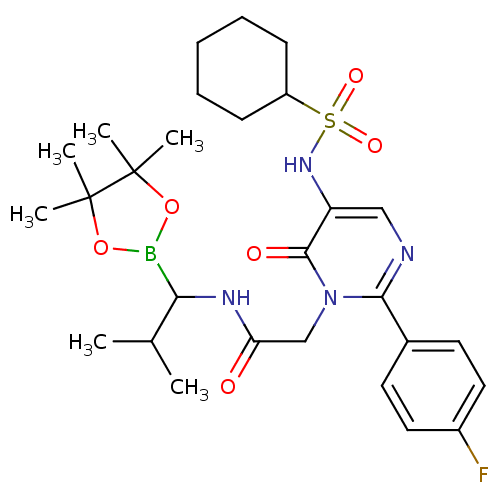
(2-[5-Cyclohexanesulfonylamino-2-(4-fluoro-phenyl)-...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(NS(=O)(=O)C2CCCCC2)c1=O)-c1ccc(F)cc1)B1OC(C)(C)C(C)(C)O1 Show InChI InChI=1S/C28H40BFN4O6S/c1-18(2)24(29-39-27(3,4)28(5,6)40-29)32-23(35)17-34-25(19-12-14-20(30)15-13-19)31-16-22(26(34)36)33-41(37,38)21-10-8-7-9-11-21/h12-16,18,21,24,33H,7-11,17H2,1-6H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of human leukocyte elastase mediated hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNA |
J Med Chem 38: 98-108 (1995)
BindingDB Entry DOI: 10.7270/Q2JW8CX7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301476
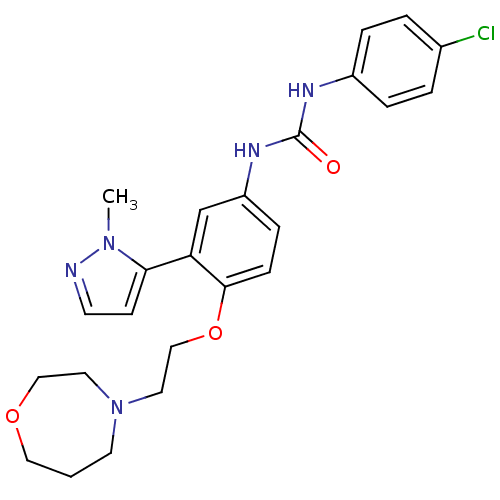
(1-(4-(2-(1,4-oxazepan-4-yl)ethoxy)-3-(1-methyl-1H-...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCCOCC1 Show InChI InChI=1S/C24H28ClN5O3/c1-29-22(9-10-26-29)21-17-20(28-24(31)27-19-5-3-18(25)4-6-19)7-8-23(21)33-16-13-30-11-2-14-32-15-12-30/h3-10,17H,2,11-16H2,1H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301495
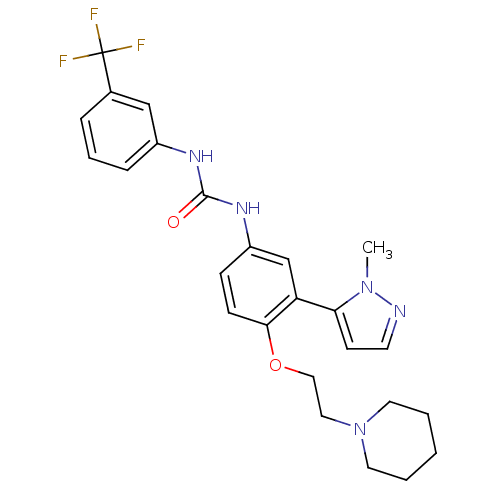
(1-(3-(1-methyl-1H-pyrazol-5-yl)-4-(2-(piperidin-1-...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2cccc(c2)C(F)(F)F)ccc1OCCN1CCCCC1 Show InChI InChI=1S/C25H28F3N5O2/c1-32-22(10-11-29-32)21-17-20(8-9-23(21)35-15-14-33-12-3-2-4-13-33)31-24(34)30-19-7-5-6-18(16-19)25(26,27)28/h5-11,16-17H,2-4,12-15H2,1H3,(H2,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301510
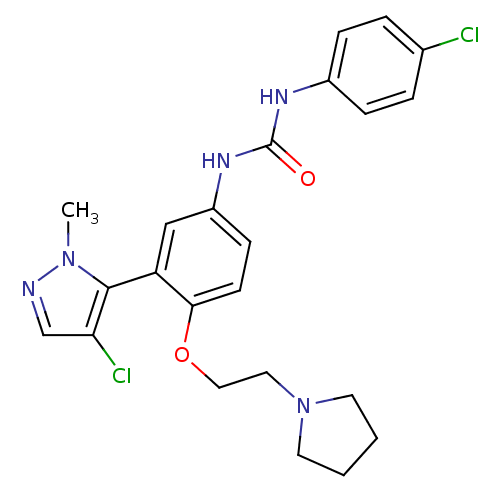
(1-(3-(4-chloro-1-methyl-1H-pyrazol-5-yl)-4-(2-(pyr...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCCC1 |(29.87,3.61,;29.84,2.07,;31.07,1.14,;30.56,-.32,;29.02,-.28,;28.09,-1.51,;28.58,1.19,;27.13,1.71,;25.8,.94,;24.47,1.71,;23.13,.94,;21.8,1.7,;21.8,3.24,;20.47,.93,;19.13,1.7,;17.8,.93,;16.46,1.7,;16.46,3.24,;15.13,4.01,;17.79,4.01,;19.13,3.25,;24.46,3.24,;25.79,4.02,;27.13,3.25,;28.46,4.02,;28.46,5.56,;29.79,6.34,;31.12,5.57,;31.6,4.11,;33.14,4.12,;33.61,5.59,;32.36,6.49,)| Show InChI InChI=1S/C23H25Cl2N5O2/c1-29-22(20(25)15-26-29)19-14-18(28-23(31)27-17-6-4-16(24)5-7-17)8-9-21(19)32-13-12-30-10-2-3-11-30/h4-9,14-15H,2-3,10-13H2,1H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301503
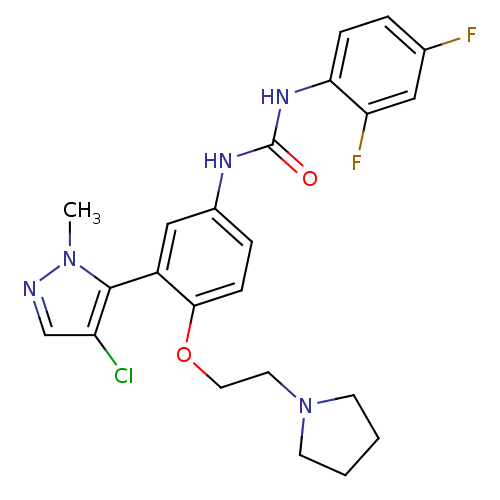
(1-(3-(4-chloro-1-methyl-1H-pyrazol-5-yl)-4-(2-(pyr...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)Nc2ccc(F)cc2F)ccc1OCCN1CCCC1 |(4.41,-24.98,;4.38,-26.52,;5.61,-27.45,;5.1,-28.91,;3.56,-28.87,;2.62,-30.1,;3.12,-27.4,;1.67,-26.88,;.34,-27.65,;-1,-26.88,;-2.33,-27.65,;-3.66,-26.88,;-3.67,-25.35,;-5,-27.66,;-6.33,-26.89,;-6.34,-25.34,;-7.67,-24.58,;-9,-25.35,;-10.33,-24.58,;-9,-26.89,;-7.67,-27.66,;-7.67,-29.2,;-1.01,-25.35,;.32,-24.57,;1.66,-25.34,;3,-24.56,;2.99,-23.03,;4.32,-22.25,;5.66,-23.02,;5.83,-24.54,;7.34,-24.85,;8.1,-23.52,;7.07,-22.38,)| Show InChI InChI=1S/C23H24ClF2N5O2/c1-30-22(18(24)14-27-30)17-13-16(5-7-21(17)33-11-10-31-8-2-3-9-31)28-23(32)29-20-6-4-15(25)12-19(20)26/h4-7,12-14H,2-3,8-11H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301509
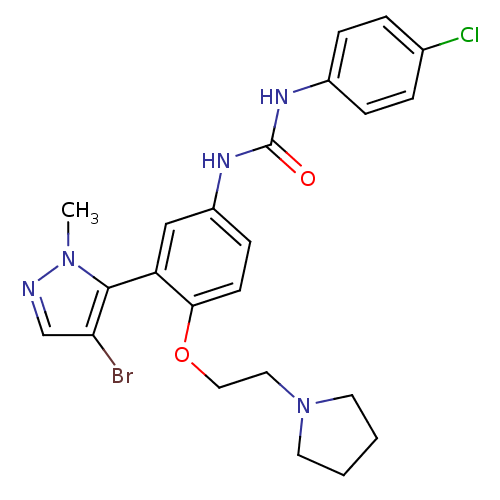
(1-(3-(4-bromo-1-methyl-1H-pyrazol-5-yl)-4-(2-(pyrr...)Show SMILES Cn1ncc(Br)c1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCCC1 |(5.77,3.8,;5.74,2.26,;6.97,1.33,;6.46,-.13,;4.92,-.09,;3.98,-1.31,;4.48,1.39,;3.03,1.9,;1.7,1.13,;.36,1.9,;-.97,1.13,;-2.3,1.9,;-2.31,3.44,;-3.64,1.13,;-4.97,1.89,;-6.31,1.12,;-7.64,1.89,;-7.64,3.44,;-8.97,4.21,;-6.31,4.21,;-4.98,3.44,;.36,3.44,;1.68,4.21,;3.03,3.44,;4.36,4.22,;4.35,5.76,;5.68,6.53,;7.02,5.77,;7.5,4.31,;9.04,4.32,;9.51,5.78,;8.26,6.68,)| Show InChI InChI=1S/C23H25BrClN5O2/c1-29-22(20(24)15-26-29)19-14-18(28-23(31)27-17-6-4-16(25)5-7-17)8-9-21(19)32-13-12-30-10-2-3-11-30/h4-9,14-15H,2-3,10-13H2,1H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091758
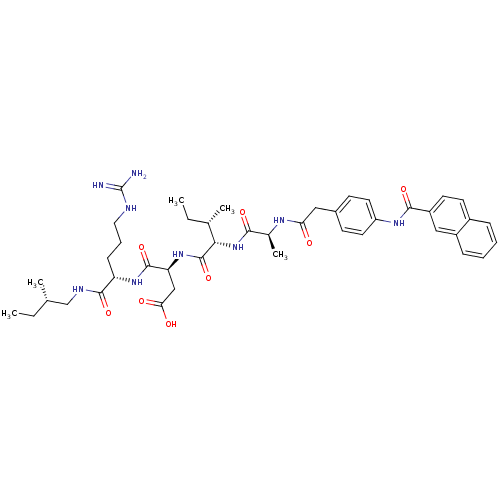
((S)-N-[(S)-4-Guanidino-1-((S)-2-methyl-butylcarbam...)Show SMILES CC[C@H](C)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)Cc1ccc(NC(=O)c2ccc3ccccc3c2)cc1)[C@@H](C)CC Show InChI InChI=1S/C43H59N9O8/c1-6-25(3)24-47-40(58)33(13-10-20-46-43(44)45)50-41(59)34(23-36(54)55)51-42(60)37(26(4)7-2)52-38(56)27(5)48-35(53)21-28-14-18-32(19-15-28)49-39(57)31-17-16-29-11-8-9-12-30(29)22-31/h8-9,11-12,14-19,22,25-27,33-34,37H,6-7,10,13,20-21,23-24H2,1-5H3,(H,47,58)(H,48,53)(H,49,57)(H,50,59)(H,51,60)(H,52,56)(H,54,55)(H4,44,45,46)/t25-,26-,27-,33-,34-,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 1
(Homo sapiens (Human)) | BDBM50091758

((S)-N-[(S)-4-Guanidino-1-((S)-2-methyl-butylcarbam...)Show SMILES CC[C@H](C)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)Cc1ccc(NC(=O)c2ccc3ccccc3c2)cc1)[C@@H](C)CC Show InChI InChI=1S/C43H59N9O8/c1-6-25(3)24-47-40(58)33(13-10-20-46-43(44)45)50-41(59)34(23-36(54)55)51-42(60)37(26(4)7-2)52-38(56)27(5)48-35(53)21-28-14-18-32(19-15-28)49-39(57)31-17-16-29-11-8-9-12-30(29)22-31/h8-9,11-12,14-19,22,25-27,33-34,37H,6-7,10,13,20-21,23-24H2,1-5H3,(H,47,58)(H,48,53)(H,49,57)(H,50,59)(H,51,60)(H,52,56)(H,54,55)(H4,44,45,46)/t25-,26-,27-,33-,34-,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP |
ACS Med Chem Lett 6: 630-4 (2015)
Article DOI: 10.1021/ml500409n
BindingDB Entry DOI: 10.7270/Q2WS8W1V |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418481
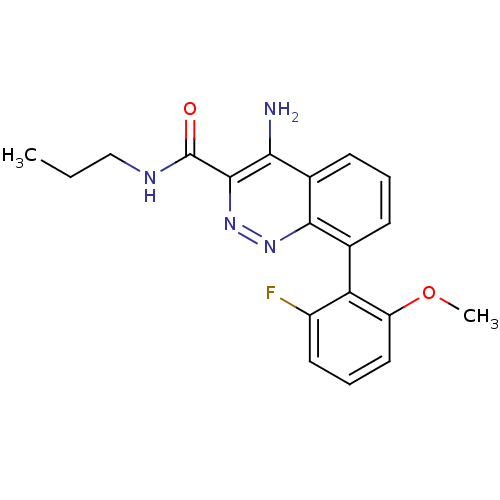
(CHEMBL1783282)Show SMILES CCCNC(=O)c1nnc2c(cccc2c1N)-c1c(F)cccc1OC |(3.52,-21.84,;2.19,-22.61,;.85,-21.84,;-.48,-22.61,;-1.81,-21.84,;-1.81,-20.3,;-3.15,-22.61,;-3.16,-24.17,;-4.5,-24.95,;-5.84,-24.18,;-7.18,-24.95,;-8.51,-24.18,;-8.51,-22.63,;-7.18,-21.86,;-5.85,-22.63,;-4.51,-21.84,;-4.52,-20.3,;-7.18,-26.49,;-5.85,-27.25,;-4.52,-26.47,;-5.85,-28.78,;-7.19,-29.56,;-8.52,-28.78,;-8.52,-27.25,;-9.85,-26.47,;-9.84,-24.93,)| Show InChI InChI=1S/C19H19FN4O2/c1-3-10-22-19(25)18-16(21)12-7-4-6-11(17(12)23-24-18)15-13(20)8-5-9-14(15)26-2/h4-9H,3,10H2,1-2H3,(H2,21,23)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301507
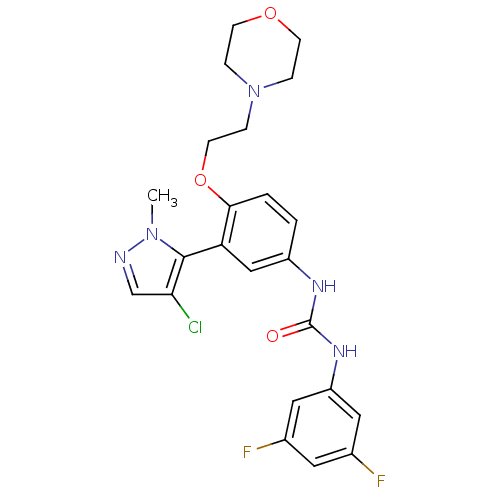
(1-(3-(4-chloro-1-methyl-1H-pyrazol-5-yl)-4-(2-morp...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)Nc2cc(F)cc(F)c2)ccc1OCCN1CCOCC1 |(4.32,-45.59,;4.29,-47.13,;5.52,-48.06,;5.01,-49.52,;3.47,-49.48,;2.54,-50.7,;3.03,-48,;1.58,-47.49,;.25,-48.26,;-1.08,-47.49,;-2.42,-48.26,;-3.75,-47.49,;-3.75,-45.95,;-5.08,-48.26,;-6.42,-47.49,;-7.75,-48.27,;-9.09,-47.5,;-10.42,-48.26,;-9.09,-45.95,;-7.76,-45.18,;-7.76,-43.64,;-6.42,-45.94,;-1.09,-45.95,;.24,-45.18,;1.58,-45.94,;2.91,-45.17,;2.91,-43.63,;4.24,-42.86,;5.57,-43.62,;6.33,-44.95,;7.86,-44.95,;8.63,-43.63,;7.87,-42.3,;6.33,-42.29,)| Show InChI InChI=1S/C23H24ClF2N5O3/c1-30-22(20(24)14-27-30)19-13-17(28-23(32)29-18-11-15(25)10-16(26)12-18)2-3-21(19)34-9-6-31-4-7-33-8-5-31/h2-3,10-14H,4-9H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301488
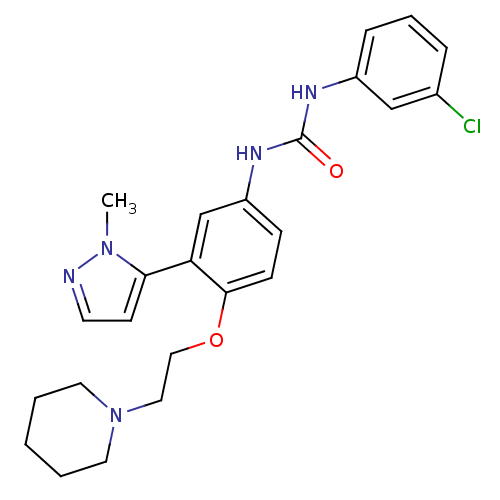
(1-(3-chlorophenyl)-3-(3-(1-methyl-1H-pyrazol-5-yl)...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2cccc(Cl)c2)ccc1OCCN1CCCCC1 Show InChI InChI=1S/C24H28ClN5O2/c1-29-22(10-11-26-29)21-17-20(28-24(31)27-19-7-5-6-18(25)16-19)8-9-23(21)32-15-14-30-12-3-2-4-13-30/h5-11,16-17H,2-4,12-15H2,1H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301496
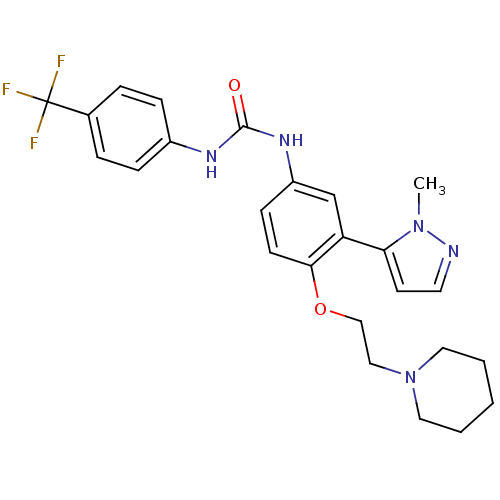
(1-(3-(1-methyl-1H-pyrazol-5-yl)-4-(2-(piperidin-1-...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2ccc(cc2)C(F)(F)F)ccc1OCCN1CCCCC1 Show InChI InChI=1S/C25H28F3N5O2/c1-32-22(11-12-29-32)21-17-20(9-10-23(21)35-16-15-33-13-3-2-4-14-33)31-24(34)30-19-7-5-18(6-8-19)25(26,27)28/h5-12,17H,2-4,13-16H2,1H3,(H2,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061047
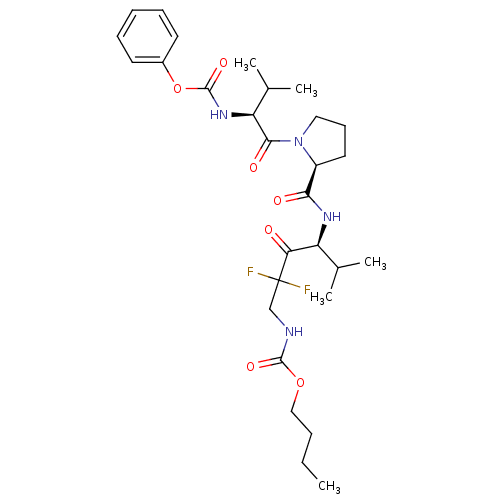
(((S)-2,2-Difluoro-5-methyl-4-{[(S)-1-((S)-3-methyl...)Show SMILES CCCCOC(=O)NCC(F)(F)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C29H42F2N4O7/c1-6-7-16-41-27(39)32-17-29(30,31)24(36)22(18(2)3)33-25(37)21-14-11-15-35(21)26(38)23(19(4)5)34-28(40)42-20-12-9-8-10-13-20/h8-10,12-13,18-19,21-23H,6-7,11,14-17H2,1-5H3,(H,32,39)(H,33,37)(H,34,40)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061028
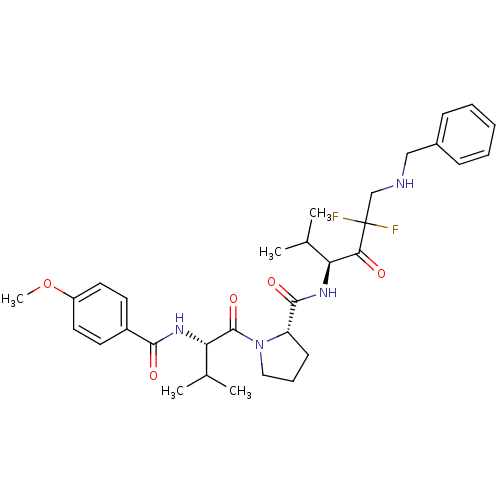
((S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-but...)Show SMILES COc1ccc(cc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNCc1ccccc1 Show InChI InChI=1S/C32H42F2N4O5/c1-20(2)26(28(39)32(33,34)19-35-18-22-10-7-6-8-11-22)36-30(41)25-12-9-17-38(25)31(42)27(21(3)4)37-29(40)23-13-15-24(43-5)16-14-23/h6-8,10-11,13-16,20-21,25-27,35H,9,12,17-19H2,1-5H3,(H,36,41)(H,37,40)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061043
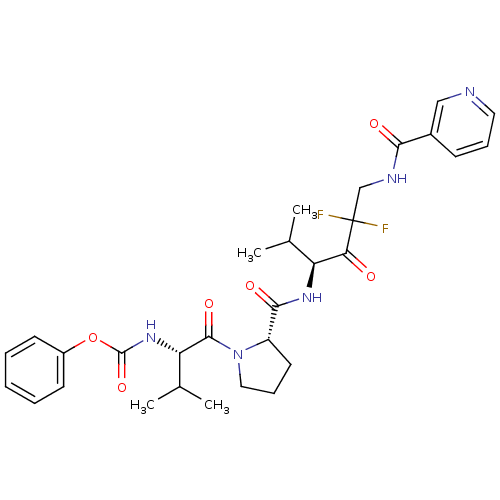
(CHEMBL106592 | [(S)-1-((S)-2-{(S)-3,3-Difluoro-1-i...)Show SMILES CC(C)[C@H](NC(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNC(=O)c1cccnc1 Show InChI InChI=1S/C30H37F2N5O6/c1-18(2)23(25(38)30(31,32)17-34-26(39)20-10-8-14-33-16-20)35-27(40)22-13-9-15-37(22)28(41)24(19(3)4)36-29(42)43-21-11-6-5-7-12-21/h5-8,10-12,14,16,18-19,22-24H,9,13,15,17H2,1-4H3,(H,34,39)(H,35,40)(H,36,42)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301504
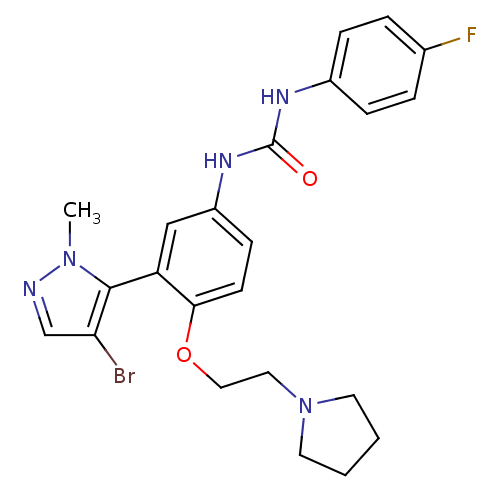
(1-(3-(4-bromo-1-methyl-1H-pyrazol-5-yl)-4-(2-(pyrr...)Show SMILES Cn1ncc(Br)c1-c1cc(NC(=O)Nc2ccc(F)cc2)ccc1OCCN1CCCC1 |(28.29,-24.64,;28.25,-26.18,;29.48,-27.11,;28.97,-28.57,;27.43,-28.53,;26.5,-29.76,;26.99,-27.06,;25.54,-26.54,;24.21,-27.31,;22.88,-26.54,;21.55,-27.31,;20.21,-26.54,;20.21,-25,;18.88,-27.32,;17.55,-26.55,;16.21,-27.32,;14.87,-26.55,;14.88,-25.01,;13.54,-24.24,;16.21,-24.23,;17.54,-25,;22.87,-25.01,;24.2,-24.23,;25.54,-25,;26.87,-24.22,;26.87,-22.68,;28.2,-21.91,;29.54,-22.68,;29.71,-24.2,;31.22,-24.51,;31.98,-23.18,;30.95,-22.04,)| Show InChI InChI=1S/C23H25BrFN5O2/c1-29-22(20(24)15-26-29)19-14-18(28-23(31)27-17-6-4-16(25)5-7-17)8-9-21(19)32-13-12-30-10-2-3-11-30/h4-9,14-15H,2-3,10-13H2,1H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418483
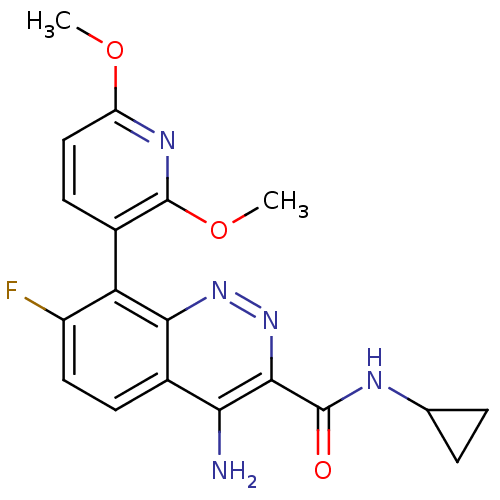
(CHEMBL1783284)Show SMILES COc1ccc(c(OC)n1)-c1c(F)ccc2c(N)c(nnc12)C(=O)NC1CC1 |(-7.37,-43.23,;-6.03,-42.46,;-6.03,-40.92,;-4.69,-40.15,;-4.69,-38.61,;-6.02,-37.85,;-7.36,-38.61,;-8.69,-37.83,;-10.03,-38.6,;-7.36,-40.15,;-6.02,-36.31,;-7.35,-35.54,;-8.69,-36.31,;-7.35,-34,;-6.02,-33.23,;-4.69,-33.99,;-3.35,-33.2,;-3.36,-31.66,;-1.99,-33.98,;-2,-35.54,;-3.34,-36.31,;-4.68,-35.54,;-.65,-33.21,;-.65,-31.67,;.68,-33.98,;2.01,-33.21,;3.55,-33.2,;2.78,-31.87,)| Show InChI InChI=1S/C19H18FN5O3/c1-27-13-8-6-10(19(23-13)28-2)14-12(20)7-5-11-15(21)17(25-24-16(11)14)18(26)22-9-3-4-9/h5-9H,3-4H2,1-2H3,(H2,21,24)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061037
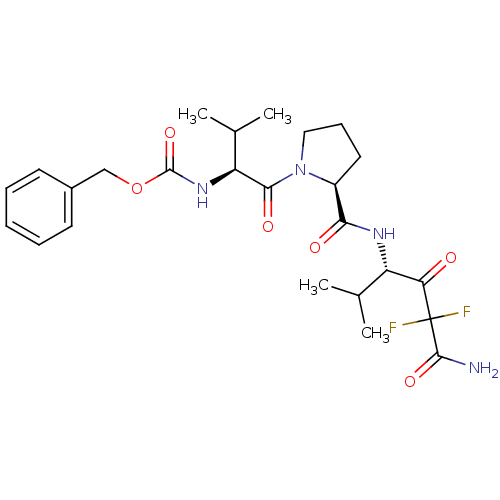
(CHEMBL302961 | {(S)-1-[(S)-2-((S)-3-Carbamoyl-3,3-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(N)=O Show InChI InChI=1S/C25H34F2N4O6/c1-14(2)18(20(32)25(26,27)23(28)35)29-21(33)17-11-8-12-31(17)22(34)19(15(3)4)30-24(36)37-13-16-9-6-5-7-10-16/h5-7,9-10,14-15,17-19H,8,11-13H2,1-4H3,(H2,28,35)(H,29,33)(H,30,36)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50416474
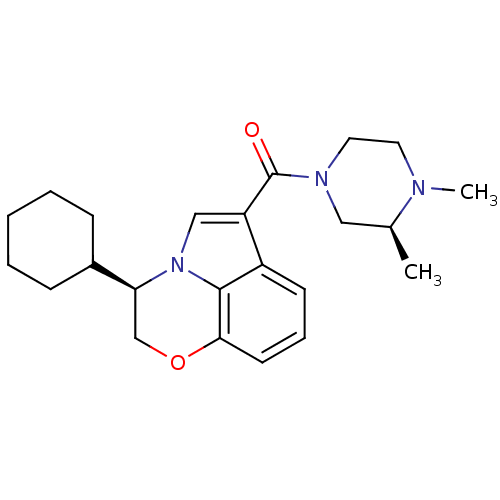
(CHEMBL1209710)Show SMILES C[C@H]1CN(CCN1C)C(=O)c1cn2[C@@H](COc3cccc1c23)C1CCCCC1 |r| Show InChI InChI=1S/C23H31N3O2/c1-16-13-25(12-11-24(16)2)23(27)19-14-26-20(17-7-4-3-5-8-17)15-28-21-10-6-9-18(19)22(21)26/h6,9-10,14,16-17,20H,3-5,7-8,11-13,15H2,1-2H3/t16-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 20: 4918-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.067
BindingDB Entry DOI: 10.7270/Q21R6RS6 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 1
(Homo sapiens (Human)) | BDBM50091752
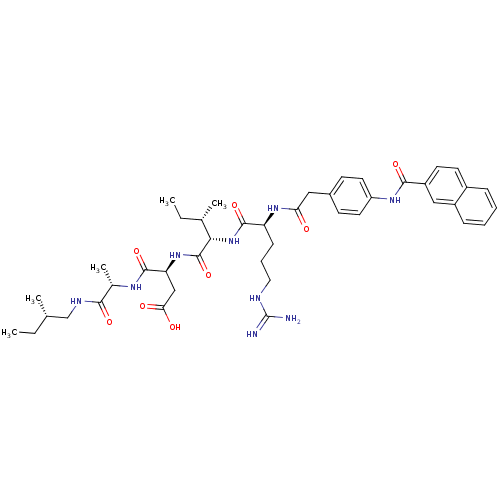
((S)-3-{(2S,3S)-2-[(S)-5-Guanidino-2-(2-{4-[(naphth...)Show SMILES CC[C@H](C)CNC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(NC(=O)c2ccc3ccccc3c2)cc1)[C@@H](C)CC Show InChI InChI=1S/C43H59N9O8/c1-6-25(3)24-47-38(56)27(5)48-41(59)34(23-36(54)55)51-42(60)37(26(4)7-2)52-40(58)33(13-10-20-46-43(44)45)50-35(53)21-28-14-18-32(19-15-28)49-39(57)31-17-16-29-11-8-9-12-30(29)22-31/h8-9,11-12,14-19,22,25-27,33-34,37H,6-7,10,13,20-21,23-24H2,1-5H3,(H,47,56)(H,48,59)(H,49,57)(H,50,53)(H,51,60)(H,52,58)(H,54,55)(H4,44,45,46)/t25-,26-,27-,33-,34-,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091752

((S)-3-{(2S,3S)-2-[(S)-5-Guanidino-2-(2-{4-[(naphth...)Show SMILES CC[C@H](C)CNC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(NC(=O)c2ccc3ccccc3c2)cc1)[C@@H](C)CC Show InChI InChI=1S/C43H59N9O8/c1-6-25(3)24-47-38(56)27(5)48-41(59)34(23-36(54)55)51-42(60)37(26(4)7-2)52-40(58)33(13-10-20-46-43(44)45)50-35(53)21-28-14-18-32(19-15-28)49-39(57)31-17-16-29-11-8-9-12-30(29)22-31/h8-9,11-12,14-19,22,25-27,33-34,37H,6-7,10,13,20-21,23-24H2,1-5H3,(H,47,56)(H,48,59)(H,49,57)(H,50,53)(H,51,60)(H,52,58)(H,54,55)(H4,44,45,46)/t25-,26-,27-,33-,34-,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418482
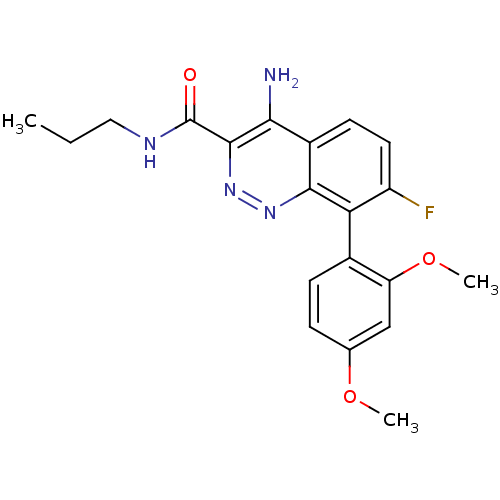
(CHEMBL1783283)Show SMILES CCCNC(=O)c1nnc2c(c(F)ccc2c1N)-c1ccc(OC)cc1OC |(30.61,-19.8,;29.28,-20.57,;27.94,-19.8,;26.61,-20.57,;25.28,-19.8,;25.28,-18.26,;23.94,-20.57,;23.93,-22.13,;22.59,-22.9,;21.25,-22.14,;19.91,-22.9,;18.58,-22.13,;17.24,-22.9,;18.58,-20.59,;19.91,-19.82,;21.24,-20.58,;22.58,-19.79,;22.57,-18.25,;19.91,-24.44,;21.24,-25.2,;21.24,-26.74,;19.9,-27.51,;19.9,-29.05,;18.57,-29.82,;18.57,-26.74,;18.57,-25.2,;17.24,-24.42,;15.9,-25.19,)| Show InChI InChI=1S/C20H21FN4O3/c1-4-9-23-20(26)19-17(22)13-7-8-14(21)16(18(13)24-25-19)12-6-5-11(27-2)10-15(12)28-3/h5-8,10H,4,9H2,1-3H3,(H2,22,24)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301514
(1-(4-chlorophenyl)-3-(3-(1-methyl-1H-pyrazol-5-yl)...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCCCC1 Show InChI InChI=1S/C24H28ClN5O2/c1-29-22(11-12-26-29)21-17-20(28-24(31)27-19-7-5-18(25)6-8-19)9-10-23(21)32-16-15-30-13-3-2-4-14-30/h5-12,17H,2-4,13-16H2,1H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301480
(1-(4-chlorophenyl)-3-(4-(2-(4-hydroxypiperidin-1-y...)Show SMILES Cn1nccc1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCC(O)CC1 Show InChI InChI=1S/C24H28ClN5O3/c1-29-22(8-11-26-29)21-16-19(28-24(32)27-18-4-2-17(25)3-5-18)6-7-23(21)33-15-14-30-12-9-20(31)10-13-30/h2-8,11,16,20,31H,9-10,12-15H2,1H3,(H2,27,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301518
(1-(4-chlorophenyl)-3-(4-(2-(diethylamino)ethoxy)-3...)Show SMILES CCN(CC)CCOc1ccc(NC(=O)Nc2ccc(Cl)cc2)cc1-c1ccnn1C Show InChI InChI=1S/C23H28ClN5O2/c1-4-29(5-2)14-15-31-22-11-10-19(16-20(22)21-12-13-25-28(21)3)27-23(30)26-18-8-6-17(24)7-9-18/h6-13,16H,4-5,14-15H2,1-3H3,(H2,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301513
(1-(3-(4-chloro-1-methyl-1H-pyrazol-5-yl)-4-(2-(pip...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)Nc2ccc(Cl)cc2)ccc1OCCN1CCCCC1 |(4.23,-16.28,;4.2,-17.82,;5.43,-18.75,;4.92,-20.21,;3.38,-20.17,;2.44,-21.39,;2.94,-18.69,;1.49,-18.18,;.16,-18.95,;-1.18,-18.18,;-2.51,-18.95,;-3.84,-18.18,;-3.85,-16.64,;-5.18,-18.95,;-6.51,-18.19,;-7.85,-18.96,;-9.18,-18.19,;-9.18,-16.64,;-10.51,-15.87,;-7.85,-15.87,;-6.52,-16.64,;-1.18,-16.64,;.14,-15.87,;1.49,-16.64,;2.82,-15.86,;2.81,-14.32,;4.14,-13.55,;5.48,-14.31,;5.48,-15.85,;6.81,-16.62,;8.14,-15.85,;8.14,-14.31,;6.8,-13.53,)| Show InChI InChI=1S/C24H27Cl2N5O2/c1-30-23(21(26)16-27-30)20-15-19(29-24(32)28-18-7-5-17(25)6-8-18)9-10-22(20)33-14-13-31-11-3-2-4-12-31/h5-10,15-16H,2-4,11-14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50306587
(6-Fluoro-N-(6-(4-methoxytetrahydro-2H-pyran-4-yl)p...)Show SMILES COC1(CCOCC1)c1ccc(NC(=O)c2cc(=O)c3cc(F)cc(-c4c(C)nn(C)c4C)c3o2)cn1 |(27.97,-16.65,;26.65,-17.44,;26.66,-18.99,;26.66,-20.53,;27.99,-21.29,;29.33,-20.52,;29.33,-18.98,;27.99,-18.21,;25.33,-18.23,;24,-19,;22.66,-18.23,;22.67,-16.69,;21.33,-15.93,;20,-16.7,;20,-18.24,;18.66,-15.93,;18.66,-14.39,;17.31,-13.61,;17.31,-12.07,;15.98,-14.4,;14.65,-13.63,;13.32,-14.4,;11.99,-13.63,;13.32,-15.95,;14.65,-16.72,;14.65,-18.27,;15.9,-19.17,;17.36,-18.7,;15.42,-20.64,;13.88,-20.64,;12.98,-21.89,;13.41,-19.17,;11.94,-18.7,;15.99,-15.94,;17.33,-16.71,;23.99,-15.92,;25.32,-16.68,)| Show InChI InChI=1S/C27H27FN4O5/c1-15-24(16(2)32(3)31-15)20-12-17(28)11-19-21(33)13-22(37-25(19)20)26(34)30-18-5-6-23(29-14-18)27(35-4)7-9-36-10-8-27/h5-6,11-14H,7-10H2,1-4H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1B receptor expressed in CHO cells |
J Med Chem 53: 1876-80 (2010)
Article DOI: 10.1021/jm901200t
BindingDB Entry DOI: 10.7270/Q25D8RZ0 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091761
((S)-N-[(S)-4-Guanidino-1-((S)-2-methyl-butylcarbam...)Show SMILES CC[C@H](C)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(NC(=O)c2ccc3ccccc3c2)cc1)[C@@H](C)CC Show InChI InChI=1S/C46H66N12O8/c1-5-27(3)26-53-41(63)34(13-9-21-51-45(47)48)56-43(65)36(25-38(60)61)57-44(66)39(28(4)6-2)58-42(64)35(14-10-22-52-46(49)50)55-37(59)23-29-15-19-33(20-16-29)54-40(62)32-18-17-30-11-7-8-12-31(30)24-32/h7-8,11-12,15-20,24,27-28,34-36,39H,5-6,9-10,13-14,21-23,25-26H2,1-4H3,(H,53,63)(H,54,62)(H,55,59)(H,56,65)(H,57,66)(H,58,64)(H,60,61)(H4,47,48,51)(H4,49,50,52)/t27-,28-,34-,35-,36-,39-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 1
(Homo sapiens (Human)) | BDBM50091757
(CHEMBL264744 | Cyclic-(Cys-Phe-Gly-Gly-Ala-Ile-Asp...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(O)=O)[C@@H](C)CC Show InChI InChI=1S/C67H107N21O22S2/c1-9-33(5)53-64(107)77-26-49(93)78-35(7)55(98)82-40(18-19-46(69)90)61(104)86-44(29-89)60(103)76-27-50(94)80-41(21-32(3)4)58(101)75-28-51(95)81-45(66(109)110)31-112-111-30-38(68)57(100)84-42(22-37-15-12-11-13-16-37)59(102)74-24-47(91)73-25-48(92)79-36(8)56(99)87-54(34(6)10-2)65(108)85-43(23-52(96)97)63(106)83-39(62(105)88-53)17-14-20-72-67(70)71/h11-13,15-16,32-36,38-45,53-54,89H,9-10,14,17-31,68H2,1-8H3,(H2,69,90)(H,73,91)(H,74,102)(H,75,101)(H,76,103)(H,77,107)(H,78,93)(H,79,92)(H,80,94)(H,81,95)(H,82,98)(H,83,106)(H,84,100)(H,85,108)(H,86,104)(H,87,99)(H,88,105)(H,96,97)(H,109,110)(H4,70,71,72)/t33-,34-,35-,36-,38+,39-,40-,41-,42-,43-,44-,45+,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091757

(CHEMBL264744 | Cyclic-(Cys-Phe-Gly-Gly-Ala-Ile-Asp...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(O)=O)[C@@H](C)CC Show InChI InChI=1S/C67H107N21O22S2/c1-9-33(5)53-64(107)77-26-49(93)78-35(7)55(98)82-40(18-19-46(69)90)61(104)86-44(29-89)60(103)76-27-50(94)80-41(21-32(3)4)58(101)75-28-51(95)81-45(66(109)110)31-112-111-30-38(68)57(100)84-42(22-37-15-12-11-13-16-37)59(102)74-24-47(91)73-25-48(92)79-36(8)56(99)87-54(34(6)10-2)65(108)85-43(23-52(96)97)63(106)83-39(62(105)88-53)17-14-20-72-67(70)71/h11-13,15-16,32-36,38-45,53-54,89H,9-10,14,17-31,68H2,1-8H3,(H2,69,90)(H,73,91)(H,74,102)(H,75,101)(H,76,103)(H,77,107)(H,78,93)(H,79,92)(H,80,94)(H,81,95)(H,82,98)(H,83,106)(H,84,100)(H,85,108)(H,86,104)(H,87,99)(H,88,105)(H,96,97)(H,109,110)(H4,70,71,72)/t33-,34-,35-,36-,38+,39-,40-,41-,42-,43-,44-,45+,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50040237
(2-Chloro-11-(4-methyl-piperazin-1-yl)-dibenzo[b,f]...)Show SMILES CN1CCN(CC1)C1=Nc2ccccc2Sc2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C18H18ClN3S/c1-21-8-10-22(11-9-21)18-14-12-13(19)6-7-16(14)23-17-5-3-2-4-15(17)20-18/h2-7,12H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB
US Patent
| Assay Description
The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... |
US Patent US8653257 (2014)
BindingDB Entry DOI: 10.7270/Q2DR2T69 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301494
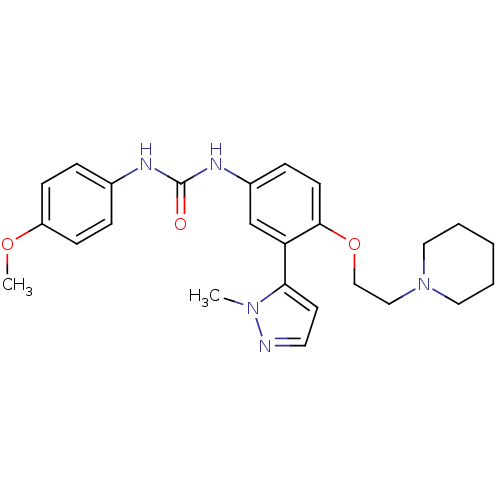
(1-(4-methoxyphenyl)-3-(3-(1-methyl-1H-pyrazol-5-yl...)Show SMILES COc1ccc(NC(=O)Nc2ccc(OCCN3CCCCC3)c(c2)-c2ccnn2C)cc1 Show InChI InChI=1S/C25H31N5O3/c1-29-23(12-13-26-29)22-18-20(28-25(31)27-19-6-9-21(32-2)10-7-19)8-11-24(22)33-17-16-30-14-4-3-5-15-30/h6-13,18H,3-5,14-17H2,1-2H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50301519
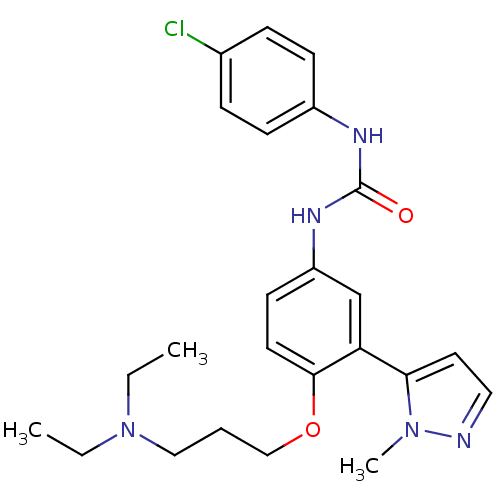
(1-(4-chlorophenyl)-3-(4-(3-(diethylamino)propoxy)-...)Show SMILES CCN(CC)CCCOc1ccc(NC(=O)Nc2ccc(Cl)cc2)cc1-c1ccnn1C Show InChI InChI=1S/C24H30ClN5O2/c1-4-30(5-2)15-6-16-32-23-12-11-20(17-21(23)22-13-14-26-29(22)3)28-24(31)27-19-9-7-18(25)8-10-19/h7-14,17H,4-6,15-16H2,1-3H3,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from 5HT2A receptor expressed in HEK cells |
Bioorg Med Chem Lett 19: 5486-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.073
BindingDB Entry DOI: 10.7270/Q20V8CWG |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418488

(CHEMBL1783285)Show SMILES COc1cc(c(OC)nn1)-c1c(F)ccc2c(N)c(nnc12)C(=O)NC1CC1 |(23.34,-39.57,;22.01,-40.34,;20.67,-39.57,;20.67,-38.03,;19.34,-37.28,;18,-38.03,;16.67,-37.26,;15.34,-38.02,;18,-39.57,;19.33,-40.35,;19.34,-35.74,;18.01,-34.97,;16.67,-35.73,;18.01,-33.42,;19.34,-32.65,;20.67,-33.41,;22.01,-32.63,;22,-31.09,;23.38,-33.4,;23.37,-34.96,;22.02,-35.74,;20.68,-34.97,;24.71,-32.63,;24.71,-31.09,;26.04,-33.4,;27.38,-32.63,;28.91,-32.63,;28.14,-31.29,)| Show InChI InChI=1S/C18H17FN6O3/c1-27-12-7-10(18(28-2)25-22-12)13-11(19)6-5-9-14(20)16(24-23-15(9)13)17(26)21-8-3-4-8/h5-8H,3-4H2,1-2H3,(H2,20,23)(H,21,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031210
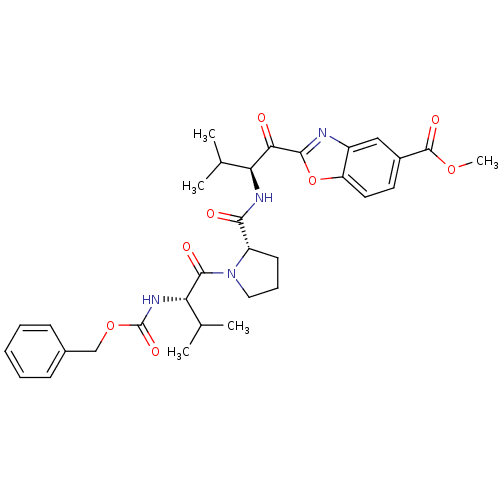
(2-((S)-2-{[(S)-1-((S)-2-Benzyloxycarbonylamino-3-m...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C32H38N4O8/c1-18(2)25(27(37)29-33-22-16-21(31(40)42-5)13-14-24(22)44-29)34-28(38)23-12-9-15-36(23)30(39)26(19(3)4)35-32(41)43-17-20-10-7-6-8-11-20/h6-8,10-11,13-14,16,18-19,23,25-26H,9,12,15,17H2,1-5H3,(H,34,38)(H,35,41)/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data