 Found 1343 hits with Last Name = 'fournier' and Initial = 'j'
Found 1343 hits with Last Name = 'fournier' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134
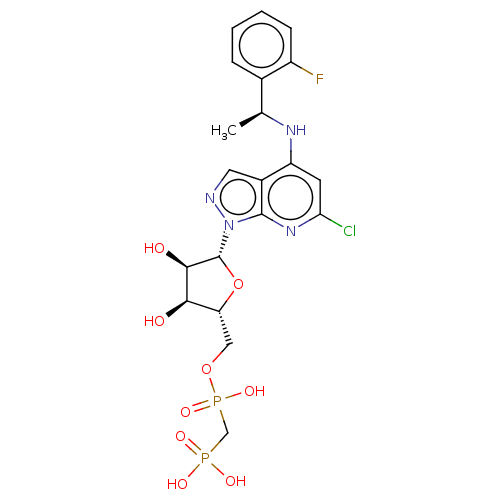
(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50485941

(CHEMBL2181004)Show SMILES COc1ccc(CN[C@H]2CCc3cc(OC)c(OC)c(OC)c3-c3ccc(OC)c(=O)cc23)cc1 |r| Show InChI InChI=1S/C28H31NO6/c1-31-19-9-6-17(7-10-19)16-29-22-12-8-18-14-25(33-3)27(34-4)28(35-5)26(18)20-11-13-24(32-2)23(30)15-21(20)22/h6-7,9-11,13-15,22,29H,8,12,16H2,1-5H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463758
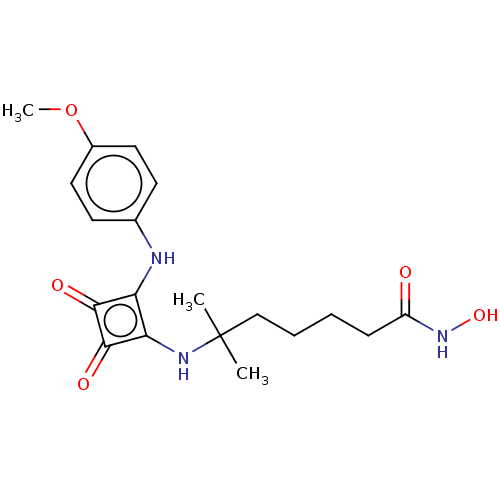
(CHEMBL4250302)Show SMILES COc1ccc(Nc2c(NC(C)(C)CCCCC(=O)NO)c(=O)c2=O)cc1 Show InChI InChI=1S/C19H25N3O5/c1-19(2,11-5-4-6-14(23)22-26)21-16-15(17(24)18(16)25)20-12-7-9-13(27-3)10-8-12/h7-10,20-21,26H,4-6,11H2,1-3H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463741
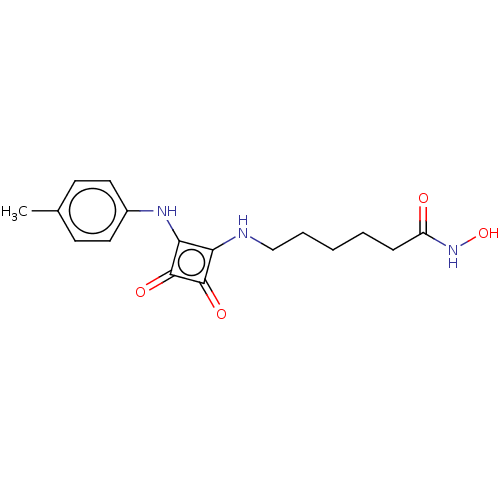
(CHEMBL4239232)Show InChI InChI=1S/C17H21N3O4/c1-11-6-8-12(9-7-11)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485945

(CHEMBL2181003)Show SMILES COc1cc2CC[C@H](NCc3ccc(cc3)[N+]([O-])=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28N2O7/c1-33-23-12-10-19-20(14-22(23)30)21(28-15-16-5-8-18(9-6-16)29(31)32)11-7-17-13-24(34-2)26(35-3)27(36-4)25(17)19/h5-6,8-10,12-14,21,28H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463759
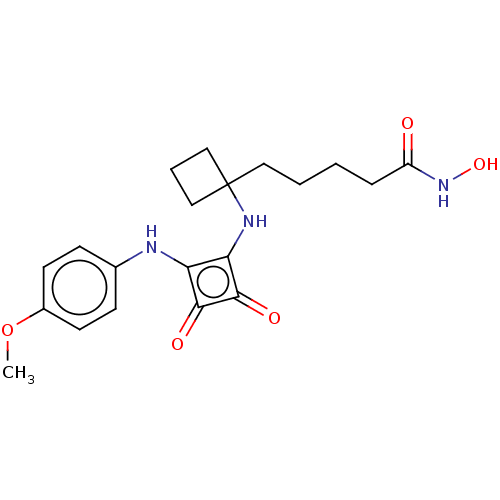
(CHEMBL4237636)Show SMILES COc1ccc(Nc2c(NC3(CCCCC(=O)NO)CCC3)c(=O)c2=O)cc1 Show InChI InChI=1S/C20H25N3O5/c1-28-14-8-6-13(7-9-14)21-16-17(19(26)18(16)25)22-20(11-4-12-20)10-3-2-5-15(24)23-27/h6-9,21-22,27H,2-5,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485942

(CHEMBL2181002)Show SMILES COc1cc2CC[C@H](NCc3cc(F)c(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H26F3NO5/c1-33-22-8-6-16-17(12-21(22)32)20(31-13-14-9-18(28)25(30)19(29)10-14)7-5-15-11-23(34-2)26(35-3)27(36-4)24(15)16/h6,8-12,20,31H,5,7,13H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463739
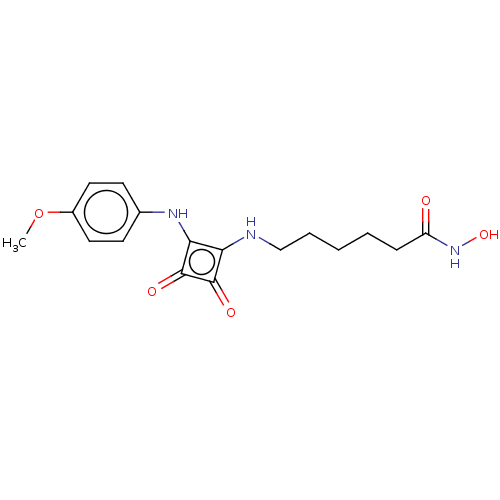
(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463736
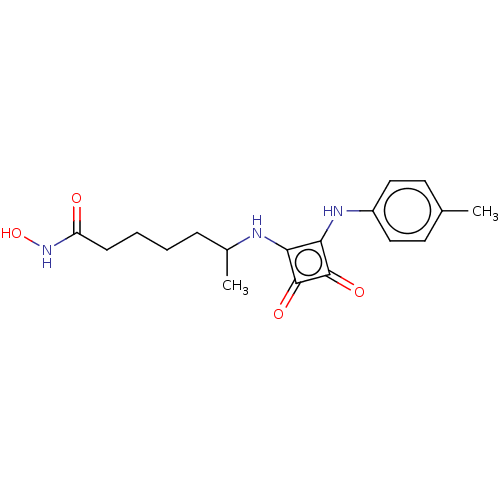
(CHEMBL4251203)Show InChI InChI=1S/C18H23N3O4/c1-11-7-9-13(10-8-11)20-16-15(17(23)18(16)24)19-12(2)5-3-4-6-14(22)21-25/h7-10,12,19-20,25H,3-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485950
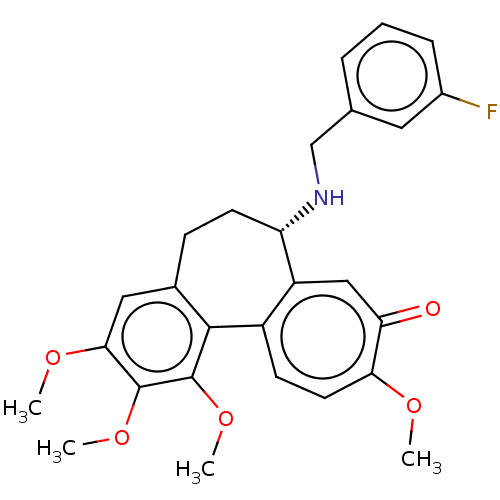
(CHEMBL2181009)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-11-9-19-20(14-22(23)30)21(29-15-16-6-5-7-18(28)12-16)10-8-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-7,9,11-14,21,29H,8,10,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485944

(CHEMBL2181006)Show SMILES COc1cc2CC[C@H](NCc3ccc(Cl)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28ClNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485943
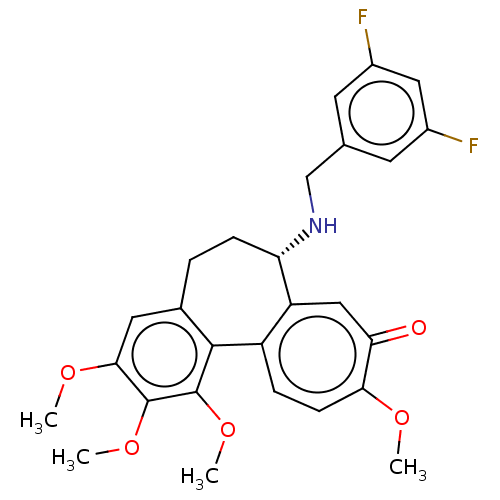
(CHEMBL2181001)Show SMILES COc1cc2CC[C@H](NCc3cc(F)cc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-8-6-19-20(13-22(23)31)21(30-14-15-9-17(28)12-18(29)10-15)7-5-16-11-24(33-2)26(34-3)27(35-4)25(16)19/h6,8-13,21,30H,5,7,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485946
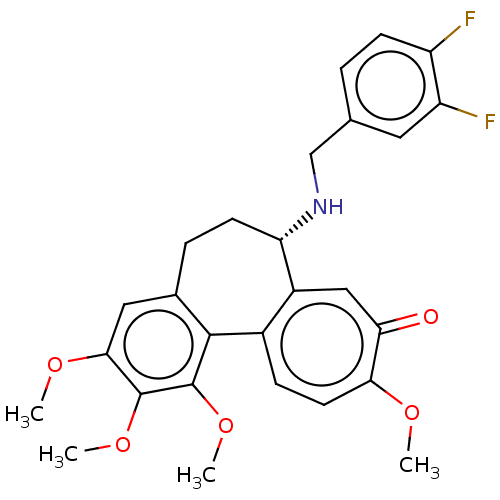
(CHEMBL2181000)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-10-7-17-18(13-22(23)31)21(30-14-15-5-8-19(28)20(29)11-15)9-6-16-12-24(33-2)26(34-3)27(35-4)25(16)17/h5,7-8,10-13,21,30H,6,9,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463756
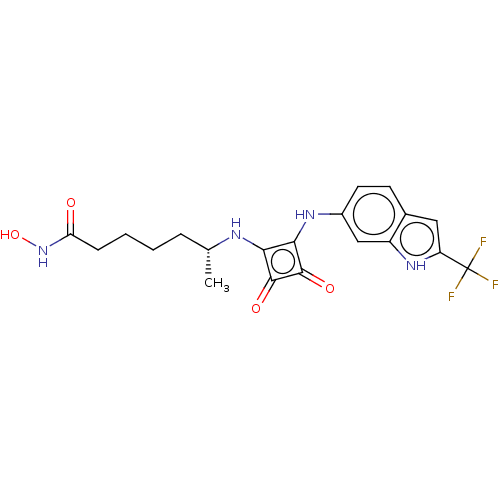
(CHEMBL4246561)Show SMILES C[C@H](CCCCC(=O)NO)Nc1c(Nc2ccc3cc([nH]c3c2)C(F)(F)F)c(=O)c1=O |r| Show InChI InChI=1S/C20H21F3N4O4/c1-10(4-2-3-5-15(28)27-31)24-16-17(19(30)18(16)29)25-12-7-6-11-8-14(20(21,22)23)26-13(11)9-12/h6-10,24-26,31H,2-5H2,1H3,(H,27,28)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485947
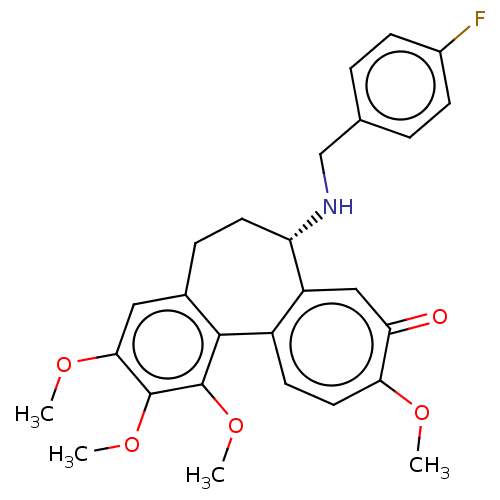
(CHEMBL2181008)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485949

(CHEMBL2180999)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3F)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-22-11-9-17-18(13-21(22)31)20(30-14-16-6-5-7-19(28)25(16)29)10-8-15-12-23(33-2)26(34-3)27(35-4)24(15)17/h5-7,9,11-13,20,30H,8,10,14H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463750
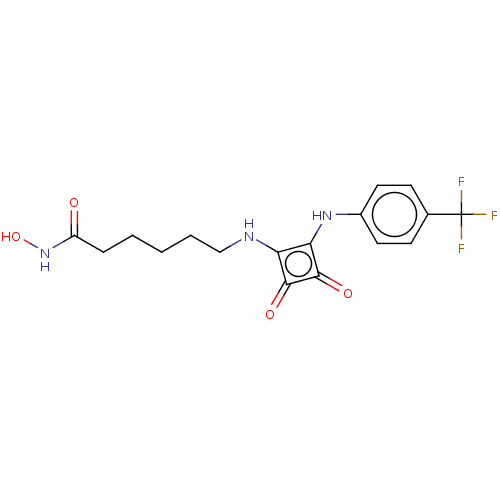
(CHEMBL4241807)Show SMILES ONC(=O)CCCCCNc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C17H18F3N3O4/c18-17(19,20)10-5-7-11(8-6-10)22-14-13(15(25)16(14)26)21-9-3-1-2-4-12(24)23-27/h5-8,21-22,27H,1-4,9H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463753
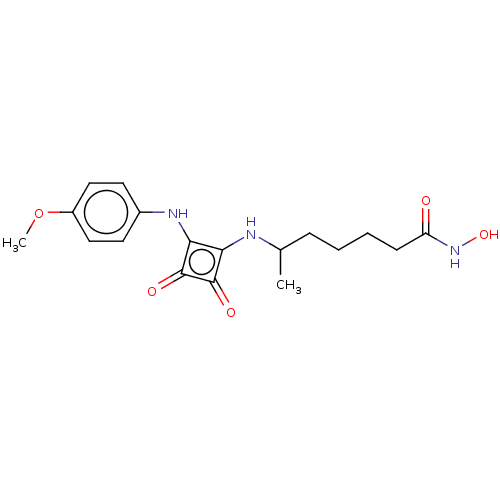
(CHEMBL4250739)Show SMILES COc1ccc(Nc2c(NC(C)CCCCC(=O)NO)c(=O)c2=O)cc1 Show InChI InChI=1S/C18H23N3O5/c1-11(5-3-4-6-14(22)21-25)19-15-16(18(24)17(15)23)20-12-7-9-13(26-2)10-8-12/h7-11,19-20,25H,3-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463752
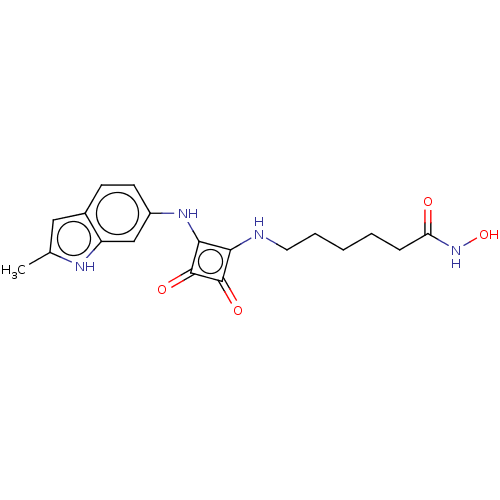
(CHEMBL4247370)Show SMILES Cc1cc2ccc(Nc3c(NCCCCCC(=O)NO)c(=O)c3=O)cc2[nH]1 Show InChI InChI=1S/C19H22N4O4/c1-11-9-12-6-7-13(10-14(12)21-11)22-17-16(18(25)19(17)26)20-8-4-2-3-5-15(24)23-27/h6-7,9-10,20-22,27H,2-5,8H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463743
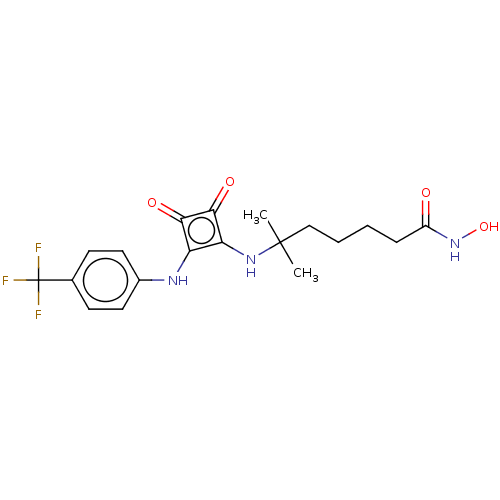
(CHEMBL4241370)Show SMILES CC(C)(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-18(2,10-4-3-5-13(26)25-29)24-15-14(16(27)17(15)28)23-12-8-6-11(7-9-12)19(20,21)22/h6-9,23-24,29H,3-5,10H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485948
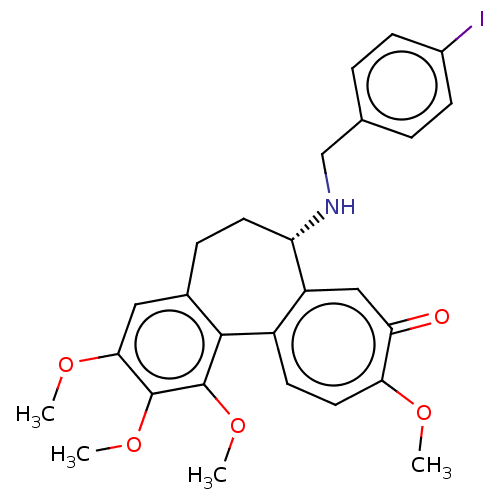
(CHEMBL2181007)Show SMILES COc1cc2CC[C@H](NCc3ccc(I)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28INO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463754
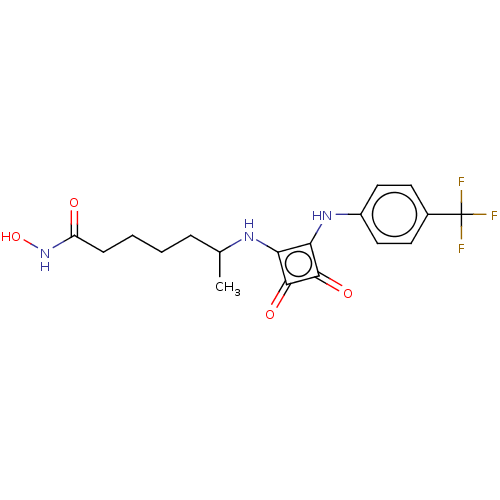
(CHEMBL4240635)Show SMILES CC(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C18H20F3N3O4/c1-10(4-2-3-5-13(25)24-28)22-14-15(17(27)16(14)26)23-12-8-6-11(7-9-12)18(19,20)21/h6-10,22-23,28H,2-5H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463740
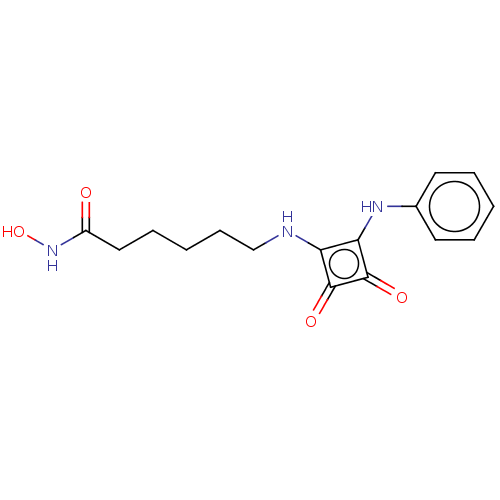
(CHEMBL4251365)Show InChI InChI=1S/C16H19N3O4/c20-12(19-23)9-5-2-6-10-17-13-14(16(22)15(13)21)18-11-7-3-1-4-8-11/h1,3-4,7-8,17-18,23H,2,5-6,9-10H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606735
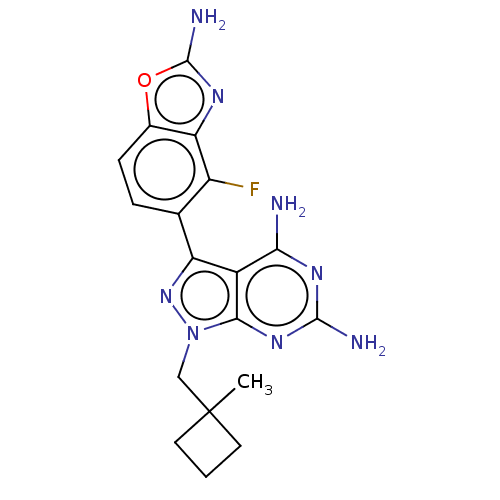
(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463739

(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485940

(CHEMBL2181005)Show SMILES COc1cc2CC[C@H](NCc3ccc(Br)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28BrNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463763
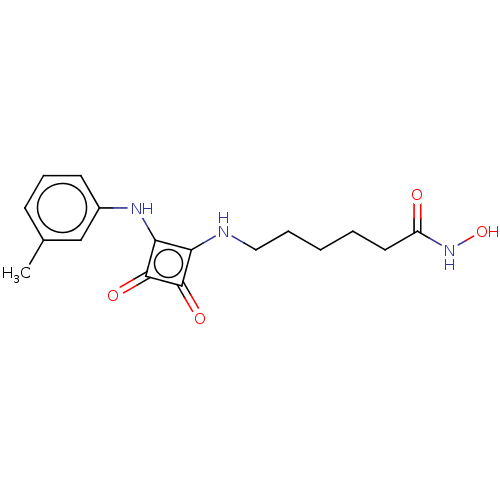
(CHEMBL4248374)Show InChI InChI=1S/C17H21N3O4/c1-11-6-5-7-12(10-11)19-15-14(16(22)17(15)23)18-9-4-2-3-8-13(21)20-24/h5-7,10,18-19,24H,2-4,8-9H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606737
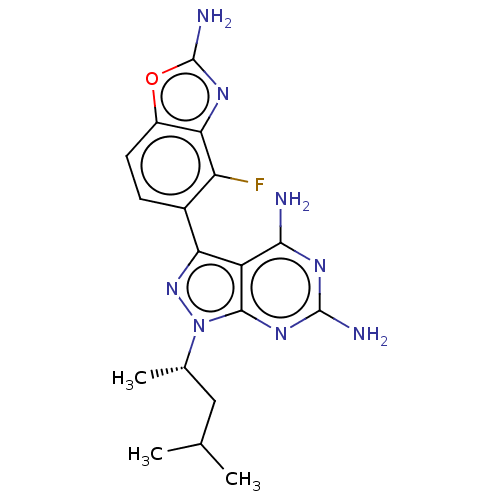
(CHEMBL5218727 | US11731973, Example 5)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327
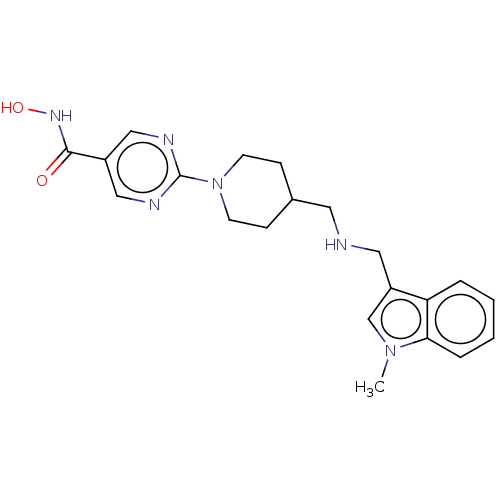
(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463751
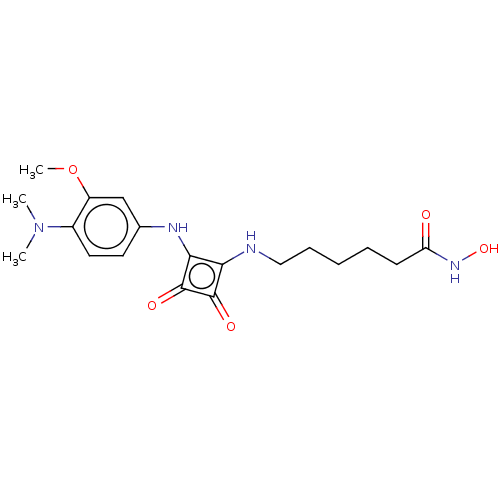
(CHEMBL4244350)Show SMILES COc1cc(Nc2c(NCCCCCC(=O)NO)c(=O)c2=O)ccc1N(C)C Show InChI InChI=1S/C19H26N4O5/c1-23(2)13-9-8-12(11-14(13)28-3)21-17-16(18(25)19(17)26)20-10-6-4-5-7-15(24)22-27/h8-9,11,20-21,27H,4-7,10H2,1-3H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606738
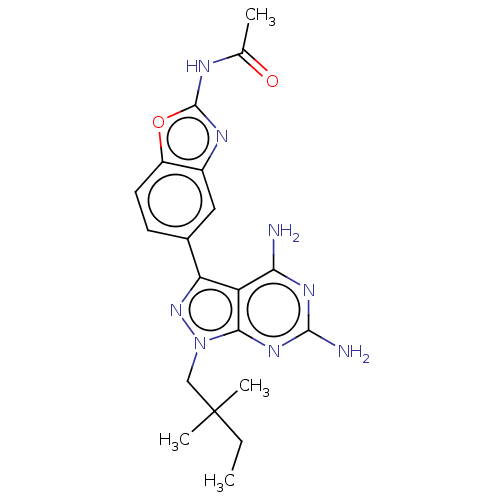
(CHEMBL5218590 | US11731973, Example 6)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(NC(C)=O)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463757
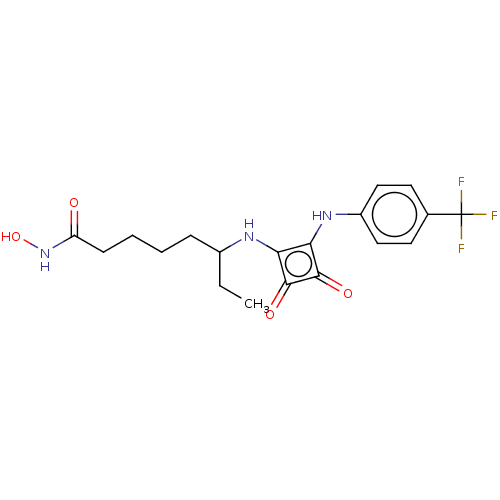
(CHEMBL4249385)Show SMILES CCC(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-2-12(5-3-4-6-14(26)25-29)23-15-16(18(28)17(15)27)24-13-9-7-11(8-10-13)19(20,21)22/h7-10,12,23-24,29H,2-6H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463764
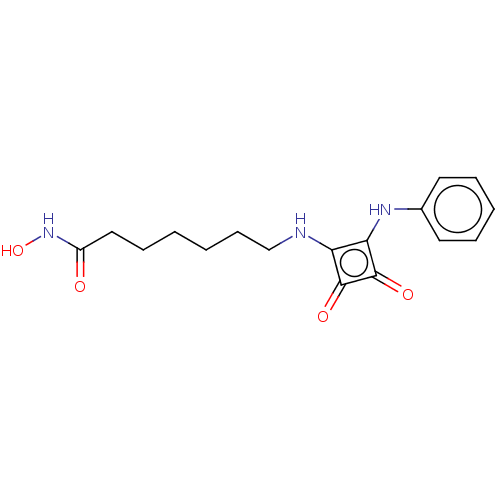
(CHEMBL4245007)Show InChI InChI=1S/C17H21N3O4/c21-13(20-24)10-6-1-2-7-11-18-14-15(17(23)16(14)22)19-12-8-4-3-5-9-12/h3-5,8-9,18-19,24H,1-2,6-7,10-11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463742
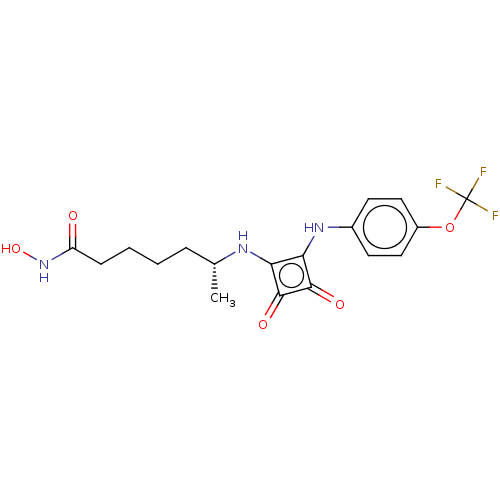
(CHEMBL4242292)Show SMILES C[C@H](CCCCC(=O)NO)Nc1c(Nc2ccc(OC(F)(F)F)cc2)c(=O)c1=O |r| Show InChI InChI=1S/C18H20F3N3O5/c1-10(4-2-3-5-13(25)24-28)22-14-15(17(27)16(14)26)23-11-6-8-12(9-7-11)29-18(19,20)21/h6-10,22-23,28H,2-5H2,1H3,(H,24,25)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606740
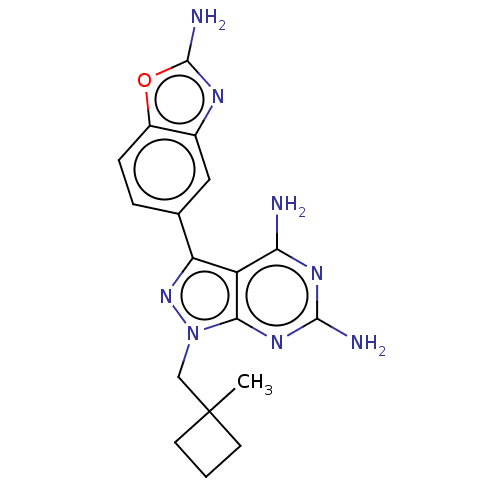
(CHEMBL5220536 | US11731973, Example 30)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606742
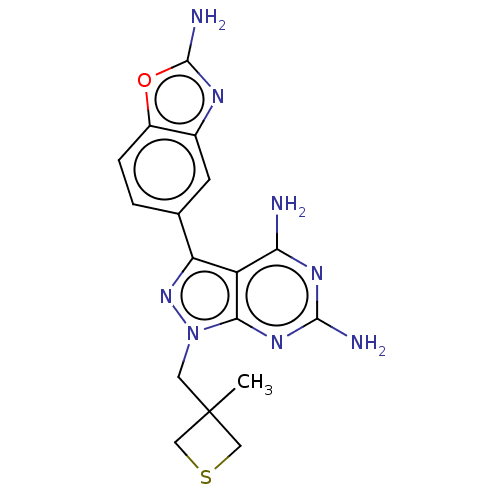
(CHEMBL5220948 | US11731973, Example 9)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CSC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM613740
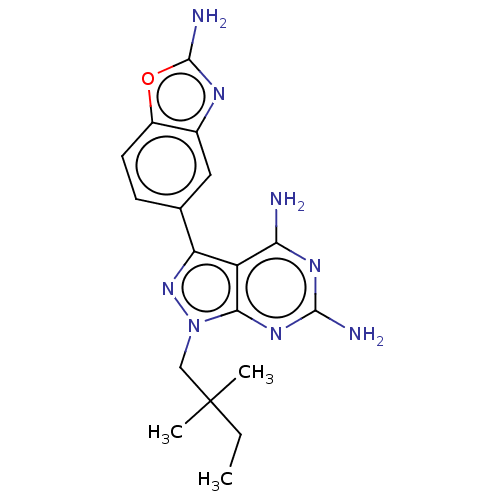
(3-(2-aminobenzoxazol-5-yl)-1-(2,2- dimethylbutyl)-...)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606739
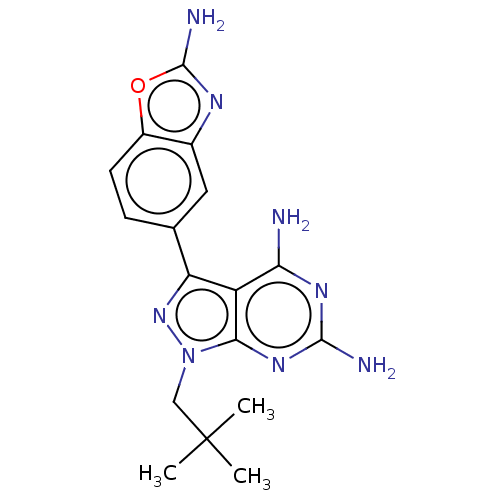
(CHEMBL5220152 | US11731973, Example 7)Show SMILES CC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463767
(CHEMBL4246919)Show InChI InChI=1S/C17H21N3O4/c1-11(7-5-6-10-13(21)20-24)18-14-15(17(23)16(14)22)19-12-8-3-2-4-9-12/h2-4,8-9,11,18-19,24H,5-7,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602541
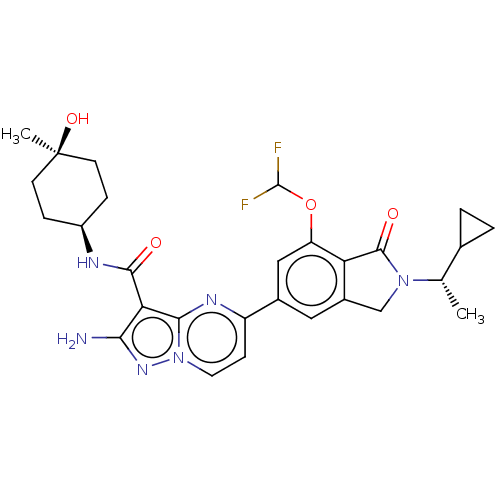
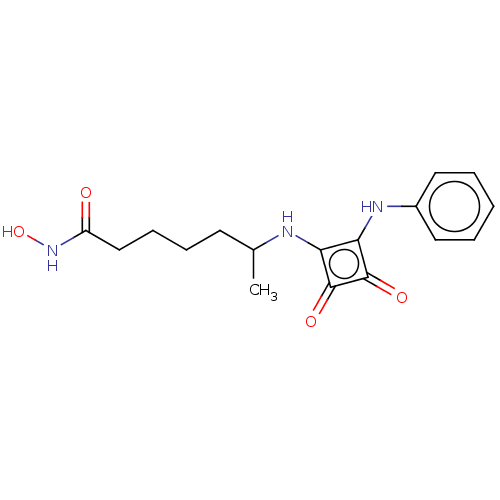
(CHEMBL5209268)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(OC(F)F)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@](C)(O)CC3)c2n1 |r,wU:30.32,1.1,33.37,(7.66,2.89,;6.89,1.55,;7.66,.22,;7.66,-1.32,;8.99,-.55,;5.35,1.55,;4.44,2.8,;2.98,2.32,;1.65,3.09,;.31,2.32,;.31,.78,;1.65,.01,;1.65,-1.53,;.32,-2.3,;.32,-3.84,;-1.02,-1.53,;2.98,.78,;4.44,.31,;4.84,-1.18,;-1.02,3.09,;-1.02,4.63,;-2.34,5.39,;-3.67,4.63,;-5.14,5.1,;-6.04,3.86,;-7.58,3.86,;-5.14,2.61,;-5.91,1.28,;-7.45,1.28,;-5.14,-.06,;-5.91,-1.39,;-5.14,-2.72,;-5.91,-4.06,;-7.45,-4.06,;-8.22,-5.39,;-8.99,-4.06,;-8.22,-2.72,;-7.45,-1.39,;-3.67,3.09,;-2.34,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606736
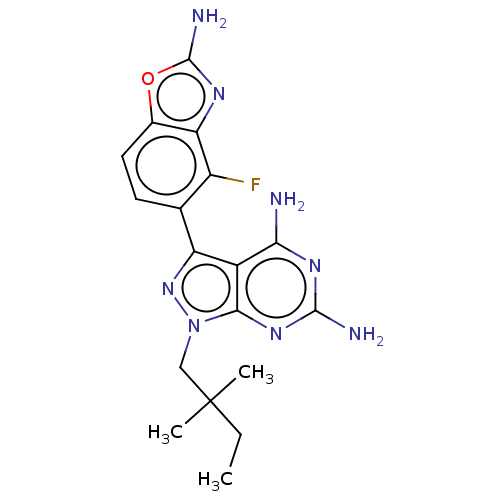
(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606741
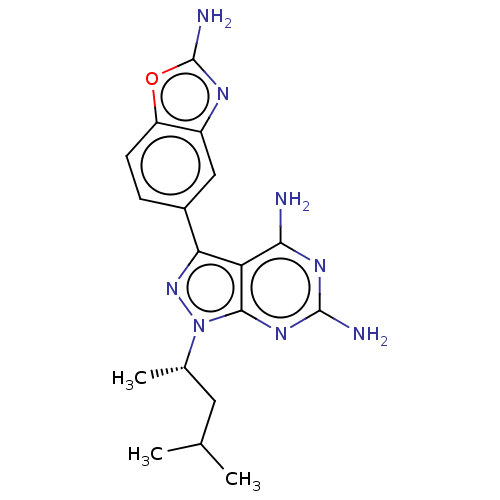
(CHEMBL5219710 | US11731973, Example 1)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50463739

(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using fluorogenic HDAC substrate after 10 mins by spectrophotometric method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM613743

(1-(2,2-dimethylpropyl)-3-(2- ethylaminobenzoxazol-...)Show SMILES CCNc1nc2cc(ccc2o1)-c1nn(CC(C)(C)C)c2nc(N)nc(N)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463749
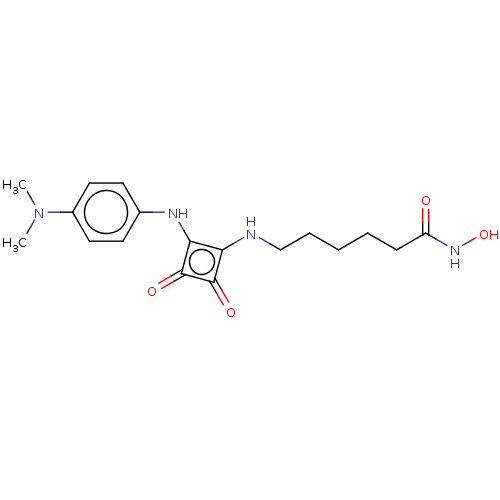
(CHEMBL4242426)Show SMILES CN(C)c1ccc(Nc2c(NCCCCCC(=O)NO)c(=O)c2=O)cc1 Show InChI InChI=1S/C18H24N4O4/c1-22(2)13-9-7-12(8-10-13)20-16-15(17(24)18(16)25)19-11-5-3-4-6-14(23)21-26/h7-10,19-20,26H,3-6,11H2,1-2H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM613734
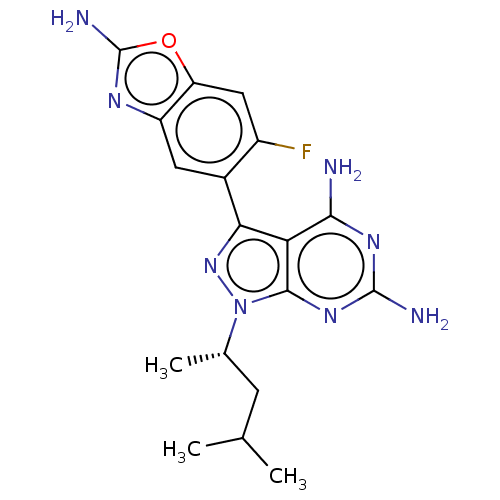
(3-(2-amino-6-fluorobenzoxazol-5-yl)- 1-((S)-1,3-di...)Show SMILES CC(C)C[C@H](C)n1nc(-c2cc3nc(N)oc3cc2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463743

(CHEMBL4241370)Show SMILES CC(C)(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-18(2,10-4-3-5-13(26)25-29)24-15-14(16(27)17(15)28)23-12-8-6-11(7-9-12)19(20,21)22/h6-9,23-24,29H,3-5,10H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50463739

(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate after 45 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM613744

(3-(2-aminobenzoxazol-5-yl)-1-(2,2- dimethylbut-3-e...)Show SMILES CC(C)(Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data