

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231459 (CHEMBL252818 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231448 (CHEMBL253022 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231440 (CHEMBL253878 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231445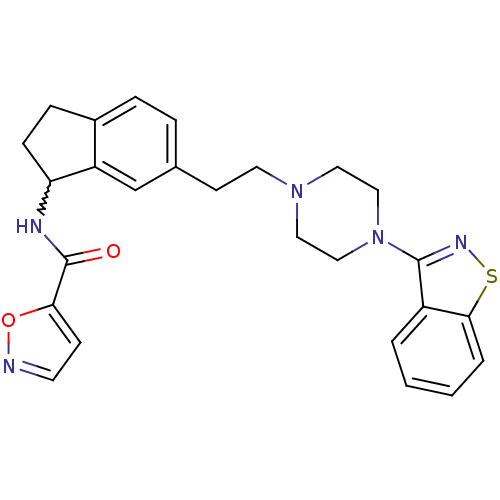 (CHEMBL251834 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231453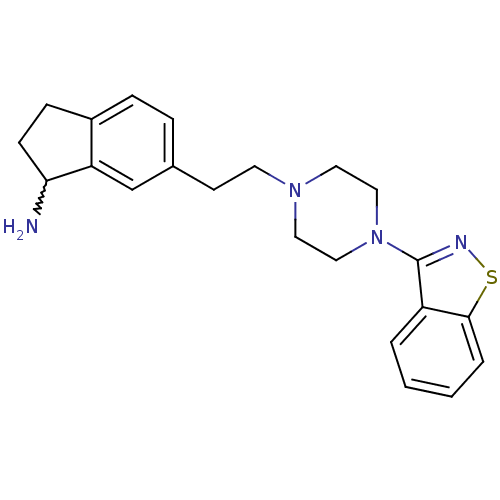 (6-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231452 (CHEMBL253439 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231449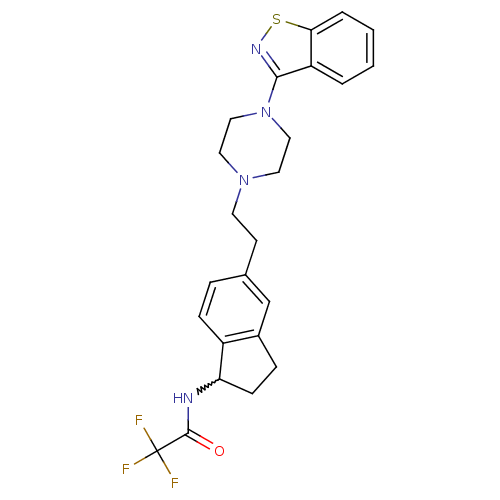 (CHEMBL398711 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486093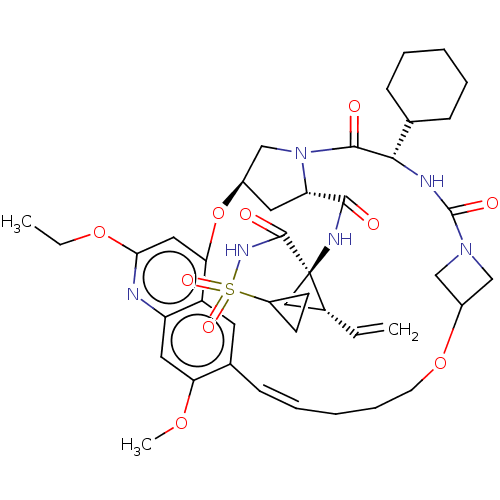 (CHEMBL2203889) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231441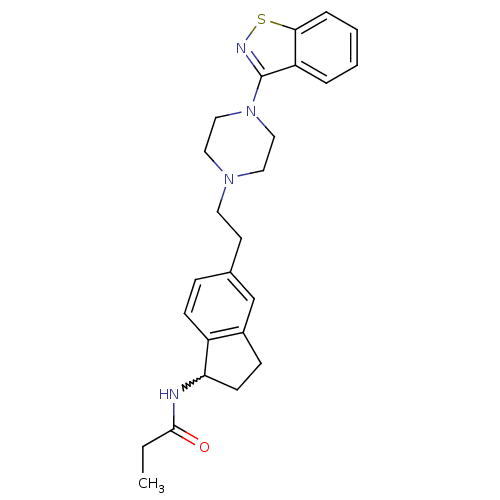 (CHEMBL252031 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231442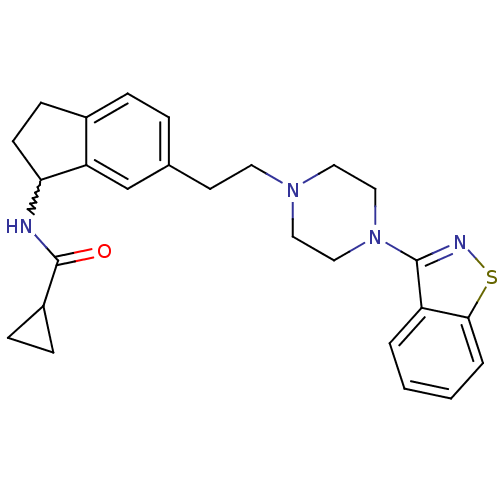 (CHEMBL253024 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231449 (CHEMBL398711 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231447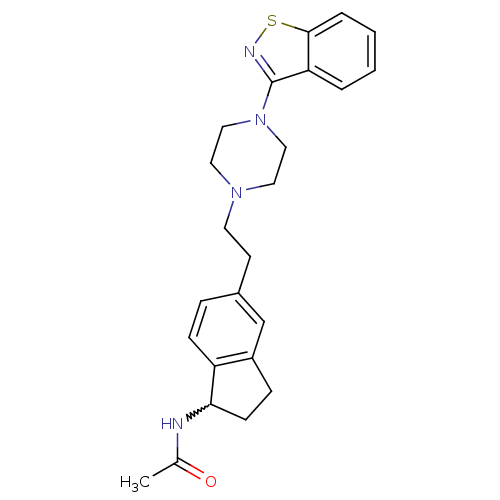 (CHEMBL252030 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486108 (CHEMBL2203884) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231451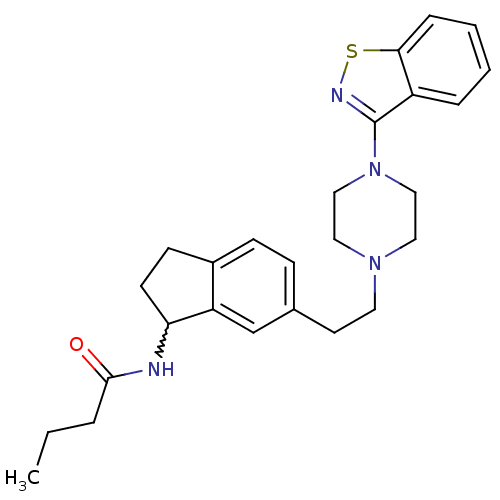 (CHEMBL400235 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231441 (CHEMBL252031 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231437 (CHEMBL252230 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231460 (CHEMBL398525 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231443 (CHEMBL252228 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50505279 (CHEMBL4436207) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibitory effect of histamine H3 antagonist on the electrically evoked contractile response of isolated guinea pig jejunum segments. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231444 (CHEMBL398744 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vertex Pharmaceuticals (Europe) Limited | Assay Description The kinase activity was determined by incubation of enzyme and its substrate, and test compound, in the presence ATP/[gamma-32P] ATP. After incubatio... | Nat Med 10: 262-7 (2004) Article DOI: 10.1038/nm1003 BindingDB Entry DOI: 10.7270/Q25M63ZF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231460 (CHEMBL398525 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231446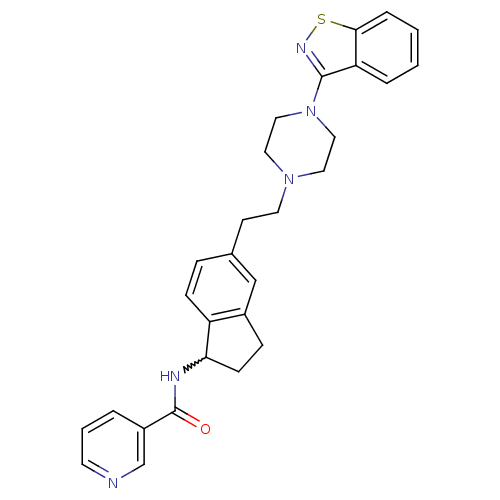 (CHEMBL252430 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486111 (CHEMBL2203888) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486101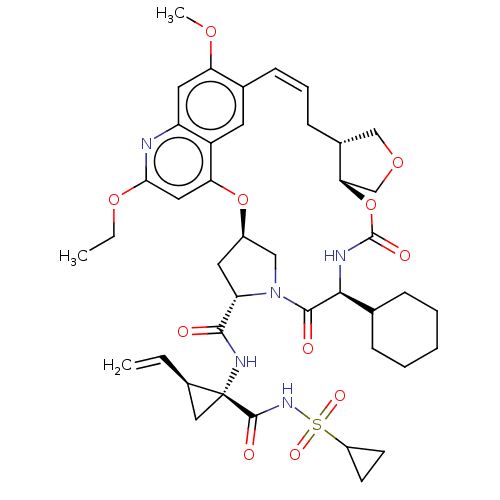 (CHEMBL2203879) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231437 (CHEMBL252230 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231457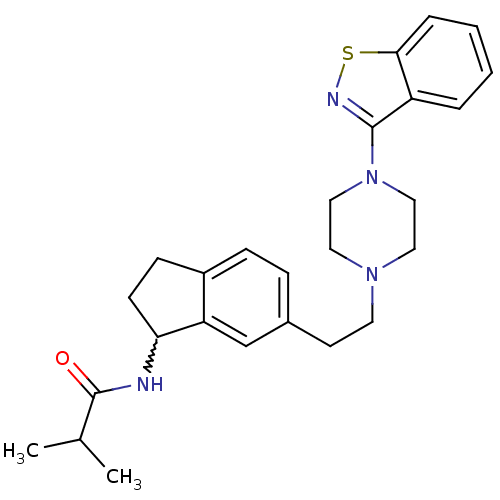 (CHEMBL400236 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486103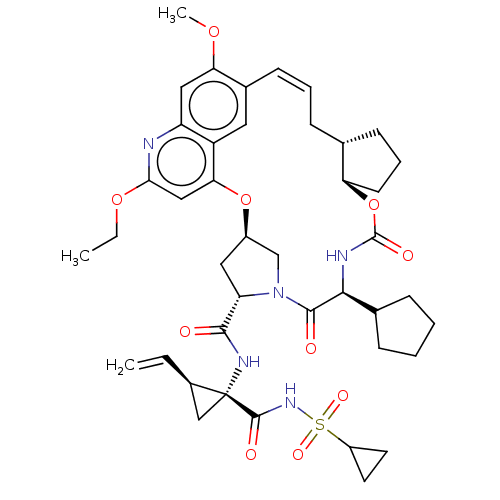 (CHEMBL2203874) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231443 (CHEMBL252228 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231438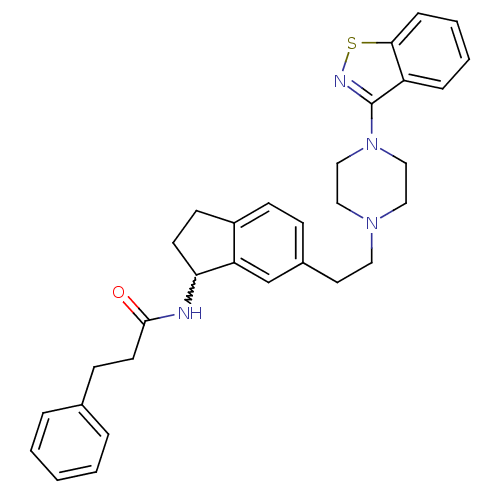 (CHEMBL252201 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231456 (CHEMBL398619 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50231449 (CHEMBL398711 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8OHDPAT from human 5HT1A receptor | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50231460 (CHEMBL398525 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8OHDPAT from human 5HT1A receptor | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50231447 (CHEMBL252030 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8OHDPAT from human 5HT1A receptor | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486092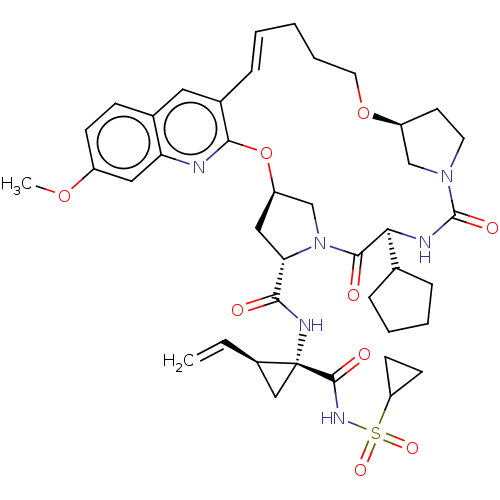 (CHEMBL2203892) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease D168V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231459 (CHEMBL252818 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231444 (CHEMBL398744 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486091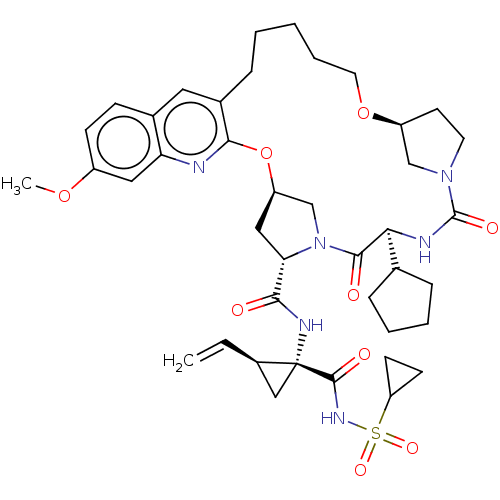 (CHEMBL2203893) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50231450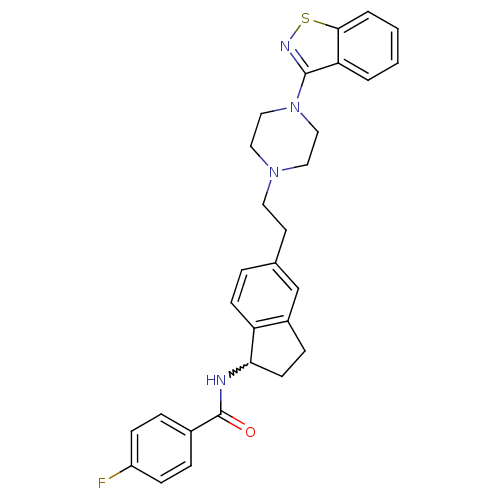 (CHEMBL252429 | N-(5-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486110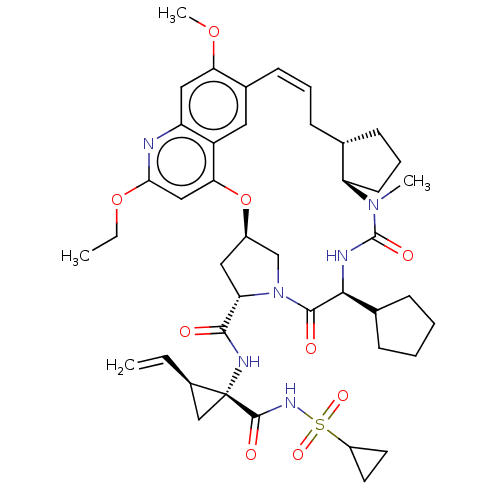 (CHEMBL2203883) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486090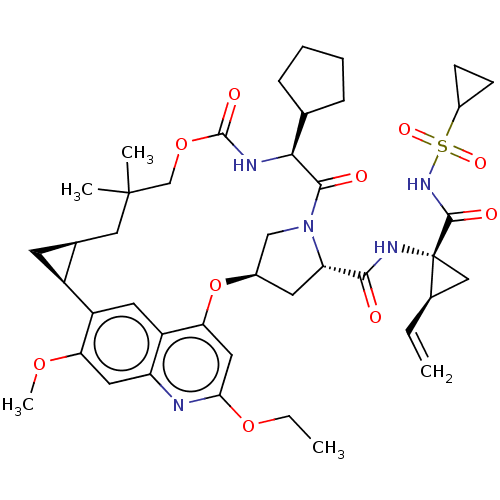 (CHEMBL2203882) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease D168V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231445 (CHEMBL251834 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486105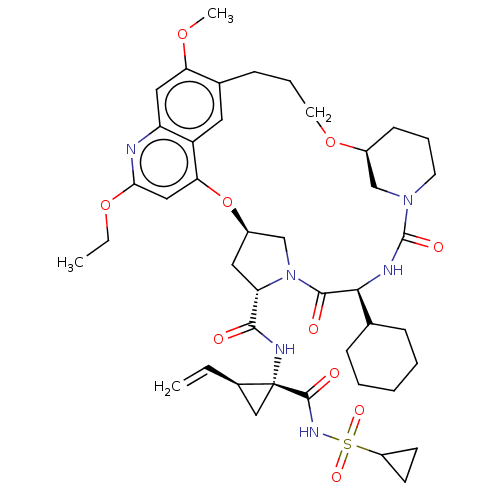 (CHEMBL2203886) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50231454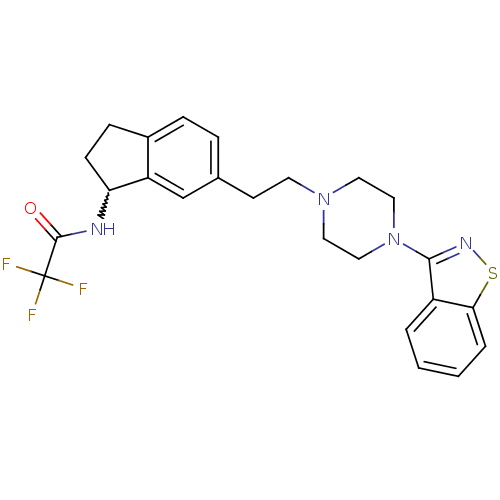 (CHEMBL252816 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from rat adrenergic alpha1A receptor expressed in fibroblast cells | Bioorg Med Chem Lett 18: 489-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.106 BindingDB Entry DOI: 10.7270/Q2611023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486097 (CHEMBL2203875) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 813 total ) | Next | Last >> |