 Found 197 hits with Last Name = 'hoshi' and Initial = 'j'
Found 197 hits with Last Name = 'hoshi' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50157339
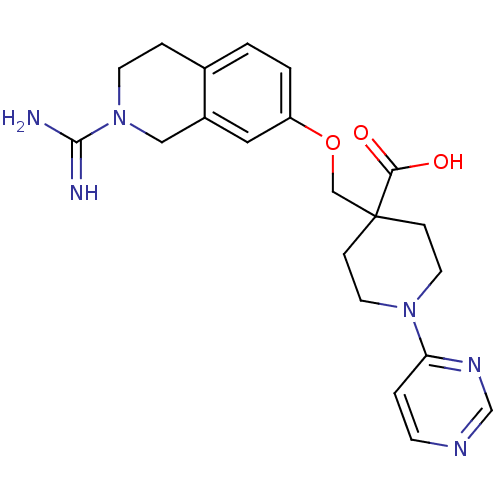
(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES NC(=N)N1CCc2ccc(OCC3(CCN(CC3)c3ccncn3)C(O)=O)cc2C1 Show InChI InChI=1S/C21H26N6O3/c22-20(23)27-8-4-15-1-2-17(11-16(15)12-27)30-13-21(19(28)29)5-9-26(10-6-21)18-3-7-24-14-25-18/h1-3,7,11,14H,4-6,8-10,12-13H2,(H3,22,23)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50068488
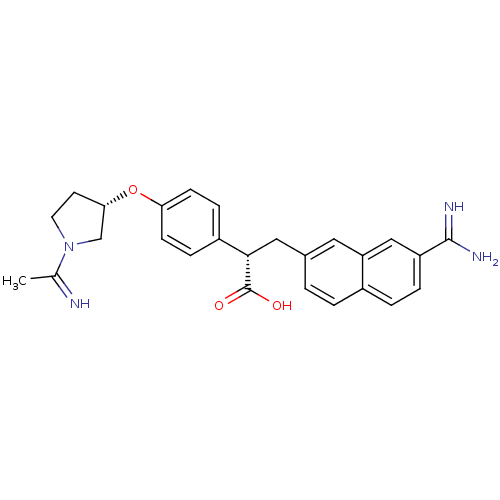
((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50068488

((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Trypsin |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50157339

(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES NC(=N)N1CCc2ccc(OCC3(CCN(CC3)c3ccncn3)C(O)=O)cc2C1 Show InChI InChI=1S/C21H26N6O3/c22-20(23)27-8-4-15-1-2-17(11-16(15)12-27)30-13-21(19(28)29)5-9-26(10-6-21)18-3-7-24-14-25-18/h1-3,7,11,14H,4-6,8-10,12-13H2,(H3,22,23)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Trypsin |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50157341
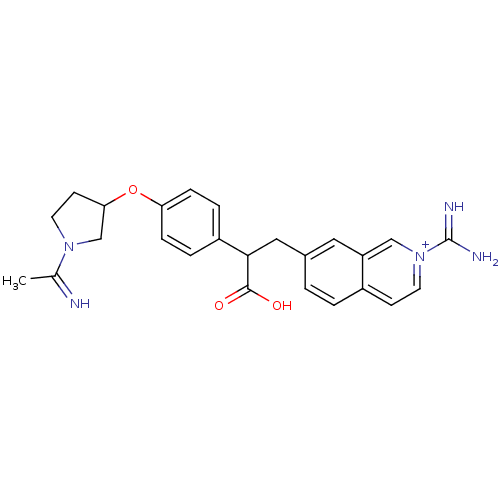
(3-(2-Carbamimidoyl-isoquinolin-7-yl)-2-{4-[1-(1-im...)Show SMILES CC(=N)N1CCC(C1)Oc1ccc(cc1)C(Cc1ccc2cc[n+](cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C25H27N5O3/c1-16(26)29-11-9-22(15-29)33-21-6-4-19(5-7-21)23(24(31)32)13-17-2-3-18-8-10-30(25(27)28)14-20(18)12-17/h2-8,10,12,14,22-23,26H,9,11,13,15H2,1H3,(H3-,27,28,31,32)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against plasmin; n=3 |
Bioorg Med Chem Lett 15: 185-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.033
BindingDB Entry DOI: 10.7270/Q26Q1Z14 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50068488

((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Plasmin |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50157335

(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES NC(=N)N1CCc2ccc(OCC3(CCN(CC3)c3ccncc3)C(O)=O)cc2C1 Show InChI InChI=1S/C22H27N5O3/c23-21(24)27-10-5-16-1-2-19(13-17(16)14-27)30-15-22(20(28)29)6-11-26(12-7-22)18-3-8-25-9-4-18/h1-4,8-9,13H,5-7,10-12,14-15H2,(H3,23,24)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against plasmin; n=3 |
Bioorg Med Chem Lett 15: 185-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.033
BindingDB Entry DOI: 10.7270/Q26Q1Z14 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50157339

(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES NC(=N)N1CCc2ccc(OCC3(CCN(CC3)c3ccncn3)C(O)=O)cc2C1 Show InChI InChI=1S/C21H26N6O3/c22-20(23)27-8-4-15-1-2-17(11-16(15)12-27)30-13-21(19(28)29)5-9-26(10-6-21)18-3-7-24-14-25-18/h1-3,7,11,14H,4-6,8-10,12-13H2,(H3,22,23)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Plasmin |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50068488

((S)-3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor II (thrombin) |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50157339

(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES NC(=N)N1CCc2ccc(OCC3(CCN(CC3)c3ccncn3)C(O)=O)cc2C1 Show InChI InChI=1S/C21H26N6O3/c22-20(23)27-8-4-15-1-2-17(11-16(15)12-27)30-13-21(19(28)29)5-9-26(10-6-21)18-3-7-24-14-25-18/h1-3,7,11,14H,4-6,8-10,12-13H2,(H3,22,23)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor II (thrombin) |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153465

(2-{3-[3-(2,5-Dioxo-4-phenylamino-2,5-dihydro-1H-py...)Show SMILES NC(=N)SCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:10| Show InChI InChI=1S/C22H21N5O2S/c23-22(24)30-12-6-11-27-13-16(15-9-4-5-10-17(15)27)18-19(21(29)26-20(18)28)25-14-7-2-1-3-8-14/h1-5,7-10,13H,6,11-12H2,(H3,23,24)(H2,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50409938

(CHEMBL2094062)Show SMILES C[C@H](NC(=O)Cn1c(CN(C(=O)c2ccc(cc2)C(O)=O)c2ccc(OC3CCN(CC3)C(N)=N)cc2)nc2cc(ccc12)C(N)=N)c1ccccc1 |r| Show InChI InChI=1S/C39H41N9O5/c1-24(25-5-3-2-4-6-25)44-35(49)23-48-33-16-11-28(36(40)41)21-32(33)45-34(48)22-47(37(50)26-7-9-27(10-8-26)38(51)52)29-12-14-30(15-13-29)53-31-17-19-46(20-18-31)39(42)43/h2-16,21,24,31H,17-20,22-23H2,1H3,(H3,40,41)(H3,42,43)(H,44,49)(H,51,52)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM17055

((18S)-18-[(dimethylamino)methyl]-17-oxa-4,14,21-tr...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |r,t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 1 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153461
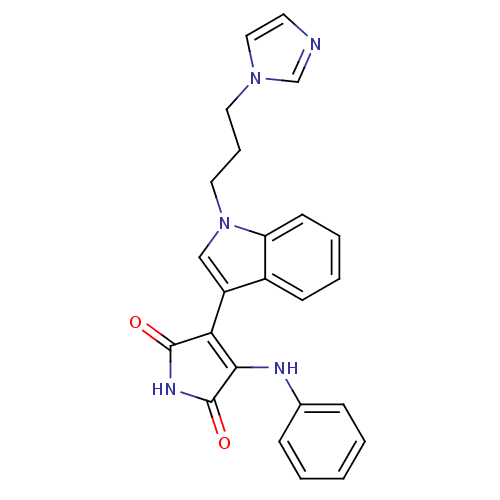
(3-(1-(3-imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anil...)Show SMILES O=C1NC(=O)C(=C1Nc1ccccc1)c1cn(CCCn2ccnc2)c2ccccc12 |c:5| Show InChI InChI=1S/C24H21N5O2/c30-23-21(22(24(31)27-23)26-17-7-2-1-3-8-17)19-15-29(20-10-5-4-9-18(19)20)13-6-12-28-14-11-25-16-28/h1-5,7-11,14-16H,6,12-13H2,(H2,26,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM17055

((18S)-18-[(dimethylamino)methyl]-17-oxa-4,14,21-tr...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |r,t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150502
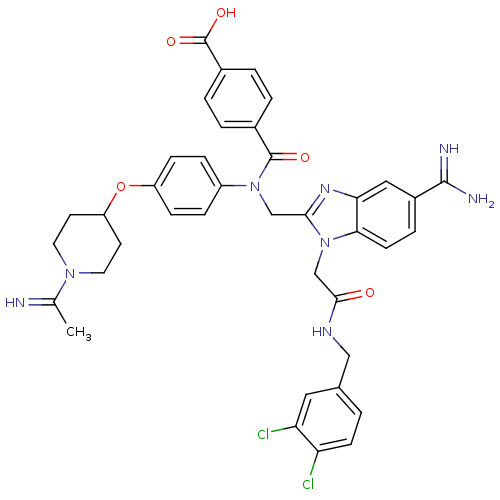
(CHEMBL537184 | N-{5-Carbamimidoyl-1-[(3,4-dichloro...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)N(Cc1nc2cc(ccc2n1CC(=O)NCc1ccc(Cl)c(Cl)c1)C(N)=N)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C39H38Cl2N8O5/c1-23(42)47-16-14-30(15-17-47)54-29-10-8-28(9-11-29)48(38(51)25-3-5-26(6-4-25)39(52)53)21-35-46-33-19-27(37(43)44)7-13-34(33)49(35)22-36(50)45-20-24-2-12-31(40)32(41)18-24/h2-13,18-19,30,42H,14-17,20-22H2,1H3,(H3,43,44)(H,45,50)(H,52,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153448

(3-(3-Chloro-phenylamino)-4-[1-(3-dimethylamino-pro...)Show SMILES CN(C)CCCn1cc(C2=C(Nc3cccc(Cl)c3)C(=O)NC2=O)c2ccccc12 |c:9| Show InChI InChI=1S/C23H23ClN4O2/c1-27(2)11-6-12-28-14-18(17-9-3-4-10-19(17)28)20-21(23(30)26-22(20)29)25-16-8-5-7-15(24)13-16/h3-5,7-10,13-14H,6,11-12H2,1-2H3,(H2,25,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150499

(CHEMBL536722 | N-(5-Carbamimidoyl-1-cyclohexylcarb...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)N(Cc1nc2cc(ccc2n1CC(=O)NC1CCCCC1)C(N)=N)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C38H44N8O5/c1-24(39)44-19-17-31(18-20-44)51-30-14-12-29(13-15-30)45(37(48)25-7-9-26(10-8-25)38(49)50)22-34-43-32-21-27(36(40)41)11-16-33(32)46(34)23-35(47)42-28-5-3-2-4-6-28/h7-16,21,28,31,39H,2-6,17-20,22-23H2,1H3,(H3,40,41)(H,42,47)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153463

(3-[1-(3-Dimethylamino-propyl)-1H-indol-3-yl]-4-phe...)Show SMILES CN(C)CCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:9| Show InChI InChI=1S/C23H24N4O2/c1-26(2)13-8-14-27-15-18(17-11-6-7-12-19(17)27)20-21(23(29)25-22(20)28)24-16-9-4-3-5-10-16/h3-7,9-12,15H,8,13-14H2,1-2H3,(H2,24,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153453

(3-[1-(4-Dimethylamino-butyl)-1H-indol-3-yl]-4-phen...)Show SMILES CN(C)CCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:10| Show InChI InChI=1S/C24H26N4O2/c1-27(2)14-8-9-15-28-16-19(18-12-6-7-13-20(18)28)21-22(24(30)26-23(21)29)25-17-10-4-3-5-11-17/h3-7,10-13,16H,8-9,14-15H2,1-2H3,(H2,25,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153452
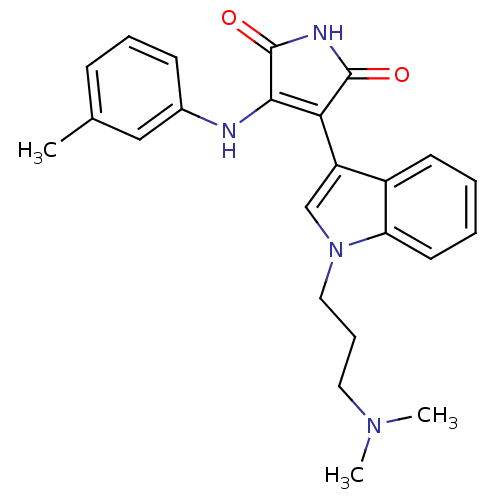
(3-[1-(3-Dimethylamino-propyl)-1H-indol-3-yl]-4-m-t...)Show SMILES CN(C)CCCn1cc(C2=C(Nc3cccc(C)c3)C(=O)NC2=O)c2ccccc12 |c:9| Show InChI InChI=1S/C24H26N4O2/c1-16-8-6-9-17(14-16)25-22-21(23(29)26-24(22)30)19-15-28(13-7-12-27(2)3)20-11-5-4-10-18(19)20/h4-6,8-11,14-15H,7,12-13H2,1-3H3,(H2,25,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153455
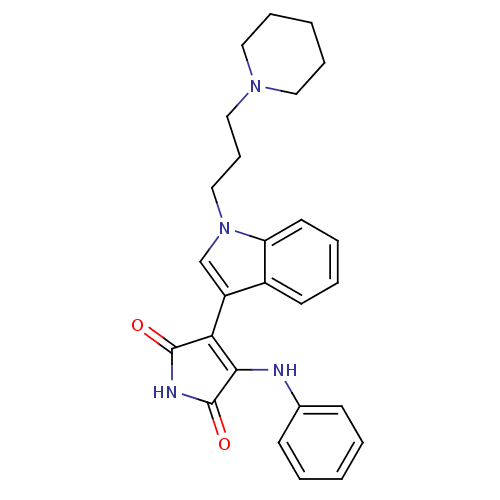
(3-Phenylamino-4-[1-(3-piperidin-1-yl-propyl)-1H-in...)Show SMILES O=C1NC(=O)C(=C1Nc1ccccc1)c1cn(CCCN2CCCCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C26H28N4O2/c31-25-23(24(26(32)28-25)27-19-10-3-1-4-11-19)21-18-30(22-13-6-5-12-20(21)22)17-9-16-29-14-7-2-8-15-29/h1,3-6,10-13,18H,2,7-9,14-17H2,(H2,27,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153466

(3-Phenylamino-4-[1-(3-pyrrolidin-1-yl-propyl)-1H-i...)Show SMILES O=C1NC(=O)C(=C1Nc1ccccc1)c1cn(CCCN2CCCC2)c2ccccc12 |c:5| Show InChI InChI=1S/C25H26N4O2/c30-24-22(23(25(31)27-24)26-18-9-2-1-3-10-18)20-17-29(21-12-5-4-11-19(20)21)16-8-15-28-13-6-7-14-28/h1-5,9-12,17H,6-8,13-16H2,(H2,26,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150496
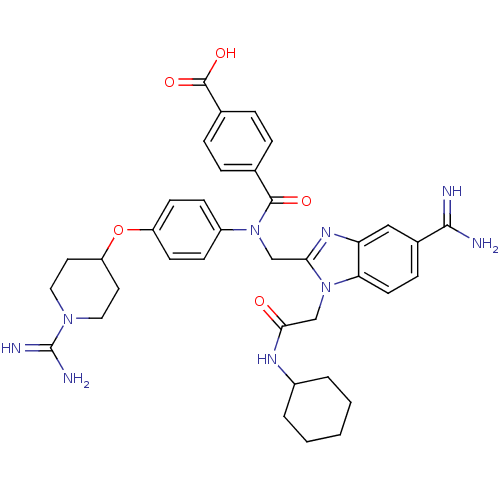
(CHEMBL536490 | N-(5-Carbamimidoyl-1-cyclohexylcarb...)Show SMILES NC(=N)N1CCC(CC1)Oc1ccc(cc1)N(Cc1nc2cc(ccc2n1CC(=O)NC1CCCCC1)C(N)=N)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C37H43N9O5/c38-34(39)25-10-15-31-30(20-25)43-32(46(31)22-33(47)42-26-4-2-1-3-5-26)21-45(35(48)23-6-8-24(9-7-23)36(49)50)27-11-13-28(14-12-27)51-29-16-18-44(19-17-29)37(40)41/h6-15,20,26,29H,1-5,16-19,21-22H2,(H3,38,39)(H3,40,41)(H,42,47)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153461

(3-(1-(3-imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anil...)Show SMILES O=C1NC(=O)C(=C1Nc1ccccc1)c1cn(CCCn2ccnc2)c2ccccc12 |c:5| Show InChI InChI=1S/C24H21N5O2/c30-23-21(22(24(31)27-23)26-17-7-2-1-3-8-17)19-15-29(20-10-5-4-9-18(19)20)13-6-12-28-14-11-25-16-28/h1-5,7-11,14-16H,6,12-13H2,(H2,26,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 1 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153457
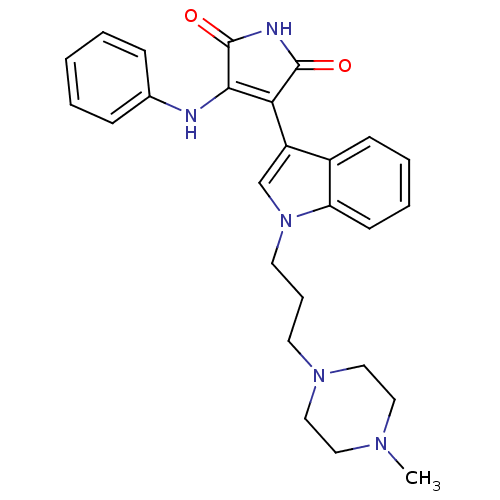
(3-{1-[3-(4-Methyl-piperazin-1-yl)-propyl]-1H-indol...)Show SMILES CN1CCN(CCCn2cc(C3=C(Nc4ccccc4)C(=O)NC3=O)c3ccccc23)CC1 |c:11| Show InChI InChI=1S/C26H29N5O2/c1-29-14-16-30(17-15-29)12-7-13-31-18-21(20-10-5-6-11-22(20)31)23-24(26(33)28-25(23)32)27-19-8-3-2-4-9-19/h2-6,8-11,18H,7,12-17H2,1H3,(H2,27,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50157335

(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES NC(=N)N1CCc2ccc(OCC3(CCN(CC3)c3ccncc3)C(O)=O)cc2C1 Show InChI InChI=1S/C22H27N5O3/c23-21(24)27-10-5-16-1-2-19(13-17(16)14-27)30-15-22(20(28)29)6-11-26(12-7-22)18-3-8-25-9-4-18/h1-4,8-9,13H,5-7,10-12,14-15H2,(H3,23,24)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50153465

(2-{3-[3-(2,5-Dioxo-4-phenylamino-2,5-dihydro-1H-py...)Show SMILES NC(=N)SCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:10| Show InChI InChI=1S/C22H21N5O2S/c23-22(24)30-12-6-11-27-13-16(15-9-4-5-10-17(15)27)18-19(21(29)26-20(18)28)25-14-7-2-1-3-8-14/h1-5,7-10,13H,6,11-12H2,(H3,23,24)(H2,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C alpha |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153458

(3-[1-(2-Dimethylamino-ethyl)-1H-indol-3-yl]-4-phen...)Show SMILES CN(C)CCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:8| Show InChI InChI=1S/C22H22N4O2/c1-25(2)12-13-26-14-17(16-10-6-7-11-18(16)26)19-20(22(28)24-21(19)27)23-15-8-4-3-5-9-15/h3-11,14H,12-13H2,1-2H3,(H2,23,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153462

(3-[1-(3-Hydroxy-propyl)-1H-indol-3-yl]-4-phenylami...)Show SMILES OCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:7| Show InChI InChI=1S/C21H19N3O3/c25-12-6-11-24-13-16(15-9-4-5-10-17(15)24)18-19(21(27)23-20(18)26)22-14-7-2-1-3-8-14/h1-5,7-10,13,25H,6,11-12H2,(H2,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150488
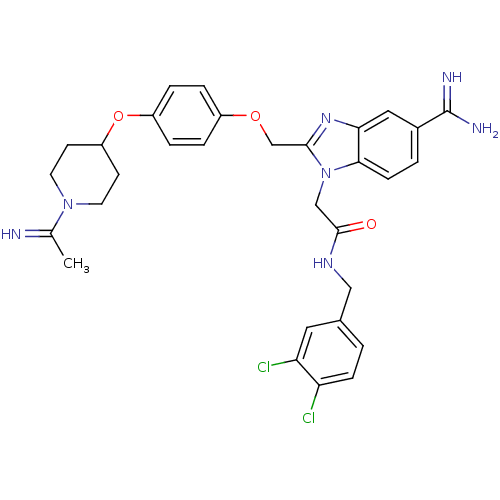
(2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(OCc2nc3cc(ccc3n2CC(=O)NCc2ccc(Cl)c(Cl)c2)C(N)=N)cc1 Show InChI InChI=1S/C31H33Cl2N7O3/c1-19(34)39-12-10-24(11-13-39)43-23-6-4-22(5-7-23)42-18-29-38-27-15-21(31(35)36)3-9-28(27)40(29)17-30(41)37-16-20-2-8-25(32)26(33)14-20/h2-9,14-15,24,34H,10-13,16-18H2,1H3,(H3,35,36)(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150492
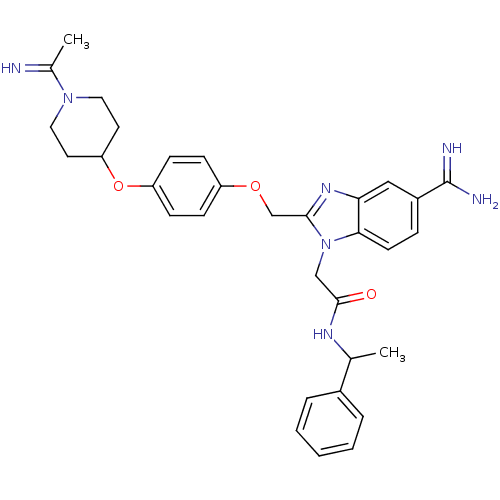
(2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...)Show SMILES CC(NC(=O)Cn1c(COc2ccc(OC3CCN(CC3)C(C)=N)cc2)nc2cc(ccc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C32H37N7O3/c1-21(23-6-4-3-5-7-23)36-31(40)19-39-29-13-8-24(32(34)35)18-28(29)37-30(39)20-41-25-9-11-26(12-10-25)42-27-14-16-38(17-15-27)22(2)33/h3-13,18,21,27,33H,14-17,19-20H2,1-2H3,(H3,34,35)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50322045
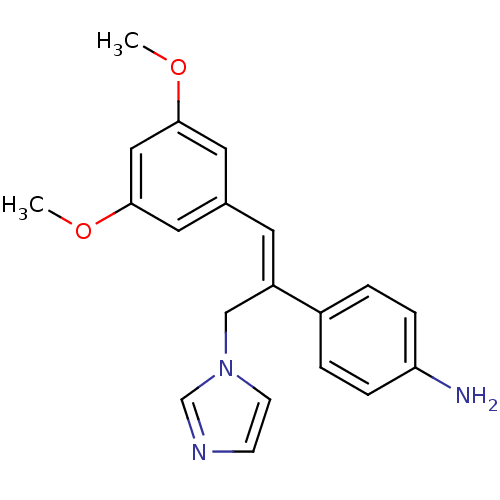
((Z)-4-(1-(3,5-Dimethoxyphenyl)-3-(1H-imidazol-1-yl...)Show InChI InChI=1S/C20H21N3O2/c1-24-19-10-15(11-20(12-19)25-2)9-17(13-23-8-7-22-14-23)16-3-5-18(21)6-4-16/h3-12,14H,13,21H2,1-2H3/b17-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase preincubated with NADP+ for 10 mins before substrate addition measured after 30 mins |
Bioorg Med Chem 18: 5352-66 (2010)
Article DOI: 10.1016/j.bmc.2010.05.042
BindingDB Entry DOI: 10.7270/Q2PK0H4P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150495
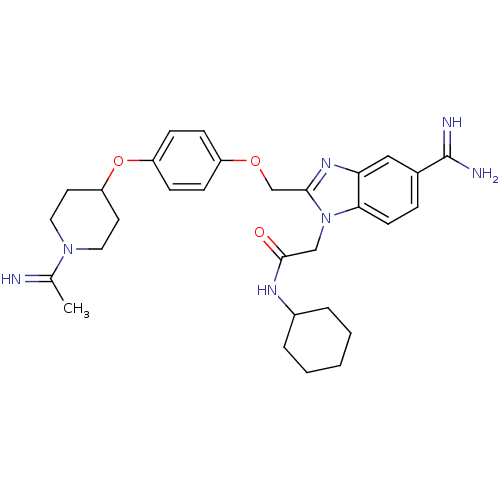
(2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(OCc2nc3cc(ccc3n2CC(=O)NC2CCCCC2)C(N)=N)cc1 Show InChI InChI=1S/C30H39N7O3/c1-20(31)36-15-13-25(14-16-36)40-24-10-8-23(9-11-24)39-19-28-35-26-17-21(30(32)33)7-12-27(26)37(28)18-29(38)34-22-5-3-2-4-6-22/h7-12,17,22,25,31H,2-6,13-16,18-19H2,1H3,(H3,32,33)(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153451
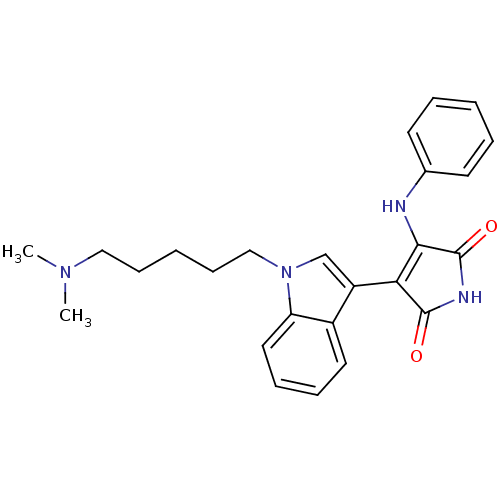
(3-[1-(5-Dimethylamino-pentyl)-1H-indol-3-yl]-4-phe...)Show SMILES CN(C)CCCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:11| Show InChI InChI=1S/C25H28N4O2/c1-28(2)15-9-4-10-16-29-17-20(19-13-7-8-14-21(19)29)22-23(25(31)27-24(22)30)26-18-11-5-3-6-12-18/h3,5-8,11-14,17H,4,9-10,15-16H2,1-2H3,(H2,26,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50157342

(7-(3,4,5,6-Tetrahydro-2H-[1,4'']bipyridinyl-4-ylme...)Show InChI InChI=1S/C21H27N5O/c22-21(23)26-12-7-17-1-2-20(13-18(17)14-26)27-15-16-5-10-25(11-6-16)19-3-8-24-9-4-19/h1-4,8-9,13,16H,5-7,10-12,14-15H2,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50153465

(2-{3-[3-(2,5-Dioxo-4-phenylamino-2,5-dihydro-1H-py...)Show SMILES NC(=N)SCCCn1cc(C2=C(Nc3ccccc3)C(=O)NC2=O)c2ccccc12 |c:10| Show InChI InChI=1S/C22H21N5O2S/c23-22(24)30-12-6-11-27-13-16(15-9-4-5-10-17(15)27)18-19(21(29)26-20(18)28)25-14-7-2-1-3-8-14/h1-5,7-10,13H,6,11-12H2,(H3,23,24)(H2,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C epsilon |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150494

(2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(OCc2nc3cc(ccc3n2CC(=O)Nc2ccc(Cl)c(Cl)c2)C(N)=N)cc1 Show InChI InChI=1S/C30H31Cl2N7O3/c1-18(33)38-12-10-23(11-13-38)42-22-6-4-21(5-7-22)41-17-28-37-26-14-19(30(34)35)2-9-27(26)39(28)16-29(40)36-20-3-8-24(31)25(32)15-20/h2-9,14-15,23,33H,10-13,16-17H2,1H3,(H3,34,35)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041218

(3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[(S)-1-(1...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)C(Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50322044
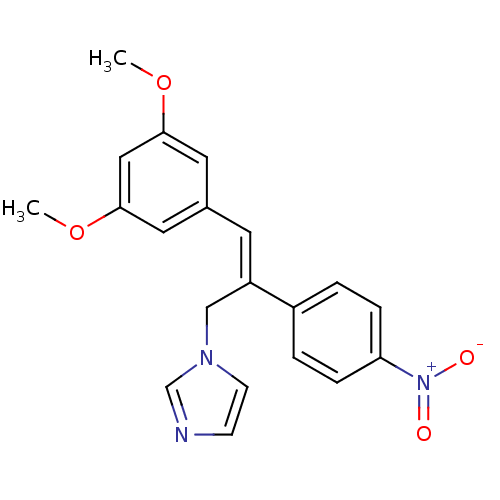
((Z)-1-(3-(3,5-Dimethoxyphenyl)-2-(4-nitrophenyl)al...)Show SMILES COc1cc(OC)cc(\C=C(/Cn2ccnc2)c2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C20H19N3O4/c1-26-19-10-15(11-20(12-19)27-2)9-17(13-22-8-7-21-14-22)16-3-5-18(6-4-16)23(24)25/h3-12,14H,13H2,1-2H3/b17-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase preincubated with NADP+ for 10 mins before substrate addition measured after 30 mins |
Bioorg Med Chem 18: 5352-66 (2010)
Article DOI: 10.1016/j.bmc.2010.05.042
BindingDB Entry DOI: 10.7270/Q2PK0H4P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50157330

(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES CCOC(=O)C1(COc2ccc3CCN(Cc3c2)C(N)=N)CCN(CC1)c1ccncc1 Show InChI InChI=1S/C24H31N5O3/c1-2-31-22(30)24(8-13-28(14-9-24)20-5-10-27-11-6-20)17-32-21-4-3-18-7-12-29(23(25)26)16-19(18)15-21/h3-6,10-11,15H,2,7-9,12-14,16-17H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50409939
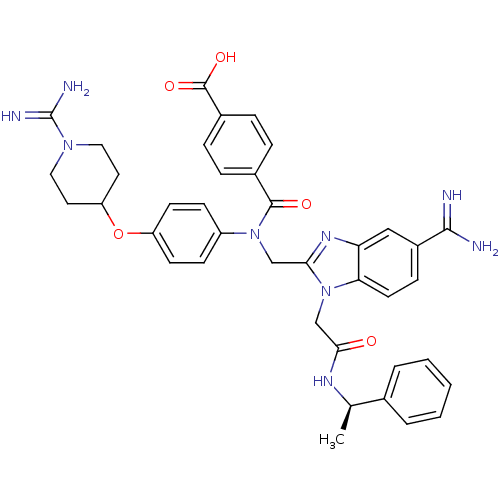
(CHEMBL2093954)Show SMILES C[C@@H](NC(=O)Cn1c(CN(C(=O)c2ccc(cc2)C(O)=O)c2ccc(OC3CCN(CC3)C(N)=N)cc2)nc2cc(ccc12)C(N)=N)c1ccccc1 |r| Show InChI InChI=1S/C39H41N9O5/c1-24(25-5-3-2-4-6-25)44-35(49)23-48-33-16-11-28(36(40)41)21-32(33)45-34(48)22-47(37(50)26-7-9-27(10-8-26)38(51)52)29-12-14-30(15-13-29)53-31-17-19-46(20-18-31)39(42)43/h2-16,21,24,31H,17-20,22-23H2,1H3,(H3,40,41)(H3,42,43)(H,44,49)(H,51,52)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150489

(CHEMBL541372 | N-{4-[1-(N-Benzyl-carbamimidoyl)-pi...)Show SMILES NC(=N)c1ccc2n(CC(=O)NC3CCCCC3)c(CN(C(=O)c3ccc(cc3)C(O)=O)c3ccc(OC4CCN(CC4)C(N)=NCc4ccccc4)cc3)nc2c1 |w:45.48| Show InChI InChI=1S/C44H49N9O5/c45-41(46)32-15-20-38-37(25-32)50-39(53(38)28-40(54)49-33-9-5-2-6-10-33)27-52(42(55)30-11-13-31(14-12-30)43(56)57)34-16-18-35(19-17-34)58-36-21-23-51(24-22-36)44(47)48-26-29-7-3-1-4-8-29/h1,3-4,7-8,11-20,25,33,36H,2,5-6,9-10,21-24,26-28H2,(H3,45,46)(H2,47,48)(H,49,54)(H,56,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150506
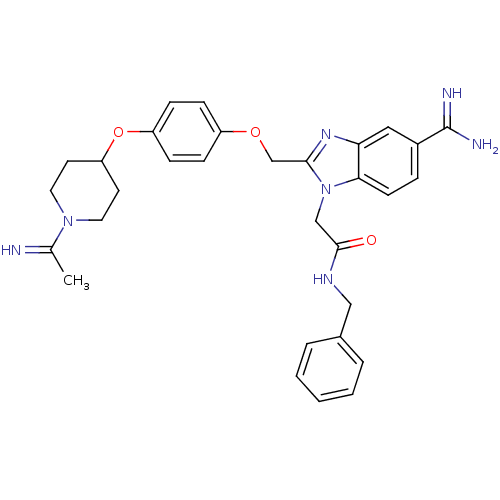
(CHEMBL556972 | N-Benzyl-2-(5-carbamimidoyl-2-{4-[1...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(OCc2nc3cc(ccc3n2CC(=O)NCc2ccccc2)C(N)=N)cc1 Show InChI InChI=1S/C31H35N7O3/c1-21(32)37-15-13-26(14-16-37)41-25-10-8-24(9-11-25)40-20-29-36-27-17-23(31(33)34)7-12-28(27)38(29)19-30(39)35-18-22-5-3-2-4-6-22/h2-12,17,26,32H,13-16,18-20H2,1H3,(H3,33,34)(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150493
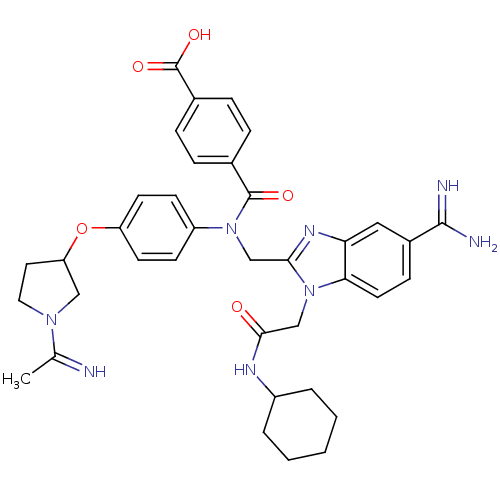
(CHEMBL553328 | N-(5-Carbamimidoyl-1-cyclohexylcarb...)Show SMILES CC(=N)N1CCC(C1)Oc1ccc(cc1)N(Cc1nc2cc(ccc2n1CC(=O)NC1CCCCC1)C(N)=N)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C37H42N8O5/c1-23(38)43-18-17-30(20-43)50-29-14-12-28(13-15-29)44(36(47)24-7-9-25(10-8-24)37(48)49)21-33-42-31-19-26(35(39)40)11-16-32(31)45(33)22-34(46)41-27-5-3-2-4-6-27/h7-16,19,27,30,38H,2-6,17-18,20-22H2,1H3,(H3,39,40)(H,41,46)(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50150497
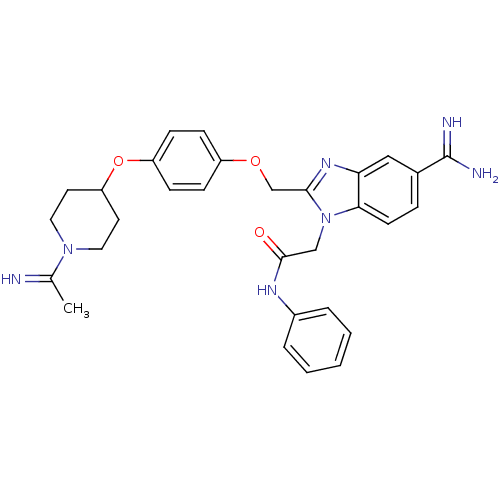
(2-(5-Carbamimidoyl-2-{4-[1-(1-imino-ethyl)-piperid...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(OCc2nc3cc(ccc3n2CC(=O)Nc2ccccc2)C(N)=N)cc1 Show InChI InChI=1S/C30H33N7O3/c1-20(31)36-15-13-25(14-16-36)40-24-10-8-23(9-11-24)39-19-28-35-26-17-21(30(32)33)7-12-27(26)37(28)18-29(38)34-22-5-3-2-4-6-22/h2-12,17,25,31H,13-16,18-19H2,1H3,(H3,32,33)(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
Bioorg Med Chem Lett 14: 4281-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.092
BindingDB Entry DOI: 10.7270/Q22808C7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50157339

(4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinolin-...)Show SMILES NC(=N)N1CCc2ccc(OCC3(CCN(CC3)c3ccncn3)C(O)=O)cc2C1 Show InChI InChI=1S/C21H26N6O3/c22-20(23)27-8-4-15-1-2-17(11-16(15)12-27)30-13-21(19(28)29)5-9-26(10-6-21)18-3-7-24-14-25-18/h1-3,7,11,14H,4-6,8-10,12-13H2,(H3,22,23)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50153464
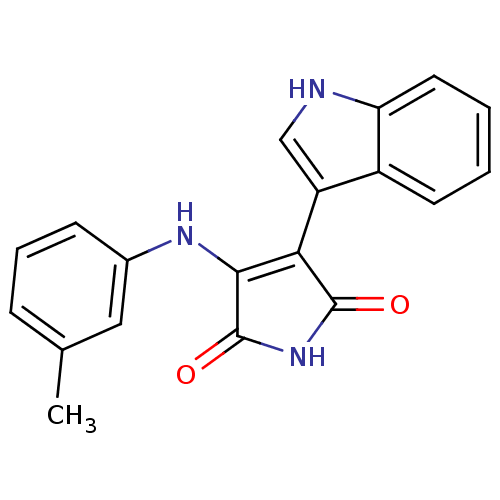
(3-(1H-Indol-3-yl)-4-m-tolylamino-pyrrole-2,5-dione...)Show SMILES Cc1cccc(NC2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c1 |t:7| Show InChI InChI=1S/C19H15N3O2/c1-11-5-4-6-12(9-11)21-17-16(18(23)22-19(17)24)14-10-20-15-8-3-2-7-13(14)15/h2-10,20H,1H3,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human Protein kinase C beta 2 |
Bioorg Med Chem Lett 14: 5171-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.061
BindingDB Entry DOI: 10.7270/Q2K35VDJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50157334
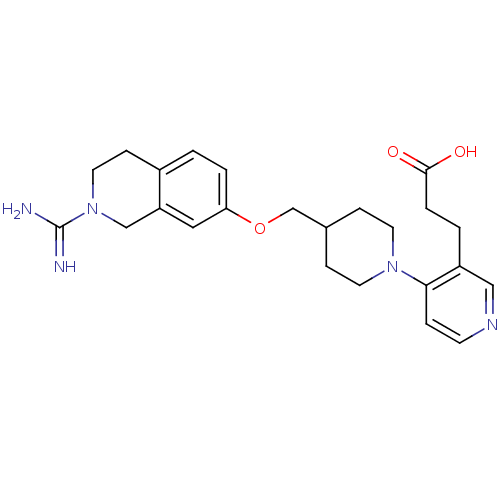
(3-[4-(2-Carbamimidoyl-1,2,3,4-tetrahydro-isoquinol...)Show SMILES NC(=N)N1CCc2ccc(OCC3CCN(CC3)c3ccncc3CCC(O)=O)cc2C1 Show InChI InChI=1S/C24H31N5O3/c25-24(26)29-12-8-18-1-3-21(13-20(18)15-29)32-16-17-6-10-28(11-7-17)22-5-9-27-14-19(22)2-4-23(30)31/h1,3,5,9,13-14,17H,2,4,6-8,10-12,15-16H2,(H3,25,26)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Coagulation factor X |
J Med Chem 48: 3586-604 (2005)
Article DOI: 10.1021/jm058160e
BindingDB Entry DOI: 10.7270/Q2CR5V36 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50240895
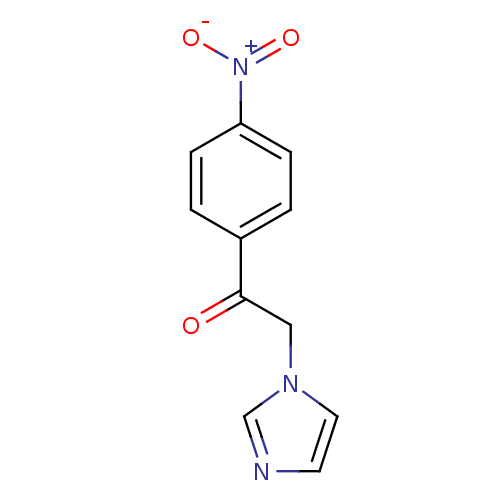
(2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | 2...)Show InChI InChI=1S/C11H9N3O3/c15-11(7-13-6-5-12-8-13)9-1-3-10(4-2-9)14(16)17/h1-6,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase preincubated with NADP+ for 10 mins before substrate addition measured after 30 mins |
Bioorg Med Chem 18: 5352-66 (2010)
Article DOI: 10.1016/j.bmc.2010.05.042
BindingDB Entry DOI: 10.7270/Q2PK0H4P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data