 Found 3437 hits with Last Name = 'iley' and Initial = 'j'
Found 3437 hits with Last Name = 'iley' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50330773
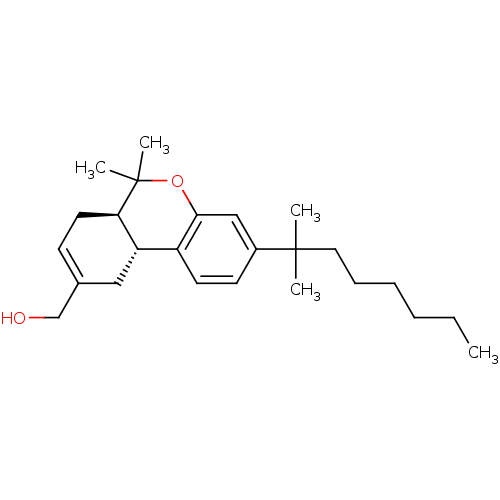
(((6aR,10aR)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a...)Show SMILES CCCCCCC(C)(C)c1ccc2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:17| Show InChI InChI=1S/C25H38O2/c1-6-7-8-9-14-24(2,3)19-11-12-20-21-15-18(17-26)10-13-22(21)25(4,5)27-23(20)16-19/h10-12,16,21-22,26H,6-9,13-15,17H2,1-5H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
Bioorg Med Chem 18: 7809-15 (2010)
Article DOI: 10.1016/j.bmc.2010.09.061
BindingDB Entry DOI: 10.7270/Q2ZK5HP7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50454329
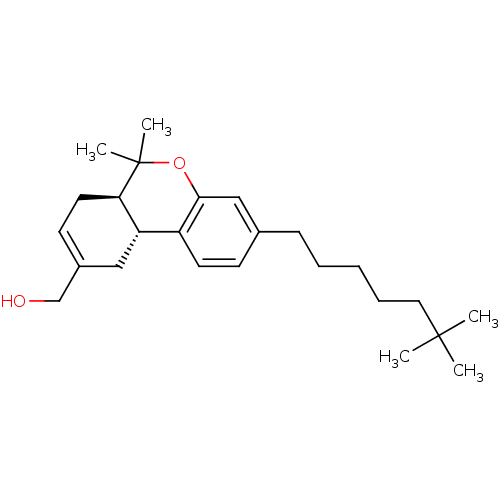
(CHEMBL2112647)Show SMILES [H][C@@]12CC(CO)=CC[C@@]1([H])C(C)(C)Oc1cc(CCCCCC(C)(C)C)ccc21 |c:5| Show InChI InChI=1S/C25H38O2/c1-24(2,3)14-8-6-7-9-18-10-12-20-21-15-19(17-26)11-13-22(21)25(4,5)27-23(20)16-18/h10-12,16,21-22,26H,6-9,13-15,17H2,1-5H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Ability to bind with Cannabinoid receptor 2 using [H]CP 55,940 as radioligand from cloned human receptor preparation |
J Med Chem 39: 3875-7 (1996)
Article DOI: 10.1021/jm960394y
BindingDB Entry DOI: 10.7270/Q2R49PVQ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50286859
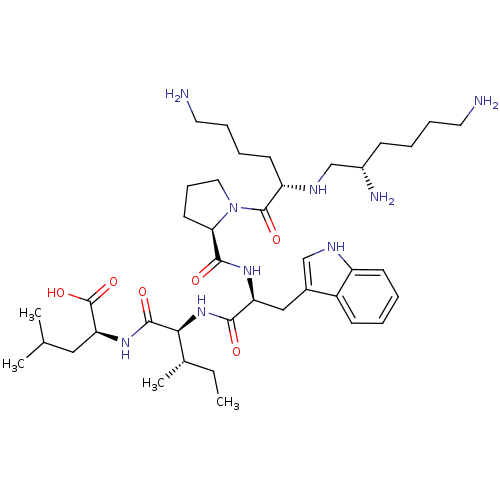
(2-{2-[(S)-2-({1-[(S)-6-Amino-2-((S)-(S)-2,6-diamin...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](CCCCN)NC[C@@H](N)CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C40H67N9O6/c1-5-26(4)35(38(52)47-33(40(54)55)21-25(2)3)48-36(50)32(22-27-23-44-30-15-7-6-14-29(27)30)46-37(51)34-17-12-20-49(34)39(53)31(16-9-11-19-42)45-24-28(43)13-8-10-18-41/h6-7,14-15,23,25-26,28,31-35,44-45H,5,8-13,16-22,24,41-43H2,1-4H3,(H,46,51)(H,47,52)(H,48,50)(H,54,55)/t26-,28-,31-,32-,33-,34+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240339
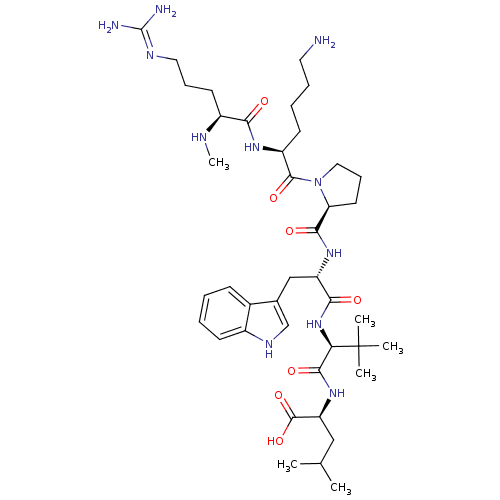
((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its binding affinity against Neurotensin Receptor after peripheral administration |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50286864

((S)-2-{(S)-2-[(S)-2-({(R)-1-[5-Guanidino-2-(3-guan...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCN1C(=O)C(CCCNC(N)=N)CCCNC(N)=N)C(C)(C)C)C(O)=O Show InChI InChI=1S/C38H61N11O6/c1-22(2)19-28(35(54)55)47-33(52)30(38(3,4)5)48-31(50)27(20-24-21-45-26-14-7-6-13-25(24)26)46-32(51)29-15-10-18-49(29)34(53)23(11-8-16-43-36(39)40)12-9-17-44-37(41)42/h6-7,13-14,21-23,27-30,45H,8-12,15-20H2,1-5H3,(H,46,51)(H,47,52)(H,48,50)(H,54,55)(H4,39,40,43)(H4,41,42,44)/t27-,28-,29+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50366427
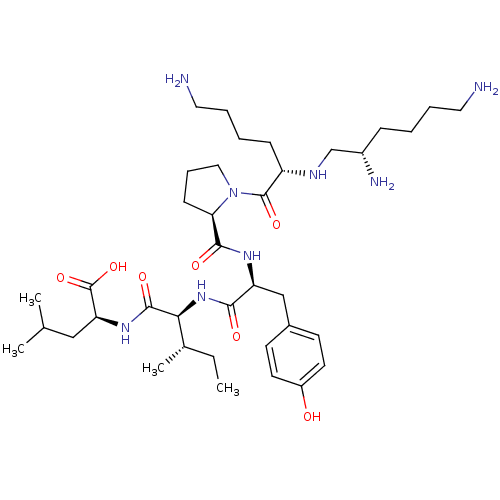
(CHEMBL1793865)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](CCCCN)NC[C@@H](N)CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H66N8O7/c1-5-25(4)33(36(50)44-31(38(52)53)21-24(2)3)45-34(48)30(22-26-14-16-28(47)17-15-26)43-35(49)32-13-10-20-46(32)37(51)29(12-7-9-19-40)42-23-27(41)11-6-8-18-39/h14-17,24-25,27,29-33,42,47H,5-13,18-23,39-41H2,1-4H3,(H,43,49)(H,44,50)(H,45,48)(H,52,53)/t25-,27-,29-,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394157
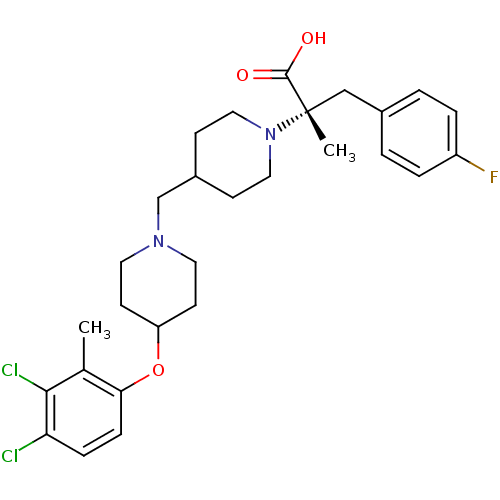
(CHEMBL2158790)Show SMILES Cc1c(Cl)c(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@](C)(Cc2ccc(F)cc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C28H35Cl2FN2O3/c1-19-25(8-7-24(29)26(19)30)36-23-11-13-32(14-12-23)18-21-9-15-33(16-10-21)28(2,27(34)35)17-20-3-5-22(31)6-4-20/h3-8,21,23H,9-18H2,1-2H3,(H,34,35)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410424
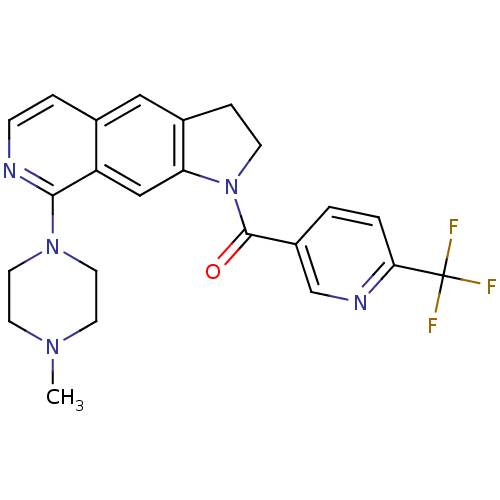
(CHEMBL199088)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(nc4)C(F)(F)F)c3cc12 Show InChI InChI=1S/C23H22F3N5O/c1-29-8-10-30(11-9-29)21-18-13-19-16(12-15(18)4-6-27-21)5-7-31(19)22(32)17-2-3-20(28-14-17)23(24,25)26/h2-4,6,12-14H,5,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by filtration assay |
Bioorg Med Chem 20: 2067-81 (2012)
Article DOI: 10.1016/j.bmc.2012.01.038
BindingDB Entry DOI: 10.7270/Q2PG1SW6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Binding affinity to displace [3H]CP-55,940 from cloned human CB2 receptor |
Bioorg Med Chem Lett 15: 4110-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.008
BindingDB Entry DOI: 10.7270/Q2KD1XGV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410426

(CHEMBL370852)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(Cl)cc4)c3cc12 Show InChI InChI=1S/C23H23ClN4O/c1-26-10-12-27(13-11-26)22-20-15-21-18(14-17(20)6-8-25-22)7-9-28(21)23(29)16-2-4-19(24)5-3-16/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50410428

(CHEMBL380812)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4cnn5ccccc45)c3cc12 Show InChI InChI=1S/C24H24N6O/c1-27-10-12-28(13-11-27)23-19-15-22-18(14-17(19)5-7-25-23)6-9-29(22)24(31)20-16-26-30-8-3-2-4-21(20)30/h2-5,7-8,14-16H,6,9-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
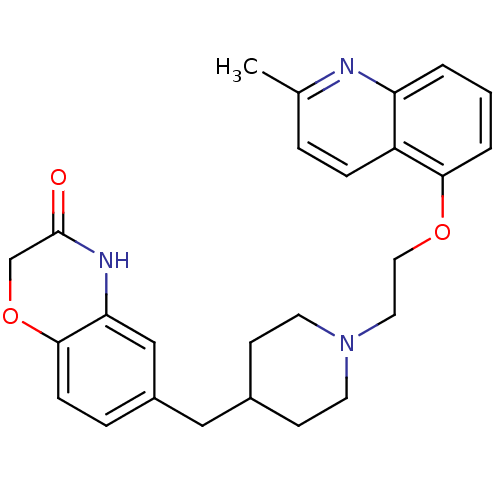
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086063
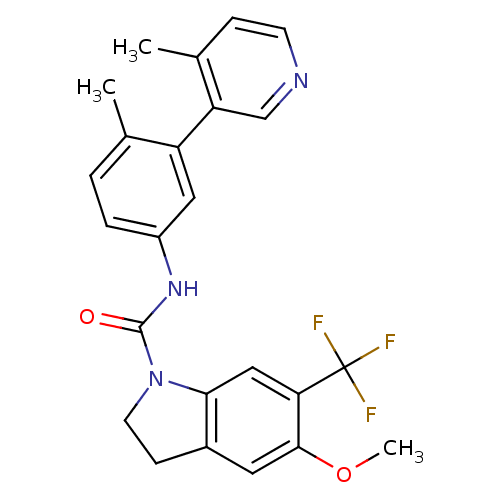
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3ccc(C)c(c3)-c3cnccc3C)c2cc1C(F)(F)F Show InChI InChI=1S/C24H22F3N3O2/c1-14-4-5-17(11-18(14)19-13-28-8-6-15(19)2)29-23(31)30-9-7-16-10-22(32-3)20(12-21(16)30)24(25,26)27/h4-6,8,10-13H,7,9H2,1-3H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303364
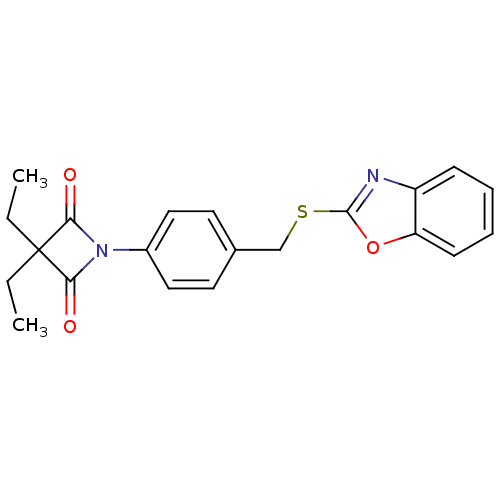
(1-(4-((Benzo[d]oxazol-2-ylthio)methyl)phenyl)-3,3-...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CSc2nc3ccccc3o2)cc1 Show InChI InChI=1S/C21H20N2O3S/c1-3-21(4-2)18(24)23(19(21)25)15-11-9-14(10-12-15)13-27-20-22-16-7-5-6-8-17(16)26-20/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218198
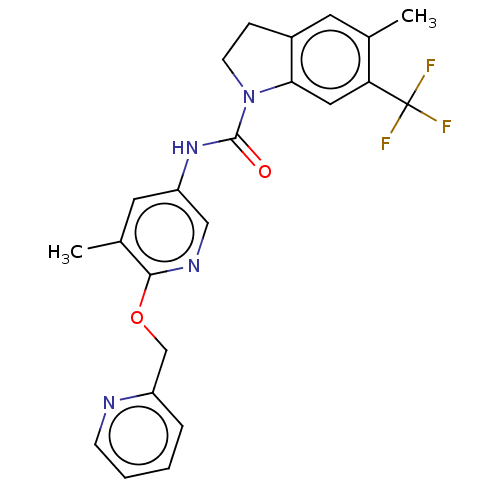
(CHEMBL55914)Show SMILES Cc1cc(NC(=O)N2CCc3cc(C)c(cc23)C(F)(F)F)cnc1OCc1ccccn1 Show InChI InChI=1S/C23H21F3N4O2/c1-14-9-16-6-8-30(20(16)11-19(14)23(24,25)26)22(31)29-18-10-15(2)21(28-12-18)32-13-17-5-3-4-7-27-17/h3-5,7,9-12H,6,8,13H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by [3H]mesulergine displacement. |
Bioorg Med Chem Lett 10: 1867-70 (2000)
BindingDB Entry DOI: 10.7270/Q2SF2ZB2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50217990

(CHEMBL54560)Show SMILES CSc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2S/c1-13-18(4-3-8-26-13)31-20-6-5-15(12-27-20)28-21(30)29-9-7-14-10-19(32-2)16(11-17(14)29)22(23,24)25/h3-6,8,10-12H,7,9H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by displacement of [3H]mesulergine |
Bioorg Med Chem Lett 10: 1863-6 (2000)
BindingDB Entry DOI: 10.7270/Q2X63Q4P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086073
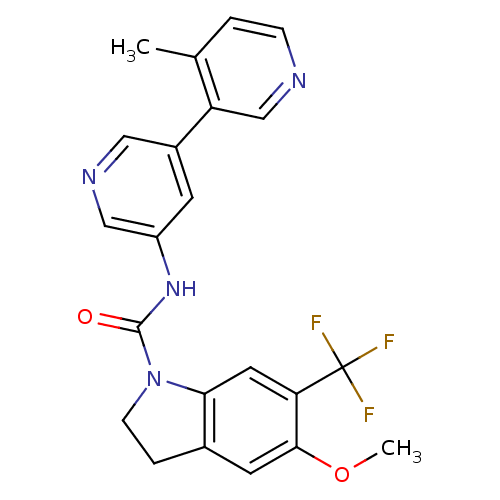
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3cncc(c3)-c3cnccc3C)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2/c1-13-3-5-26-12-17(13)15-7-16(11-27-10-15)28-21(30)29-6-4-14-8-20(31-2)18(9-19(14)29)22(23,24)25/h3,5,7-12H,4,6H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50286868
((1S,2S,4S)-2-{2-[(S)-2-({1-[6-Amino-2-(2,6-diamino...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](CCCCN)NC[C@@H](N)CCCCN)C(C)(C)C)C(O)=O Show InChI InChI=1S/C38H66N8O7/c1-24(2)21-30(37(52)53)44-35(50)32(38(3,4)5)45-33(48)29(22-25-14-16-27(47)17-15-25)43-34(49)31-13-10-20-46(31)36(51)28(12-7-9-19-40)42-23-26(41)11-6-8-18-39/h14-17,24,26,28-32,42,47H,6-13,18-23,39-41H2,1-5H3,(H,43,49)(H,44,50)(H,45,48)(H,52,53)/t26-,28-,29-,30-,31+,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222900
(CHEMBL267062)Show SMILES O=C1N(CCN2CCC(CC2)c2c[nH]c3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-21-8-2-1-6-19(21)7-5-13-28(25)17-16-27-14-11-20(12-15-27)23-18-26-24-10-4-3-9-22(23)24/h1-4,6,8-10,18,20,26H,5,7,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50071414
((6aR,10aR)-3-((1S,2R)-1,2-Dimethyl-heptyl)-6,6,9-t...)Show SMILES CCCCC[C@@H](C)[C@H](C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:17| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-17(3)18(4)19-14-22(26)24-20-13-16(2)11-12-21(20)25(5,6)27-23(24)15-19/h11,14-15,17-18,20-21,26H,7-10,12-13H2,1-6H3/t17-,18+,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for the cannabinoid brain receptor (CB1) using [3H]- CP-55,940 |
Bioorg Med Chem Lett 8: 2223-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5F78 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50071413
((6aR,10aR)-3-((1S,2R)-1,2-Dimethyl-heptyl)-9-hydro...)Show SMILES CCCCC[C@@H](C)[C@H](C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-16(2)17(3)19-13-22(27)24-20-12-18(15-26)10-11-21(20)25(4,5)28-23(24)14-19/h10,13-14,16-17,20-21,26-27H,6-9,11-12,15H2,1-5H3/t16-,17+,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for the cannabinoid brain receptor (CB1) using [3H]- CP-55,940 |
Bioorg Med Chem Lett 8: 2223-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5F78 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086064
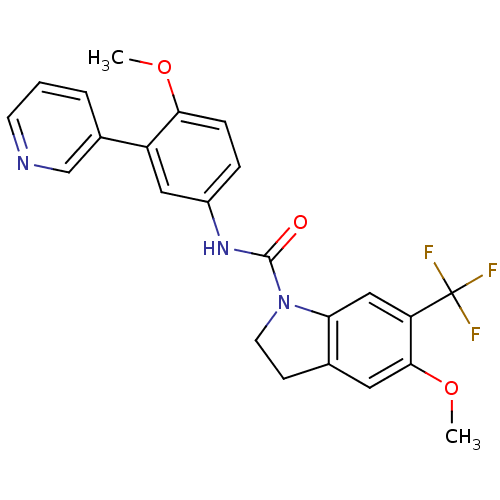
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1ccc(NC(=O)N2CCc3cc(OC)c(cc23)C(F)(F)F)cc1-c1cccnc1 Show InChI InChI=1S/C23H20F3N3O3/c1-31-20-6-5-16(11-17(20)15-4-3-8-27-13-15)28-22(30)29-9-7-14-10-21(32-2)18(12-19(14)29)23(24,25)26/h3-6,8,10-13H,7,9H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086068
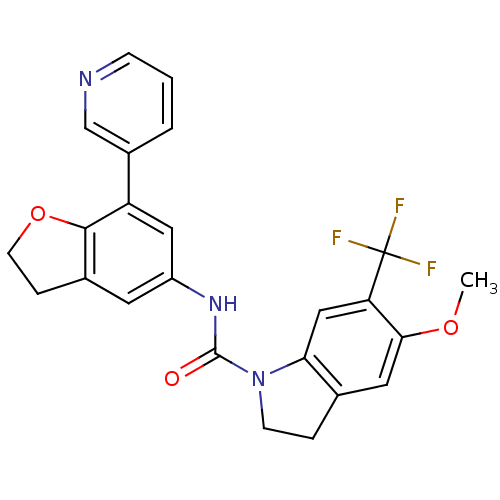
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3cc4CCOc4c(c3)-c3cccnc3)c2cc1C(F)(F)F Show InChI InChI=1S/C24H20F3N3O3/c1-32-21-10-14-4-7-30(20(14)12-19(21)24(25,26)27)23(31)29-17-9-15-5-8-33-22(15)18(11-17)16-3-2-6-28-13-16/h2-3,6,9-13H,4-5,7-8H2,1H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303371

(1-(6-((Benzo[d]thiazol-2-ylthio)methyl)pyridin-3-y...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CSc2nc3ccccc3s2)nc1 Show InChI InChI=1S/C20H19N3O2S2/c1-3-20(4-2)17(24)23(18(20)25)14-10-9-13(21-11-14)12-26-19-22-15-7-5-6-8-16(15)27-19/h5-11H,3-4,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394117
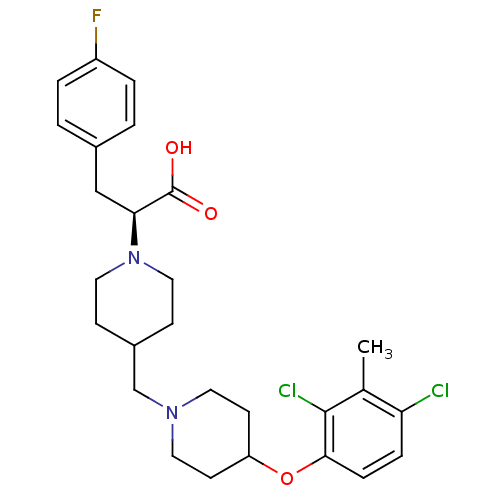
(CHEMBL2158783)Show SMILES Cc1c(Cl)ccc(OC2CCN(CC3CCN(CC3)[C@@H](Cc3ccc(F)cc3)C(O)=O)CC2)c1Cl |r| Show InChI InChI=1S/C27H33Cl2FN2O3/c1-18-23(28)6-7-25(26(18)29)35-22-10-12-31(13-11-22)17-20-8-14-32(15-9-20)24(27(33)34)16-19-2-4-21(30)5-3-19/h2-7,20,22,24H,8-17H2,1H3,(H,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394116
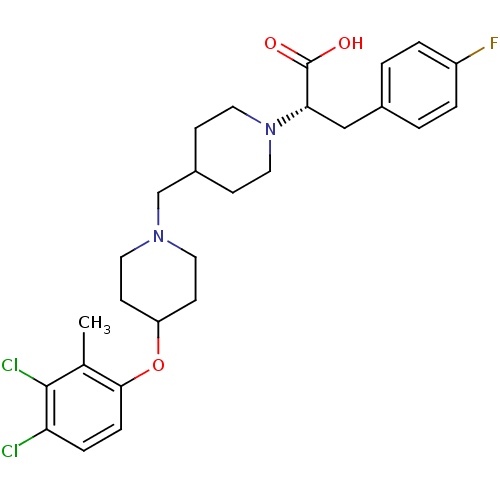
(CHEMBL2158784)Show SMILES Cc1c(Cl)c(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@H](Cc2ccc(F)cc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C27H33Cl2FN2O3/c1-18-25(7-6-23(28)26(18)29)35-22-10-12-31(13-11-22)17-20-8-14-32(15-9-20)24(27(33)34)16-19-2-4-21(30)5-3-19/h2-7,20,22,24H,8-17H2,1H3,(H,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410426

(CHEMBL370852)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(Cl)cc4)c3cc12 Show InChI InChI=1S/C23H23ClN4O/c1-26-10-12-27(13-11-26)22-20-15-21-18(14-17(20)6-8-25-22)7-9-28(21)23(29)16-2-4-19(24)5-3-16/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410424

(CHEMBL199088)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(nc4)C(F)(F)F)c3cc12 Show InChI InChI=1S/C23H22F3N5O/c1-29-8-10-30(11-9-29)21-18-13-19-16(12-15(18)4-6-27-21)5-7-31(19)22(32)17-2-3-20(28-14-17)23(24,25)26/h2-4,6,12-14H,5,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410477

(CHEMBL54719)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(OCc4ccccn4)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2/c1-14-10-15-7-9-29(19(15)11-18(14)22(23,24)25)21(30)28-16-5-6-20(27-12-16)31-13-17-4-2-3-8-26-17/h2-6,8,10-12H,7,9,13H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithkline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 2C receptor expressed in HEK 293 cells using [3H]mesulergine |
Bioorg Med Chem Lett 15: 4989-93 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.004
BindingDB Entry DOI: 10.7270/Q2Z89DNZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410427
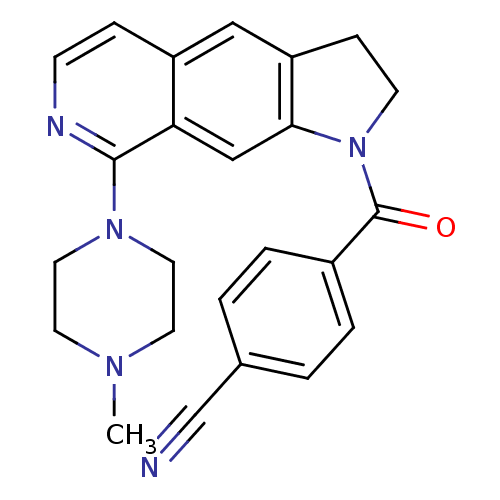
(CHEMBL194647)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(cc4)C#N)c3cc12 Show InChI InChI=1S/C24H23N5O/c1-27-10-12-28(13-11-27)23-21-15-22-20(14-19(21)6-8-26-23)7-9-29(22)24(30)18-4-2-17(16-25)3-5-18/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218192

(CHEMBL54707)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(OCc4cnccn4)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C21H18F3N5O2/c1-13-8-14-4-7-29(18(14)9-17(13)21(22,23)24)20(30)28-15-2-3-19(27-11-15)31-12-16-10-25-5-6-26-16/h2-3,5-6,8-11H,4,7,12H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by [3H]mesulergine displacement. |
Bioorg Med Chem Lett 10: 1867-70 (2000)
BindingDB Entry DOI: 10.7270/Q2SF2ZB2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50217138

(CHEMBL90433)Show SMILES CCCN(CCCCNC(=O)c1ccc(cc1)-c1ccccc1)C1CCc2nc(N)sc2C1 Show InChI InChI=1S/C27H34N4OS/c1-2-17-31(23-14-15-24-25(19-23)33-27(28)30-24)18-7-6-16-29-26(32)22-12-10-21(11-13-22)20-8-4-3-5-9-20/h3-5,8-13,23H,2,6-7,14-19H2,1H3,(H2,28,30)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Tested for the ability to displace [125I]iodosulpiride from human cloned Dopamine receptor D3, expressed in CHO cells |
Bioorg Med Chem Lett 9: 2715-20 (1999)
BindingDB Entry DOI: 10.7270/Q23X88TZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217828

(CHEMBL413707)Show SMILES Fc1ccc(OC2CCN(CC[C@H]3CCCN3S(=O)(=O)c3ccc4cc[nH]c4c3)CC2)cc1 Show InChI InChI=1S/C25H30FN3O3S/c26-20-4-6-22(7-5-20)32-23-11-16-28(17-12-23)15-10-21-2-1-14-29(21)33(30,31)24-8-3-19-9-13-27-25(19)18-24/h3-9,13,18,21,23,27H,1-2,10-12,14-17H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50217974
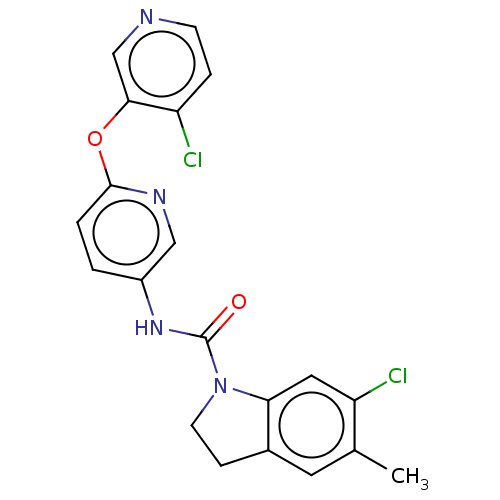
(CHEMBL55207)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cnccc4Cl)nc3)c2cc1Cl Show InChI InChI=1S/C20H16Cl2N4O2/c1-12-8-13-5-7-26(17(13)9-16(12)22)20(27)25-14-2-3-19(24-10-14)28-18-11-23-6-4-15(18)21/h2-4,6,8-11H,5,7H2,1H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by displacement of [3H]mesulergine |
Bioorg Med Chem Lett 10: 1863-6 (2000)
BindingDB Entry DOI: 10.7270/Q2X63Q4P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50410477

(CHEMBL54719)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(OCc4ccccn4)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O2/c1-14-10-15-7-9-29(19(15)11-18(14)22(23,24)25)21(30)28-16-5-6-20(27-12-16)31-13-17-4-2-3-8-26-17/h2-6,8,10-12H,7,9,13H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by [3H]mesulergine displacement. |
Bioorg Med Chem Lett 10: 1867-70 (2000)
BindingDB Entry DOI: 10.7270/Q2SF2ZB2 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240845
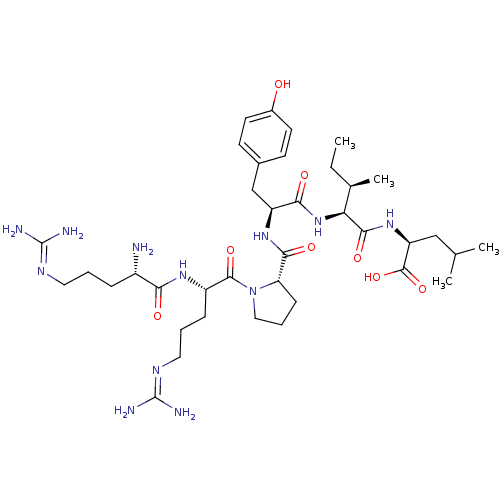
((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Neurotensin Receptor |
Bioorg Med Chem Lett 5: 997-1002 (1995)
Article DOI: 10.1016/0960-894X(95)00155-M
BindingDB Entry DOI: 10.7270/Q2GQ6Z76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50053357
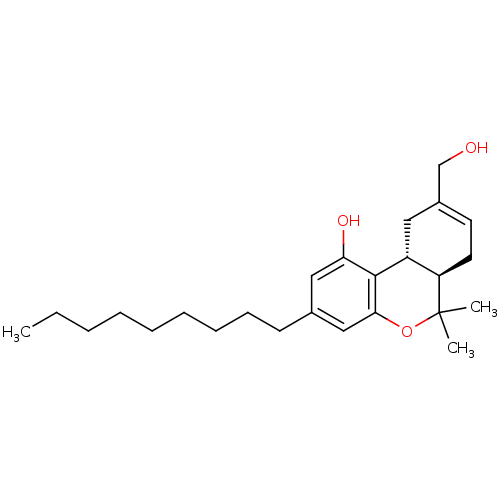
((6aR,10aR)-9-Hydroxymethyl-6,6-dimethyl-3-nonyl-6a...)Show SMILES CCCCCCCCCc1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H38O3/c1-4-5-6-7-8-9-10-11-18-15-22(27)24-20-14-19(17-26)12-13-21(20)25(2,3)28-23(24)16-18/h12,15-16,20-21,26-27H,4-11,13-14,17H2,1-3H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Ability to bind with Cannabinoid receptor 2 using [H]CP 55,940 as radioligand from cloned human receptor preparation |
J Med Chem 39: 3875-7 (1996)
Article DOI: 10.1021/jm960394y
BindingDB Entry DOI: 10.7270/Q2R49PVQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384584
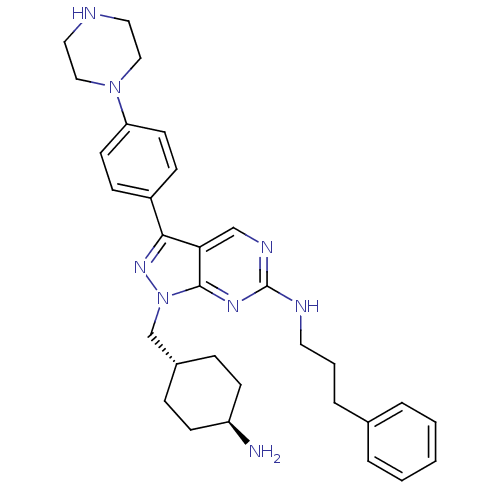
(CHEMBL2036807 | US9744172, Compound UNC607A)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(cc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(41.81,-28.1,;42.85,-26.96,;44.35,-27.28,;45.39,-26.14,;44.91,-24.68,;45.94,-23.54,;45.47,-22.07,;46.38,-20.82,;45.47,-19.56,;45.94,-18.1,;47.45,-17.78,;47.93,-16.32,;46.9,-15.17,;45.38,-15.5,;44.91,-16.96,;47.36,-13.71,;48.87,-13.39,;49.35,-11.94,;48.32,-10.79,;46.81,-11.11,;46.33,-12.57,;43.99,-20.04,;42.66,-19.28,;41.33,-20.05,;41.33,-21.6,;39.99,-22.36,;38.66,-21.59,;38.66,-20.05,;37.33,-19.28,;37.33,-17.74,;38.67,-16.98,;38.67,-15.44,;37.34,-14.67,;36,-15.45,;36,-16.98,;42.66,-22.37,;43.99,-21.6,;43.41,-24.35,;42.38,-25.49,)| Show InChI InChI=1S/C31H40N8/c32-26-12-8-24(9-13-26)22-39-30-28(21-35-31(36-30)34-16-4-7-23-5-2-1-3-6-23)29(37-39)25-10-14-27(15-11-25)38-19-17-33-18-20-38/h1-3,5-6,10-11,14-15,21,24,26,33H,4,7-9,12-13,16-20,22,32H2,(H,34,35,36)/t24-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222781
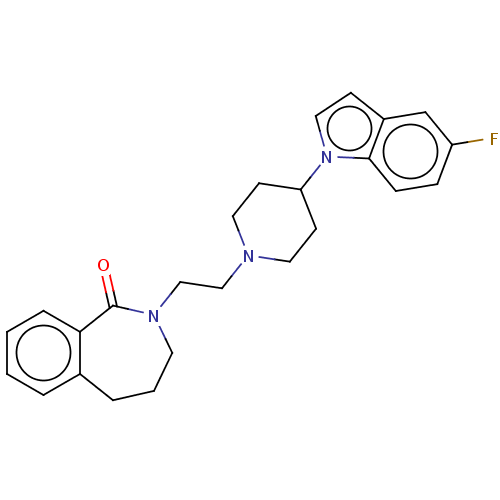
(CHEMBL9951)Show SMILES Fc1ccc2n(ccc2c1)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C25H28FN3O/c26-21-7-8-24-20(18-21)9-15-29(24)22-10-13-27(14-11-22)16-17-28-12-3-5-19-4-1-2-6-23(19)25(28)30/h1-2,4,6-9,15,18,22H,3,5,10-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor in Sprague-Dawley rat brain membrane |
Bioorg Med Chem 16: 322-35 (2008)
Article DOI: 10.1016/j.bmc.2007.09.033
BindingDB Entry DOI: 10.7270/Q22V2H0T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086053
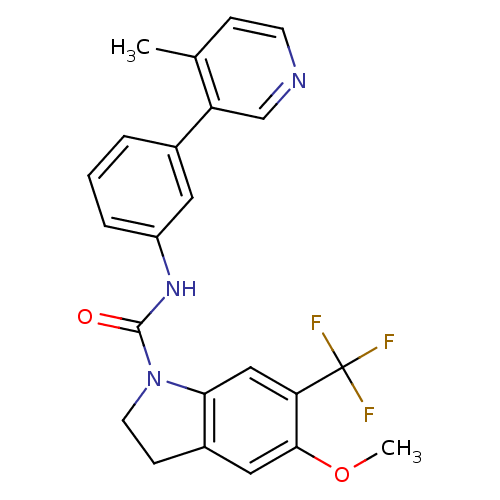
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3cccc(c3)-c3cnccc3C)c2cc1C(F)(F)F Show InChI InChI=1S/C23H20F3N3O2/c1-14-6-8-27-13-18(14)15-4-3-5-17(10-15)28-22(30)29-9-7-16-11-21(31-2)19(12-20(16)29)23(24,25)26/h3-6,8,10-13H,7,9H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086059
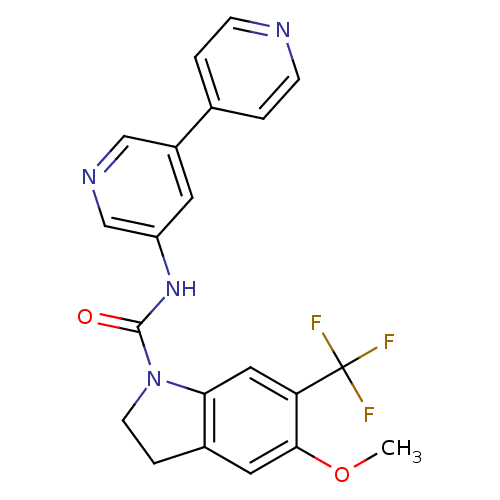
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3cncc(c3)-c3ccncc3)c2cc1C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-30-19-9-14-4-7-28(18(14)10-17(19)21(22,23)24)20(29)27-16-8-15(11-26-12-16)13-2-5-25-6-3-13/h2-3,5-6,8-12H,4,7H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001275
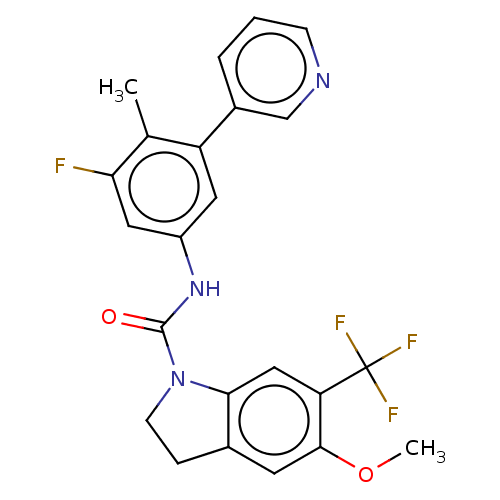
(CHEMBL280465)Show SMILES COc1cc2CCN(C(=O)Nc3cc(F)c(C)c(c3)-c3cccnc3)c2cc1C(F)(F)F Show InChI InChI=1S/C23H19F4N3O2/c1-13-17(15-4-3-6-28-12-15)9-16(10-19(13)24)29-22(31)30-7-5-14-8-21(32-2)18(11-20(14)30)23(25,26)27/h3-4,6,8-12H,5,7H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086055
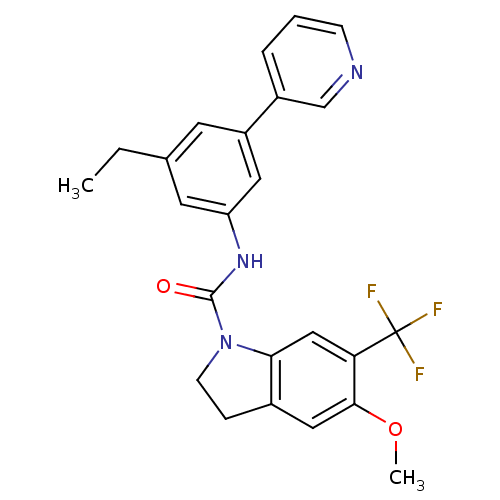
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES CCc1cc(NC(=O)N2CCc3cc(OC)c(cc23)C(F)(F)F)cc(c1)-c1cccnc1 Show InChI InChI=1S/C24H22F3N3O2/c1-3-15-9-18(17-5-4-7-28-14-17)11-19(10-15)29-23(31)30-8-6-16-12-22(32-2)20(13-21(16)30)24(25,26)27/h4-5,7,9-14H,3,6,8H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086067
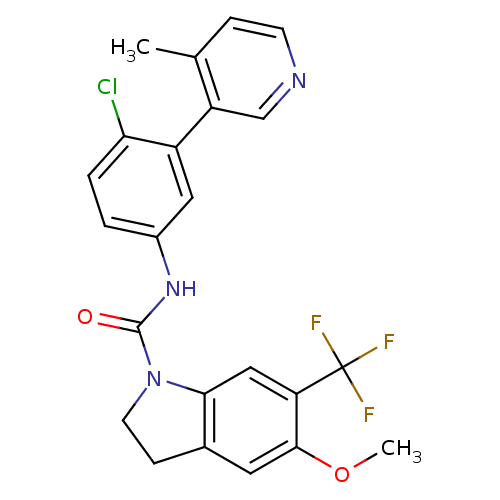
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3ccc(Cl)c(c3)-c3cnccc3C)c2cc1C(F)(F)F Show InChI InChI=1S/C23H19ClF3N3O2/c1-13-5-7-28-12-17(13)16-10-15(3-4-19(16)24)29-22(31)30-8-6-14-9-21(32-2)18(11-20(14)30)23(25,26)27/h3-5,7,9-12H,6,8H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM234270
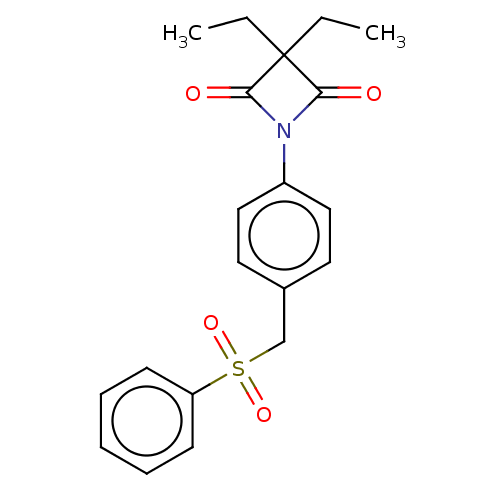
(4-oxo-β-lactam (3))Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C20H21NO4S/c1-3-20(4-2)18(22)21(19(20)23)16-12-10-15(11-13-16)14-26(24,25)17-8-6-5-7-9-17/h5-13H,3-4,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | -52.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Lisbon
| Assay Description
The inhibition of the HLE was studied at 25°C by continuously monitoring the absorbance at 410 nm for 20 min of a solution prepared by mixing 10 _... |
J Enzyme Inhib Med Chem 26: 169-75 (2011)
Article DOI: 10.3109/14756366.2010.486794
BindingDB Entry DOI: 10.7270/Q2B56HM0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086071
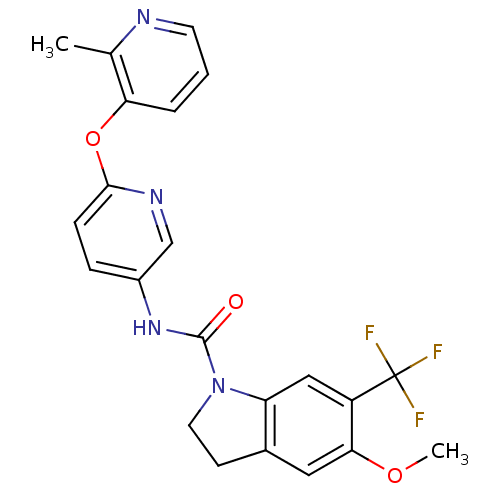
(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O3/c1-13-18(4-3-8-26-13)32-20-6-5-15(12-27-20)28-21(30)29-9-7-14-10-19(31-2)16(11-17(14)29)22(23,24)25/h3-6,8,10-12H,7,9H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086071

(5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...)Show SMILES COc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1C(F)(F)F Show InChI InChI=1S/C22H19F3N4O3/c1-13-18(4-3-8-26-13)32-20-6-5-15(12-27-20)28-21(30)29-9-7-14-10-19(31-2)16(11-17(14)29)22(23,24)25/h3-6,8,10-12H,7,9H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by displacement of [3H]mesulergine |
Bioorg Med Chem Lett 10: 1863-6 (2000)
BindingDB Entry DOI: 10.7270/Q2X63Q4P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data