

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM80642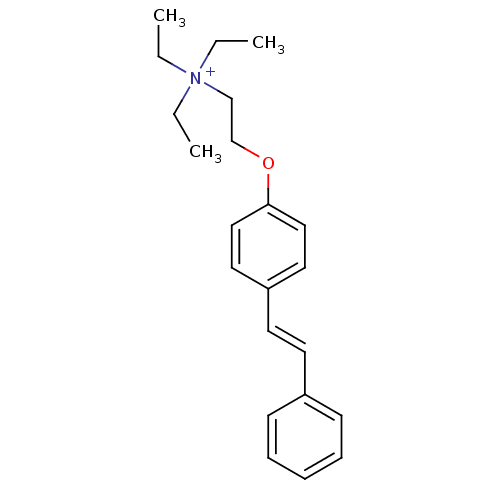 (CHEMBL191491 | MG 624 | MLS002172460 | SMR00125409...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466608 (CHEMBL4285772) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466614 (CHEMBL4291079) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466613 (CHEMBL4287656) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50001695 (CHEMBL3238085) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha3beta4 nAChR expressed in HEK293 cells after 2 hrs by betaplate counting analysis | J Med Chem 57: 3511-21 (2014) Article DOI: 10.1021/jm500183r BindingDB Entry DOI: 10.7270/Q2ZC84CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466606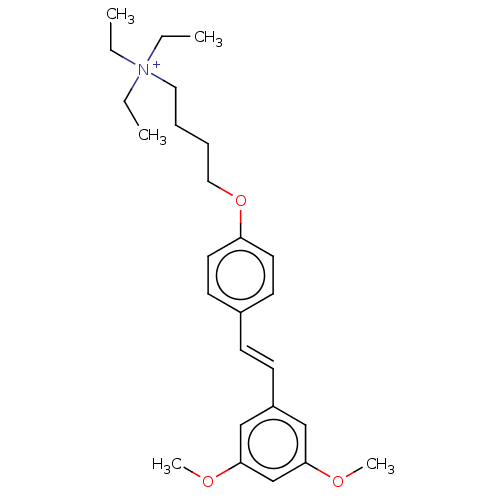 (CHEMBL4280452) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466607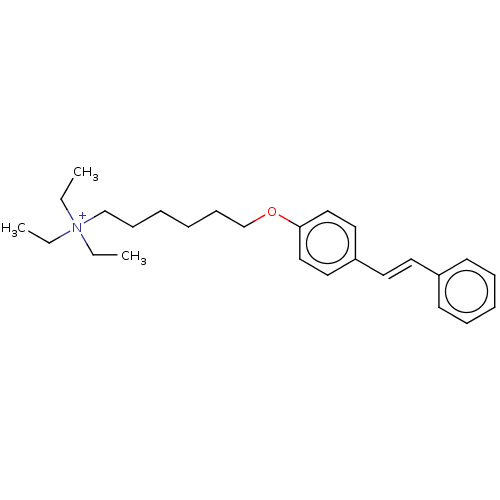 (CHEMBL4293646) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466603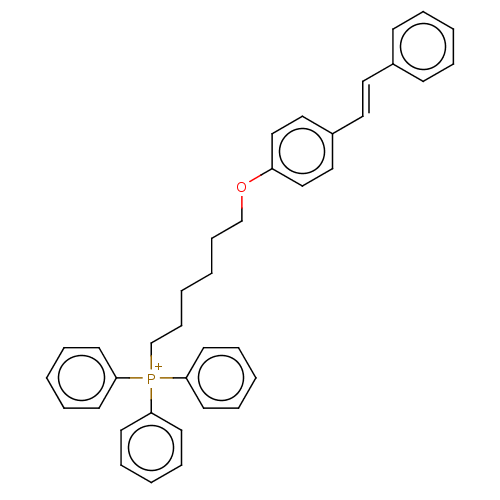 (CHEMBL4277852) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 559 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466615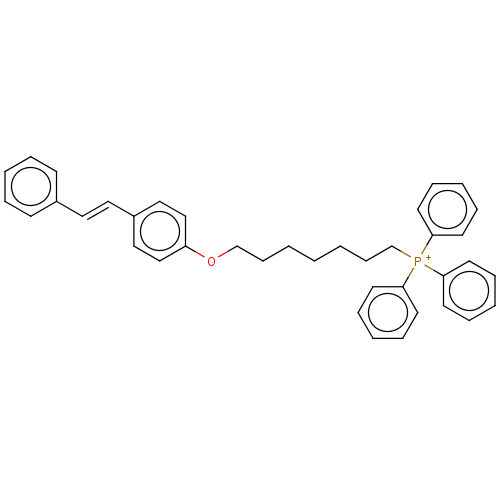 (CHEMBL4291927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50001698 (CHEMBL3238083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha3beta4 nAChR expressed in HEK293 cells after 2 hrs by betaplate counting analysis | J Med Chem 57: 3511-21 (2014) Article DOI: 10.1021/jm500183r BindingDB Entry DOI: 10.7270/Q2ZC84CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50001696 (CHEMBL3238023) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha3beta4 nAChR expressed in HEK293 cells after 2 hrs by betaplate counting analysis | J Med Chem 57: 3511-21 (2014) Article DOI: 10.1021/jm500183r BindingDB Entry DOI: 10.7270/Q2ZC84CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466611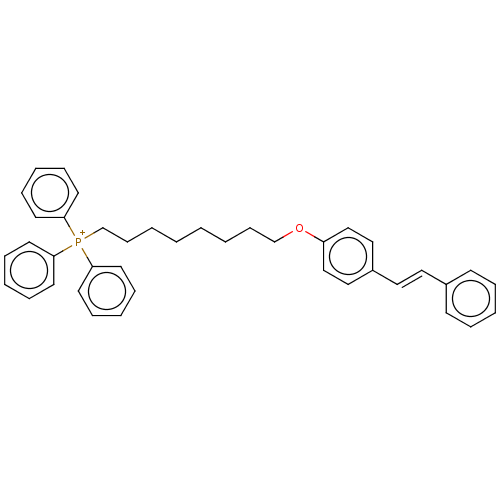 (CHEMBL4281312) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 909 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50001701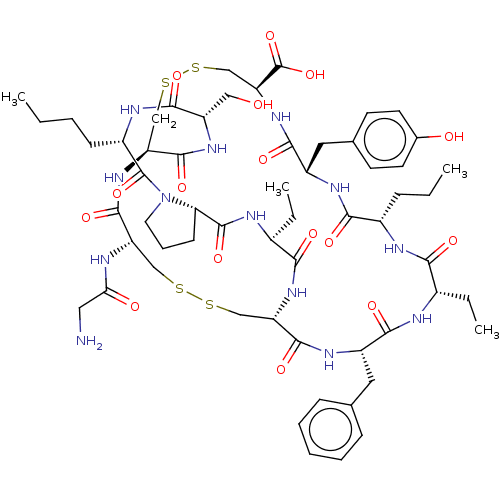 (CHEMBL3238086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha3beta4 nAChR expressed in HEK293 cells after 2 hrs by betaplate counting analysis | J Med Chem 57: 3511-21 (2014) Article DOI: 10.1021/jm500183r BindingDB Entry DOI: 10.7270/Q2ZC84CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466616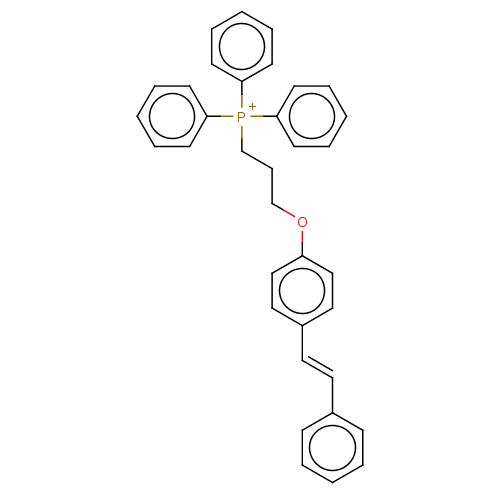 (CHEMBL4288118) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 984 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50001702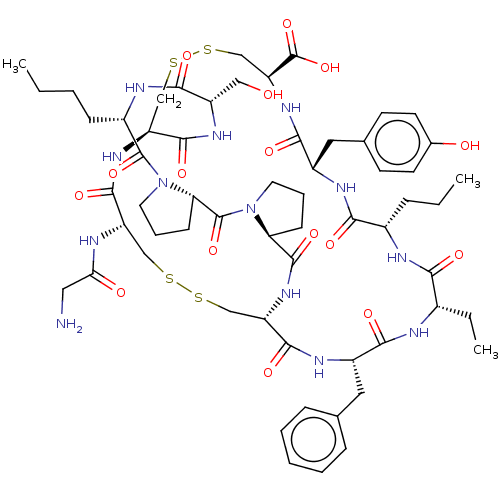 (CHEMBL3238084) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatadine from rat alpha3beta4 nAChR expressed in HEK293 cells after 2 hrs by betaplate counting analysis | J Med Chem 57: 3511-21 (2014) Article DOI: 10.1021/jm500183r BindingDB Entry DOI: 10.7270/Q2ZC84CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466612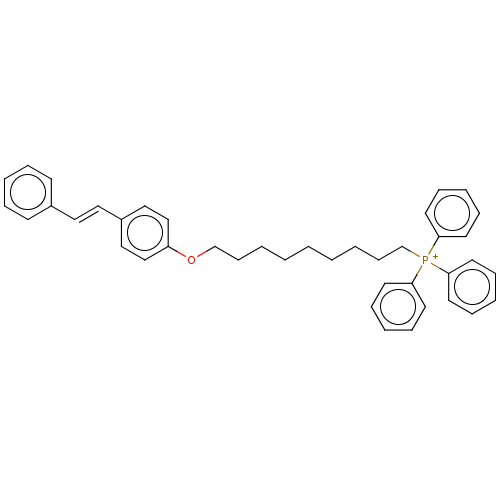 (CHEMBL4290427) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466609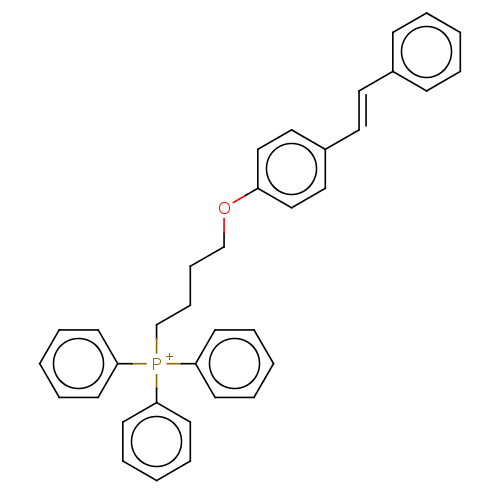 (CHEMBL4284717) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466605 (CHEMBL4276776) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466604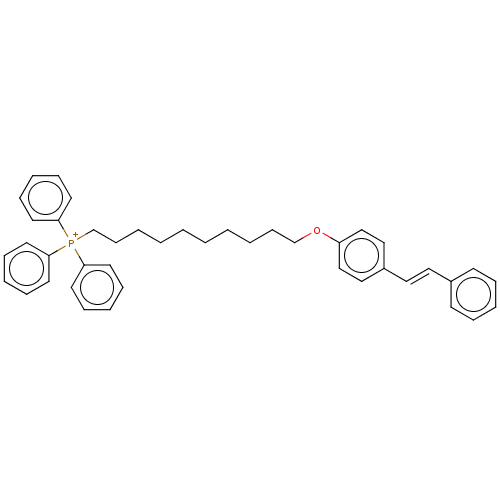 (CHEMBL4280206) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50466610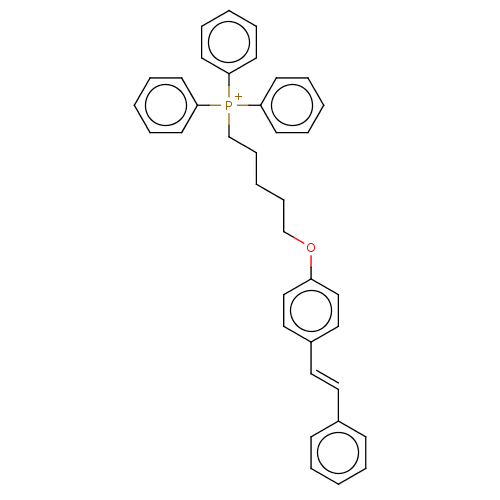 (CHEMBL4288506) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from human alpha7 nAChR expressed in human SH-SY5Y cell membranes after 30 mins by gamma counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50466614 (CHEMBL4291079) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]-epibatidine from human alpha4beta2 nAChR expressed in HEK293 cell membranes after 30 mins by beta counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50466604 (CHEMBL4280206) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]-epibatidine from human alpha4beta2 nAChR expressed in HEK293 cell membranes after 30 mins by beta counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50466609 (CHEMBL4284717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]-epibatidine from human alpha4beta2 nAChR expressed in HEK293 cell membranes after 30 mins by beta counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50466607 (CHEMBL4293646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]-epibatidine from human alpha4beta2 nAChR expressed in HEK293 cell membranes after 30 mins by beta counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM80642 (CHEMBL191491 | MG 624 | MLS002172460 | SMR00125409...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]-epibatidine from human alpha4beta2 nAChR expressed in HEK293 cell membranes after 30 mins by beta counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50466608 (CHEMBL4285772) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]-epibatidine from human alpha4beta2 nAChR expressed in HEK293 cell membranes after 30 mins by beta counting analysis | J Med Chem 61: 10531-10544 (2018) Article DOI: 10.1021/acs.jmedchem.8b01052 BindingDB Entry DOI: 10.7270/Q2PV6P2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-1 (Homo sapiens (Human)) | BDBM50199337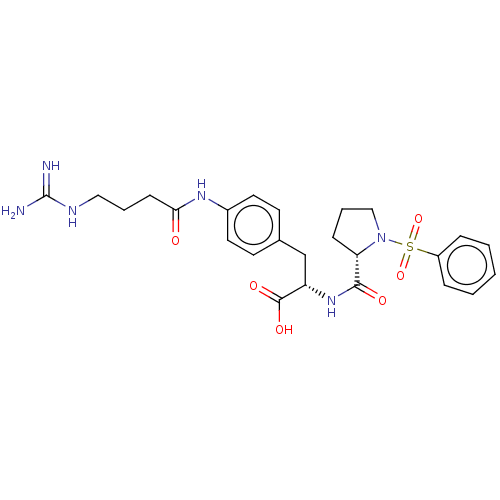 (CHEMBL3962660 | US10131658, Compound 6) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibition of integrin alphaVbeta1 (unknown origin)-mediated CHO cell adhesion to fibronectin preincubated for 15 to 30 mins followed by 60 min incub... | ACS Med Chem Lett 7: 902-907 (2016) Article DOI: 10.1021/acsmedchemlett.6b00196 BindingDB Entry DOI: 10.7270/Q2XP76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-1 (Homo sapiens (Human)) | BDBM50199337 (CHEMBL3962660 | US10131658, Compound 6) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibition of integrin alphaVbeta1 (unknown origin)-mediated CHO cell adhesion to fibronectin preincubated for 15 to 30 mins followed by 60 min incub... | ACS Med Chem Lett 7: 902-907 (2016) Article DOI: 10.1021/acsmedchemlett.6b00196 BindingDB Entry DOI: 10.7270/Q2XP76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor; alpha9/alpha10 (Homo sapiens (Human)) | BDBM50606633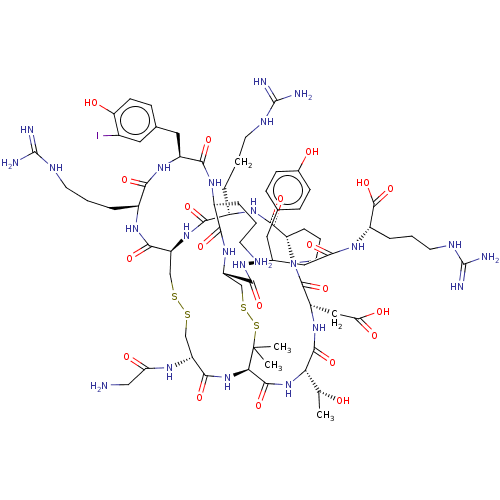 (CHEMBL5219936) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00512 BindingDB Entry DOI: 10.7270/Q20G3Q8V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-1 (Homo sapiens (Human)) | BDBM50199345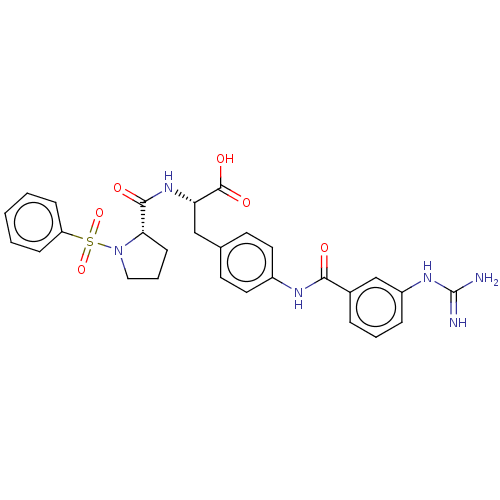 (CHEMBL3893700 | US10131658, Compound 201) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibition of integrin alphaVbeta1 (unknown origin)-mediated CHO cell adhesion to fibronectin preincubated for 15 to 30 mins followed by 60 min incub... | ACS Med Chem Lett 7: 902-907 (2016) Article DOI: 10.1021/acsmedchemlett.6b00196 BindingDB Entry DOI: 10.7270/Q2XP76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-1 (Homo sapiens (Human)) | BDBM50199345 (CHEMBL3893700 | US10131658, Compound 201) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco Curated by ChEMBL | Assay Description Inhibition of integrin alphaVbeta1 (unknown origin)-mediated CHO cell adhesion to fibronectin preincubated for 15 to 30 mins followed by 60 min incub... | ACS Med Chem Lett 7: 902-907 (2016) Article DOI: 10.1021/acsmedchemlett.6b00196 BindingDB Entry DOI: 10.7270/Q2XP76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50448963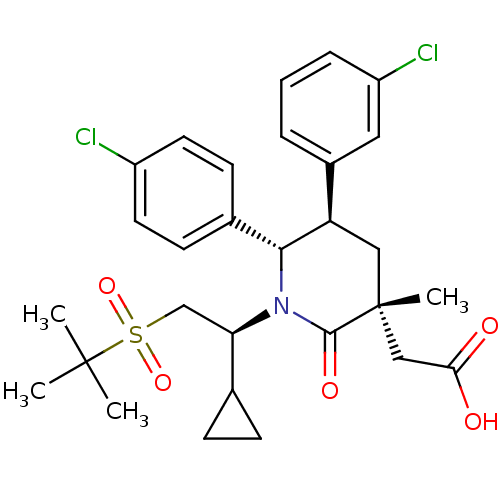 (CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2472-88 (2014) Article DOI: 10.1021/jm401767k BindingDB Entry DOI: 10.7270/Q2C24XZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215410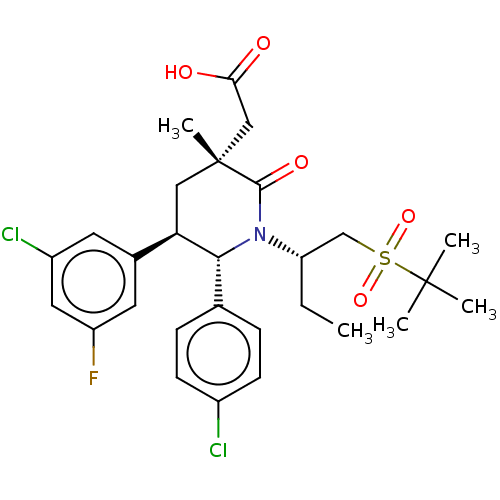 (US9296736, 398 | US9593129, Example 398) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008749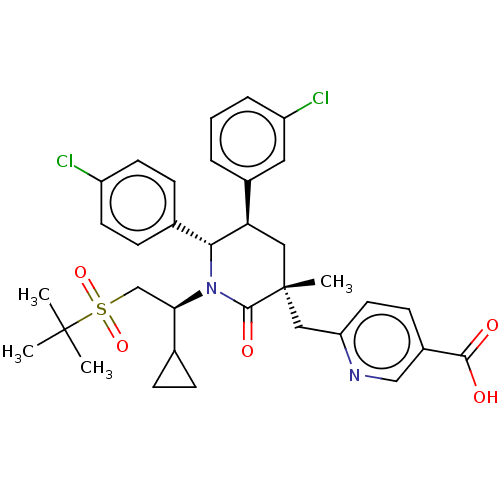 (CHEMBL3236356) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008748 (CHEMBL3236357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50448936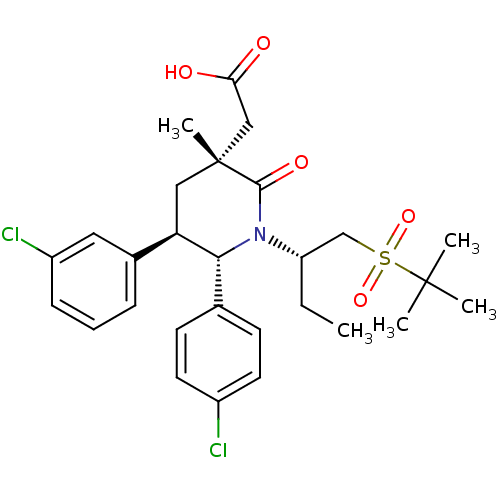 (CHEMBL3125527 | US9296736, 342 | US9593129, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008753 (CHEMBL3236358) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50448963 (CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008756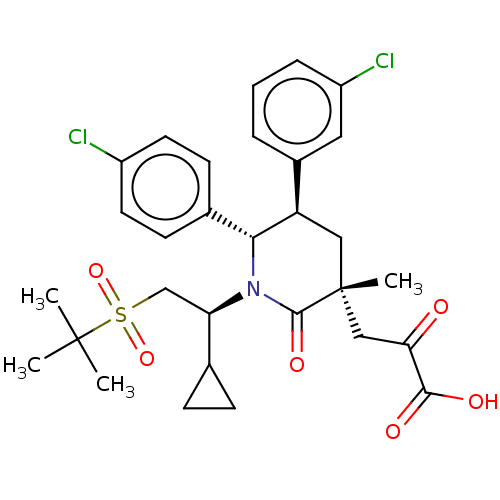 (CHEMBL3236361) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008760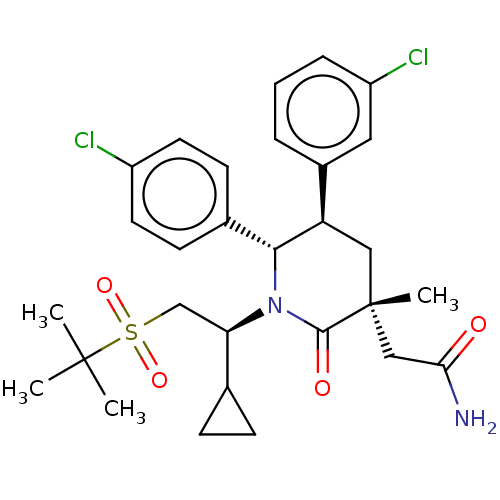 (CHEMBL3236364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008762 (CHEMBL3236635) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008763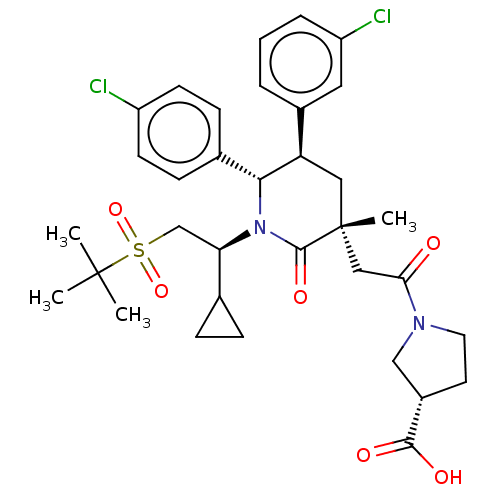 (CHEMBL3236636) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50008750 (CHEMBL3236639) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to GST-thrombin-tagged human MDM2 (1 to 188) expressed in Escherichia coli assessed as inhibition of interaction with p53 in serum f... | J Med Chem 57: 2963-88 (2014) Article DOI: 10.1021/jm401911v BindingDB Entry DOI: 10.7270/Q2862J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215340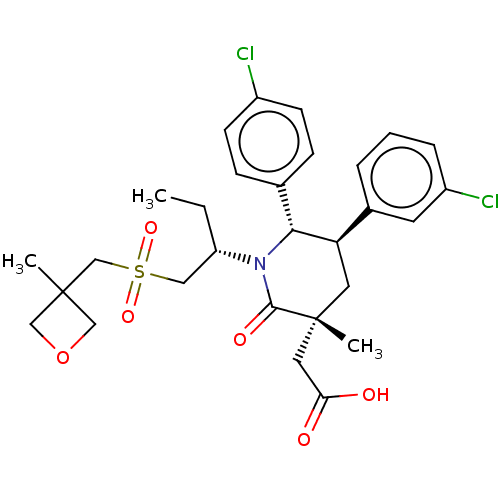 (US9296736, 308 | US9593129, Example 308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215346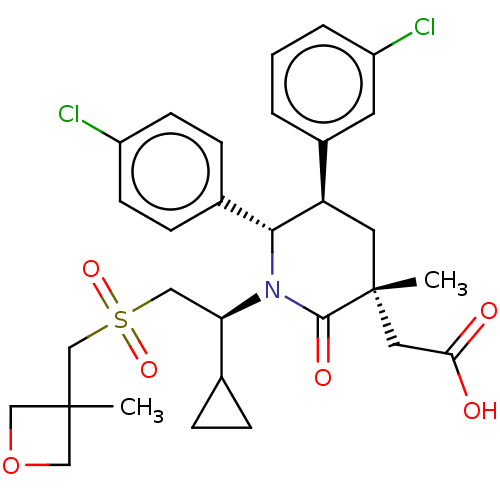 (US9296736, 314 | US9593129, Example 314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215366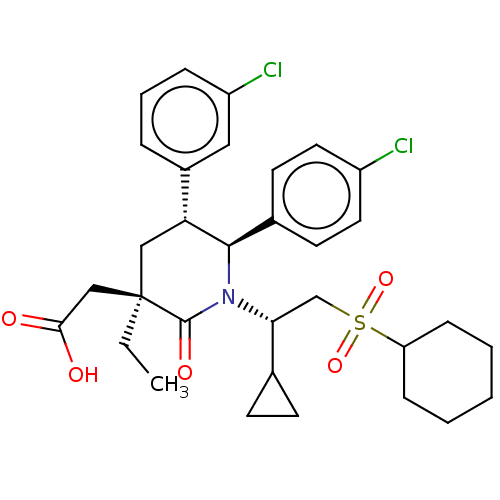 (US9296736, 334 | US9593129, Example 334) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50448936 (CHEMBL3125527 | US9296736, 342 | US9593129, Exampl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215373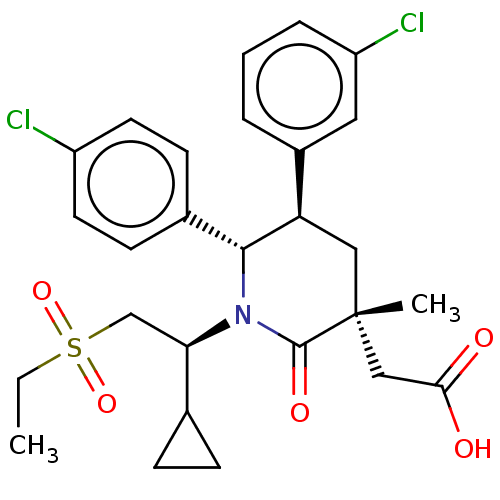 (US9296736, 349 | US9593129, Example 349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50448963 (CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215375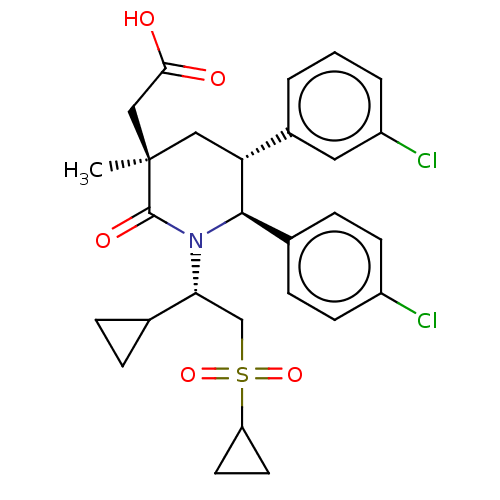 (US9296736, 353 | US9593129, Example 353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2078 total ) | Next | Last >> |