 Found 1271 hits with Last Name = 'pelletier' and Initial = 'j'
Found 1271 hits with Last Name = 'pelletier' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50243699
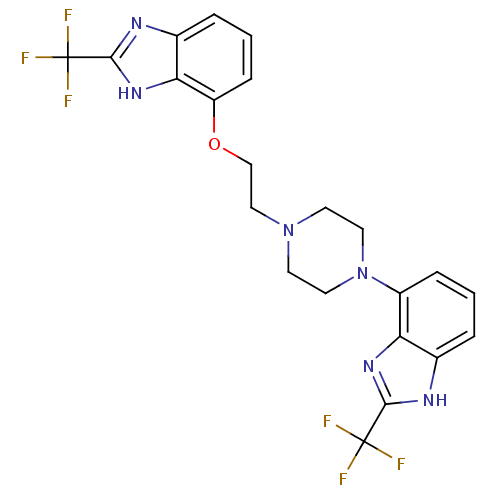
(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1D receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545315
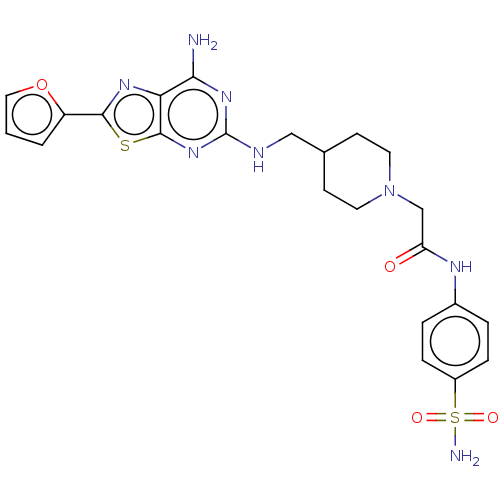
(CHEMBL4646158)Show SMILES Nc1nc(NCC2CCN(CC(=O)Nc3ccc(cc3)S(N)(=O)=O)CC2)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C23H26N8O4S2/c24-20-19-22(36-21(28-19)17-2-1-11-35-17)30-23(29-20)26-12-14-7-9-31(10-8-14)13-18(32)27-15-3-5-16(6-4-15)37(25,33)34/h1-6,11,14H,7-10,12-13H2,(H,27,32)(H2,25,33,34)(H3,24,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50243700
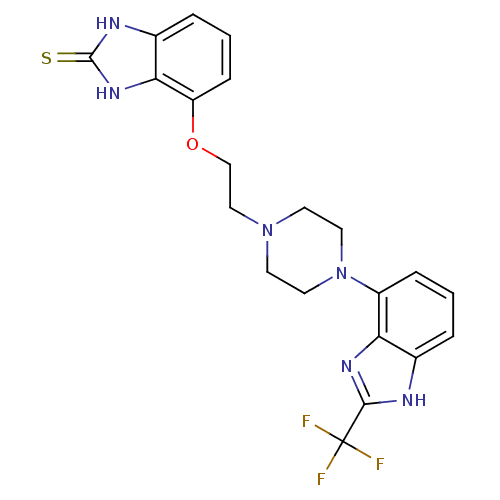
(4-(2-(4-(2-(trifluoromethyl)-1H-benzo[d]imidazol-4...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(CCOc2cccc3[nH]c(=S)[nH]c23)CC1 Show InChI InChI=1S/C21H21F3N6OS/c22-21(23,24)19-25-13-3-1-5-15(17(13)27-19)30-9-7-29(8-10-30)11-12-31-16-6-2-4-14-18(16)28-20(32)26-14/h1-6H,7-12H2,(H,25,27)(H2,26,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1676
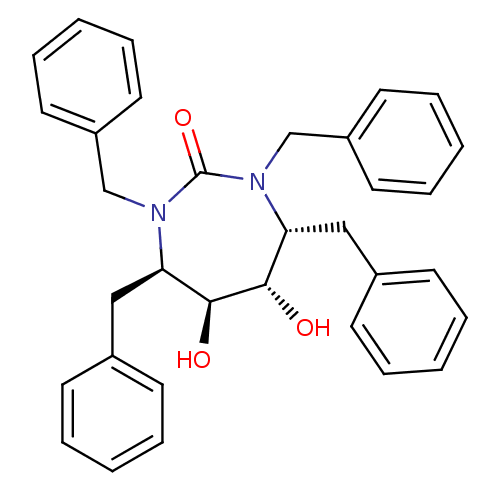
((4R,5S,6S,7R)-1,3,4,7-tetrabenzyl-5,6-dihydroxy-1,...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccccc2)C(=O)N(Cc2ccccc2)[C@@H]1Cc1ccccc1 |r| Show InChI InChI=1S/C33H34N2O3/c36-31-29(21-25-13-5-1-6-14-25)34(23-27-17-9-3-10-18-27)33(38)35(24-28-19-11-4-12-20-28)30(32(31)37)22-26-15-7-2-8-16-26/h1-20,29-32,36-37H,21-24H2/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC |
Bioorg Med Chem Lett 8: 3615-20 (1999)
BindingDB Entry DOI: 10.7270/Q2VX0FP1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545313
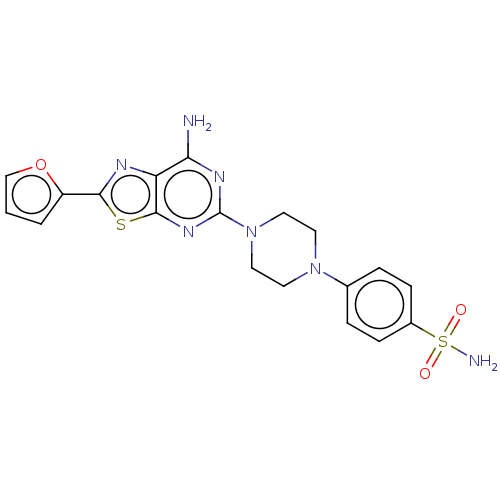
(CHEMBL4644153)Show SMILES Nc1nc(nc2sc(nc12)-c1ccco1)N1CCN(CC1)c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H19N7O3S2/c20-16-15-18(30-17(22-15)14-2-1-11-29-14)24-19(23-16)26-9-7-25(8-10-26)12-3-5-13(6-4-12)31(21,27)28/h1-6,11H,7-10H2,(H2,20,23,24)(H2,21,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1682
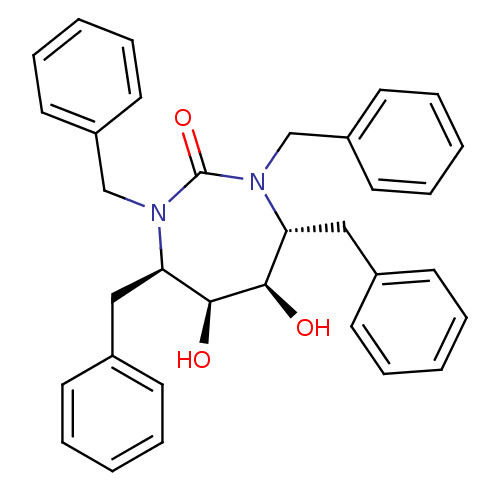
((4R,5S,6R,7R)-1,3,4,7-tetrabenzyl-5,6-dihydroxy-1,...)Show SMILES O[C@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccccc2)C(=O)N(Cc2ccccc2)[C@@H]1Cc1ccccc1 |r| Show InChI InChI=1S/C33H34N2O3/c36-31-29(21-25-13-5-1-6-14-25)34(23-27-17-9-3-10-18-27)33(38)35(24-28-19-11-4-12-20-28)30(32(31)37)22-26-15-7-2-8-16-26/h1-20,29-32,36-37H,21-24H2/t29-,30-,31-,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC |
Bioorg Med Chem Lett 8: 3615-20 (1999)
BindingDB Entry DOI: 10.7270/Q2VX0FP1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50073327

((1S,2R,3S,4R,8R,9R)-4-Benzyl-9-benzyloxy-2,3-dihyd...)Show SMILES O[C@H]1[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N2[C@@H]3CC[C@]2(C[C@H]3OCc2ccccc2)[C@H]1O Show InChI InChI=1S/C32H34N4O4/c37-29-27(16-21-7-3-1-4-8-21)35(19-23-11-12-25-24(15-23)18-33-34-25)31(39)36-26-13-14-32(36,30(29)38)17-28(26)40-20-22-9-5-2-6-10-22/h1-12,15,18,26-30,37-38H,13-14,16-17,19-20H2,(H,33,34)/t26-,27-,28-,29+,30+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC |
Bioorg Med Chem Lett 8: 3615-20 (1999)
BindingDB Entry DOI: 10.7270/Q2VX0FP1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50243699

(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453241
(CHEMBL4206625)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)OP(O)(=O)OP(S)(=S)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C18H26N4O19P4S4/c23-9-1-3-21(17(29)19-9)15-13(27)11(25)7(37-15)5-35-44(46,47)40-42(31,32)39-43(33,34)41-45(48,49)36-6-8-12(26)14(28)16(38-8)22-4-2-10(24)20-18(22)30/h1-4,7-8,11-16,25-28H,5-6H2,(H,31,32)(H,33,34)(H,46,47)(H,48,49)(H,19,23,29)(H,20,24,30)/t7-,8-,11-,12-,13-,14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537592

(CHEMBL4632881)Show SMILES C1CN(CCN1c1cc(-c2ccncc2)c(nn1)-c1ccccc1)c1ncccn1 Show InChI InChI=1S/C23H21N7/c1-2-5-19(6-3-1)22-20(18-7-11-24-12-8-18)17-21(27-28-22)29-13-15-30(16-14-29)23-25-9-4-10-26-23/h1-12,17H,13-16H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545319
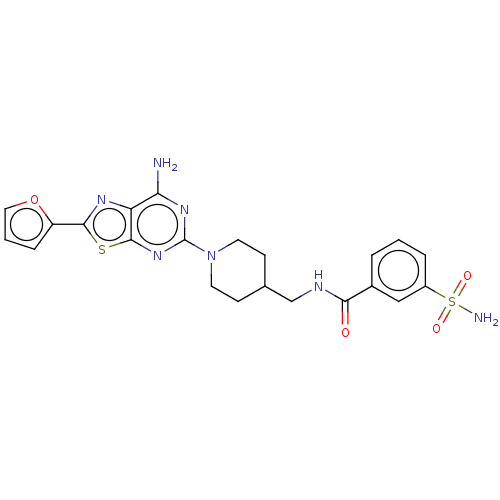
(CHEMBL4646403)Show SMILES Nc1nc(nc2sc(nc12)-c1ccco1)N1CCC(CNC(=O)c2cccc(c2)S(N)(=O)=O)CC1 Show InChI InChI=1S/C22H23N7O4S2/c23-18-17-21(34-20(26-17)16-5-2-10-33-16)28-22(27-18)29-8-6-13(7-9-29)12-25-19(30)14-3-1-4-15(11-14)35(24,31)32/h1-5,10-11,13H,6-9,12H2,(H,25,30)(H2,23,27,28)(H2,24,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545316
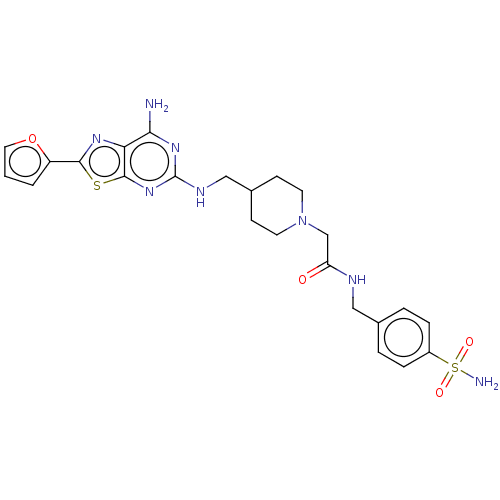
(CHEMBL4633630)Show SMILES Nc1nc(NCC2CCN(CC(=O)NCc3ccc(cc3)S(N)(=O)=O)CC2)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C24H28N8O4S2/c25-21-20-23(37-22(29-20)18-2-1-11-36-18)31-24(30-21)28-13-16-7-9-32(10-8-16)14-19(33)27-12-15-3-5-17(6-4-15)38(26,34)35/h1-6,11,16H,7-10,12-14H2,(H,27,33)(H2,26,34,35)(H3,25,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545317

(CHEMBL4645283)Show SMILES Nc1nc(NCC2CCN(CC(=O)NCCc3ccc(cc3)S(N)(=O)=O)CC2)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C25H30N8O4S2/c26-22-21-24(38-23(30-21)19-2-1-13-37-19)32-25(31-22)29-14-17-8-11-33(12-9-17)15-20(34)28-10-7-16-3-5-18(6-4-16)39(27,35)36/h1-6,13,17H,7-12,14-15H2,(H,28,34)(H2,27,35,36)(H3,26,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50243699

(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1B receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447108

(CHEMBL3112881)Show InChI InChI=1S/C11H7ClFNO2S/c12-11-7-2-1-6(13)5-9(7)17-8(11)3-4-10(15)14-16/h1-5,16H,(H,14,15)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A LC assessed as cleavage of SNAP-25 (141 to 206) after 30 mins by LC-MS analysis |
J Med Chem 57: 669-76 (2014)
Article DOI: 10.1021/jm4012164
BindingDB Entry DOI: 10.7270/Q2GH9KDZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545314

(CHEMBL4643397)Show SMILES Nc1nc(NCCN2CCN(CC2)c2ccc(cc2)S(N)(=O)=O)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C21H24N8O3S2/c22-18-17-20(33-19(25-17)16-2-1-13-32-16)27-21(26-18)24-7-8-28-9-11-29(12-10-28)14-3-5-15(6-4-14)34(23,30)31/h1-6,13H,7-12H2,(H2,23,30,31)(H3,22,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521018
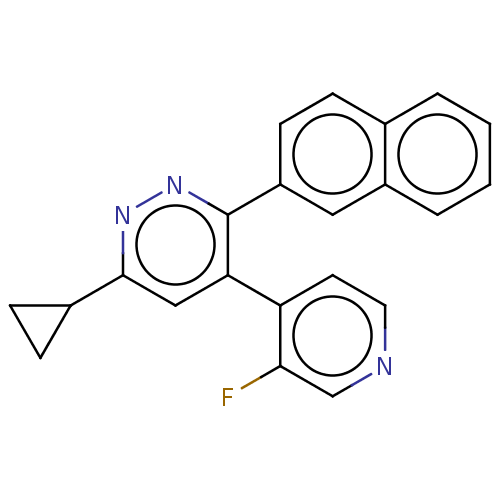
(US11149020, Compound 10 (MW-167)) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545311
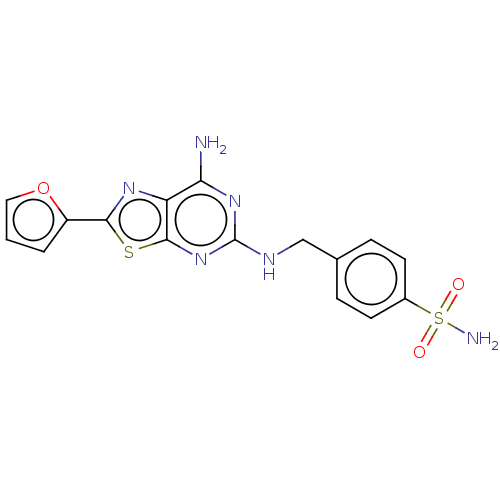
(CHEMBL4643898)Show SMILES Nc1nc(NCc2ccc(cc2)S(N)(=O)=O)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C16H14N6O3S2/c17-13-12-15(26-14(20-12)11-2-1-7-25-11)22-16(21-13)19-8-9-3-5-10(6-4-9)27(18,23)24/h1-7H,8H2,(H2,18,23,24)(H3,17,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50545314

(CHEMBL4643397)Show SMILES Nc1nc(NCCN2CCN(CC2)c2ccc(cc2)S(N)(=O)=O)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C21H24N8O3S2/c22-18-17-20(33-19(25-17)16-2-1-13-32-16)27-21(26-18)24-7-8-28-9-11-29(12-10-28)14-3-5-15(6-4-14)34(23,30)31/h1-6,13H,7-12H2,(H2,23,30,31)(H3,22,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521021
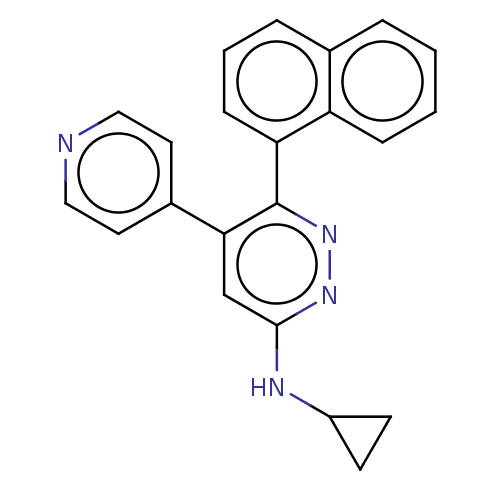
(US11149020, Compound 13 (MW-107)) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521019
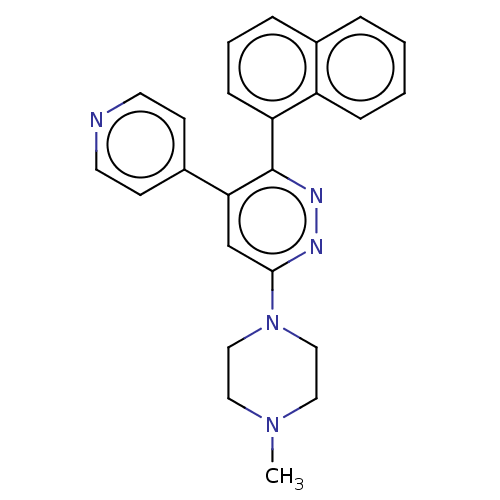
(US11149020, Compound 11 (MW-122))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1cccc2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537600

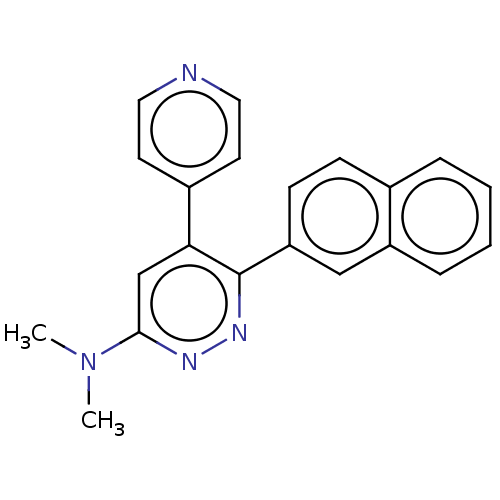
(CHEMBL4129018 | US11149020, Compound 27 (MW-150))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C24H23N5/c1-28-12-14-29(15-13-28)23-17-22(19-8-10-25-11-9-19)24(27-26-23)21-7-6-18-4-2-3-5-20(18)16-21/h2-11,16-17H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521025

(US11149020, Compound 16 (MW-200)) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521045

(US11149020, Compound 36 (MW-164))Show SMILES CC1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537600

(CHEMBL4129018 | US11149020, Compound 27 (MW-150))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C24H23N5/c1-28-12-14-29(15-13-28)23-17-22(19-8-10-25-11-9-19)24(27-26-23)21-7-6-18-4-2-3-5-20(18)16-21/h2-11,16-17H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537599
(CHEMBL4648060 | US11149020, Compound 2 (MW-108))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-9-11-22-12-10-16)21(24-23-20)18-8-7-15-5-3-4-6-17(15)13-18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537599

(CHEMBL4648060 | US11149020, Compound 2 (MW-108))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-9-11-22-12-10-16)21(24-23-20)18-8-7-15-5-3-4-6-17(15)13-18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50545315

(CHEMBL4646158)Show SMILES Nc1nc(NCC2CCN(CC(=O)Nc3ccc(cc3)S(N)(=O)=O)CC2)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C23H26N8O4S2/c24-20-19-22(36-21(28-19)17-2-1-11-35-17)30-23(29-20)26-12-14-7-9-31(10-8-14)13-18(32)27-15-3-5-16(6-4-15)37(25,33)34/h1-6,11,14H,7-10,12-13H2,(H,27,32)(H2,25,33,34)(H3,24,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521017
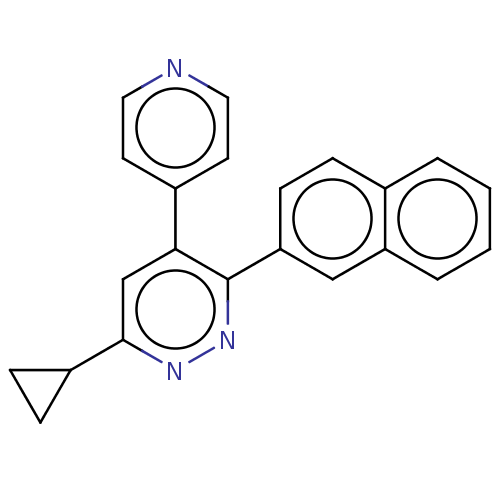
(US11149020, Compound 9 (MW-125)) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537598

(CHEMBL4646628 | US11149020, Compound 1 (MW-181))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-10-12-22-13-11-16)21(24-23-20)18-9-5-7-15-6-3-4-8-17(15)18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236L |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537598

(CHEMBL4646628 | US11149020, Compound 1 (MW-181))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-10-12-22-13-11-16)21(24-23-20)18-9-5-7-15-6-3-4-8-17(15)18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521016

(US11149020, Compound 7 (MW-077)) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50244213
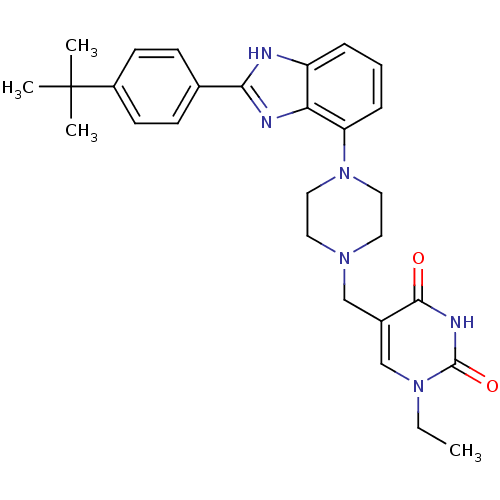
(5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CCn1cc(CN2CCN(CC2)c2cccc3[nH]c(nc23)-c2ccc(cc2)C(C)(C)C)c(=O)[nH]c1=O Show InChI InChI=1S/C28H34N6O2/c1-5-33-18-20(26(35)31-27(33)36)17-32-13-15-34(16-14-32)23-8-6-7-22-24(23)30-25(29-22)19-9-11-21(12-10-19)28(2,3)4/h6-12,18H,5,13-17H2,1-4H3,(H,29,30)(H,31,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50545311

(CHEMBL4643898)Show SMILES Nc1nc(NCc2ccc(cc2)S(N)(=O)=O)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C16H14N6O3S2/c17-13-12-15(26-14(20-12)11-2-1-7-25-11)22-16(21-13)19-8-9-3-5-10(6-4-9)27(18,23)24/h1-7H,8H2,(H2,18,23,24)(H3,17,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453240
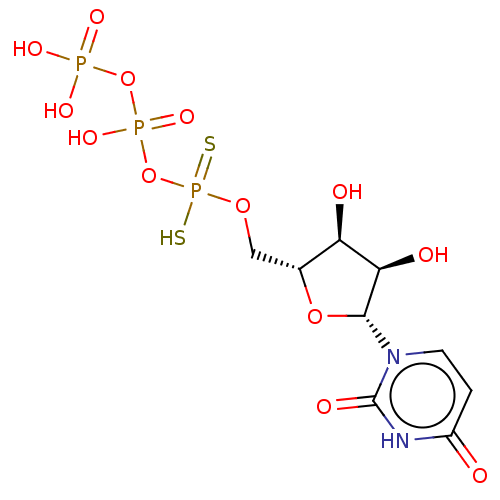
(CHEMBL4218881)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)OP(O)(O)=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H15N2O13P3S2/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(22-8)3-21-27(28,29)24-26(19,20)23-25(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,28,29)(H,10,12,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521024

(US11149020, Compound 15 (MW-156)) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545322
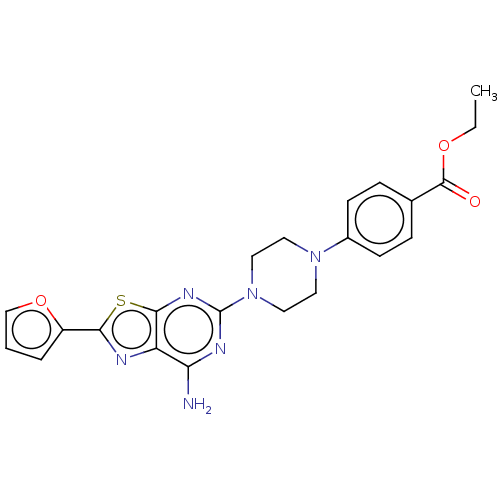
(CHEMBL4642713)Show SMILES CCOC(=O)c1ccc(cc1)N1CCN(CC1)c1nc(N)c2nc(sc2n1)-c1ccco1 Show InChI InChI=1S/C22H22N6O3S/c1-2-30-21(29)14-5-7-15(8-6-14)27-9-11-28(12-10-27)22-25-18(23)17-20(26-22)32-19(24-17)16-4-3-13-31-16/h3-8,13H,2,9-12H2,1H3,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545312
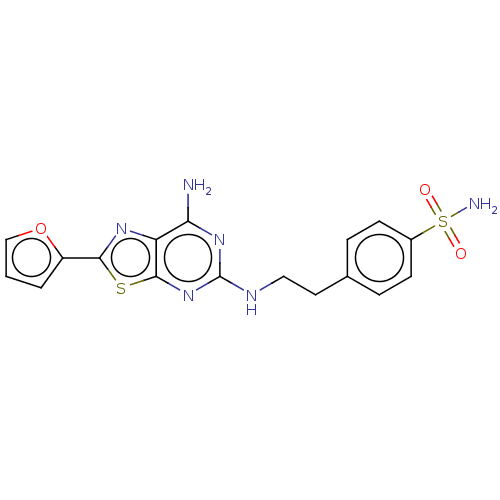
(CHEMBL4632532)Show SMILES Nc1nc(NCCc2ccc(cc2)S(N)(=O)=O)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C17H16N6O3S2/c18-14-13-16(27-15(21-13)12-2-1-9-26-12)23-17(22-14)20-8-7-10-3-5-11(6-4-10)28(19,24)25/h1-6,9H,7-8H2,(H2,19,24,25)(H3,18,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50537598

(CHEMBL4646628 | US11149020, Compound 1 (MW-181))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-10-12-22-13-11-16)21(24-23-20)18-9-5-7-15-6-3-4-8-17(15)18/h3-14H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50256882
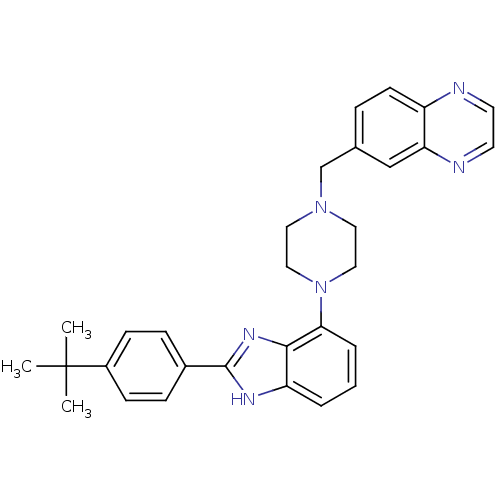
(6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)-c1nc2c(cccc2[nH]1)N1CCN(Cc2ccc3nccnc3c2)CC1 Show InChI InChI=1S/C30H32N6/c1-30(2,3)23-10-8-22(9-11-23)29-33-25-5-4-6-27(28(25)34-29)36-17-15-35(16-18-36)20-21-7-12-24-26(19-21)32-14-13-31-24/h4-14,19H,15-18,20H2,1-3H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human neurokinin NK2 receptor |
J Med Chem 52: 2148-52 (2009)
Article DOI: 10.1021/jm801572m
BindingDB Entry DOI: 10.7270/Q2ZC82RT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM521047
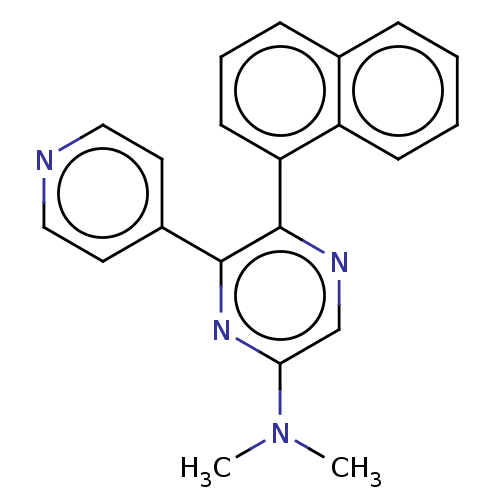
(N,N-dimethyl-5-(naphthalen-1-yl)-6-(pyridin-4-yl)p...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2251NBP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50545313

(CHEMBL4644153)Show SMILES Nc1nc(nc2sc(nc12)-c1ccco1)N1CCN(CC1)c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H19N7O3S2/c20-16-15-18(30-17(22-15)14-2-1-11-29-14)24-19(23-16)26-9-7-25(8-10-26)12-3-5-13(6-4-12)31(21,27)28/h1-6,11H,7-10H2,(H2,20,23,24)(H2,21,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545318
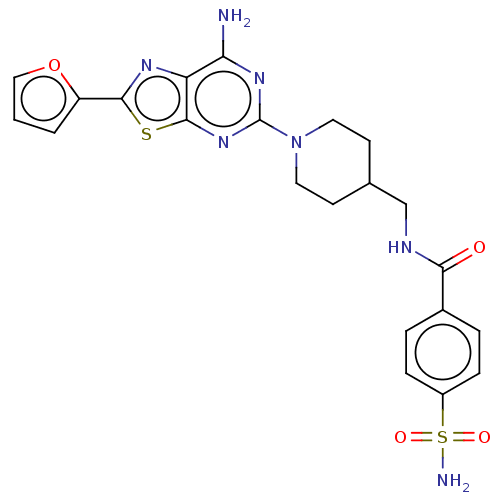
(CHEMBL4647074)Show SMILES Nc1nc(nc2sc(nc12)-c1ccco1)N1CCC(CNC(=O)c2ccc(cc2)S(N)(=O)=O)CC1 Show InChI InChI=1S/C22H23N7O4S2/c23-18-17-21(34-20(26-17)16-2-1-11-33-16)28-22(27-18)29-9-7-13(8-10-29)12-25-19(30)14-3-5-15(6-4-14)35(24,31)32/h1-6,11,13H,7-10,12H2,(H,25,30)(H2,23,27,28)(H2,24,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 488 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50545320
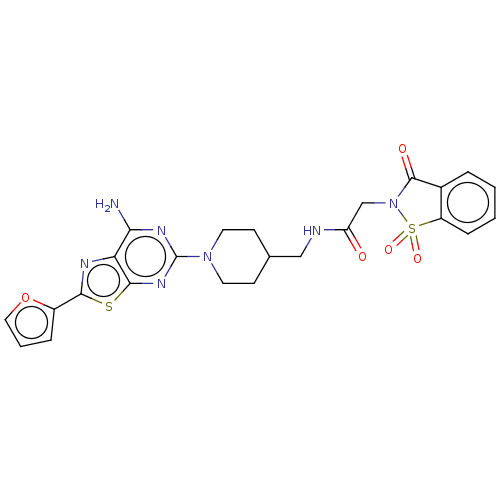
(CHEMBL4632626)Show SMILES Nc1nc(nc2sc(nc12)-c1ccco1)N1CCC(CNC(=O)CN2C(=O)c3ccccc3S2(=O)=O)CC1 Show InChI InChI=1S/C24H23N7O5S2/c25-20-19-22(37-21(27-19)16-5-3-11-36-16)29-24(28-20)30-9-7-14(8-10-30)12-26-18(32)13-31-23(33)15-4-1-2-6-17(15)38(31,34)35/h1-6,11,14H,7-10,12-13H2,(H,26,32)(H2,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 488 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50442939
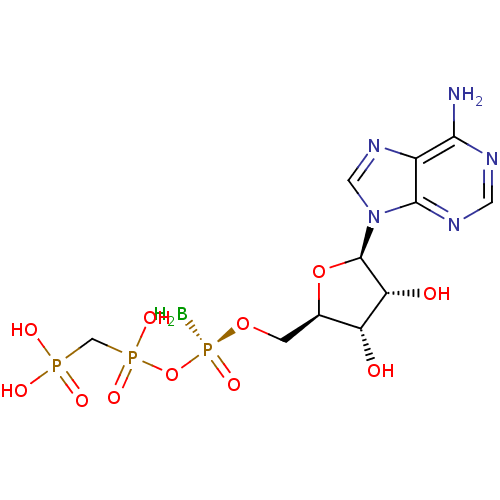
(CHEMBL3087158)Show SMILES B[P@@](=O)(OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)CP(O)(O)=O |r| Show InChI InChI=1S/C11H19BN5O11P3/c12-31(25,28-30(23,24)4-29(20,21)22)26-1-5-7(18)8(19)11(27-5)17-3-16-6-9(13)14-2-15-10(6)17/h2-3,5,7-8,11,18-19H,1,4,12H2,(H,23,24)(H2,13,14,15)(H2,20,21,22)/t5-,7-,8-,11-,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50545319

(CHEMBL4646403)Show SMILES Nc1nc(nc2sc(nc12)-c1ccco1)N1CCC(CNC(=O)c2cccc(c2)S(N)(=O)=O)CC1 Show InChI InChI=1S/C22H23N7O4S2/c23-18-17-21(34-20(26-17)16-5-2-10-33-16)28-22(27-18)29-8-6-13(7-9-29)12-25-19(30)14-3-1-4-15(11-14)35(24,31)32/h1-5,10-11,13H,6-9,12H2,(H,25,30)(H2,23,27,28)(H2,24,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 568 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50545313

(CHEMBL4644153)Show SMILES Nc1nc(nc2sc(nc12)-c1ccco1)N1CCN(CC1)c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H19N7O3S2/c20-16-15-18(30-17(22-15)14-2-1-11-29-14)24-19(23-16)26-9-7-25(8-10-26)12-3-5-13(6-4-12)31(21,27)28/h1-6,11H,7-10H2,(H2,20,23,24)(H2,21,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 582 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]AB-MEGA from human A3R expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
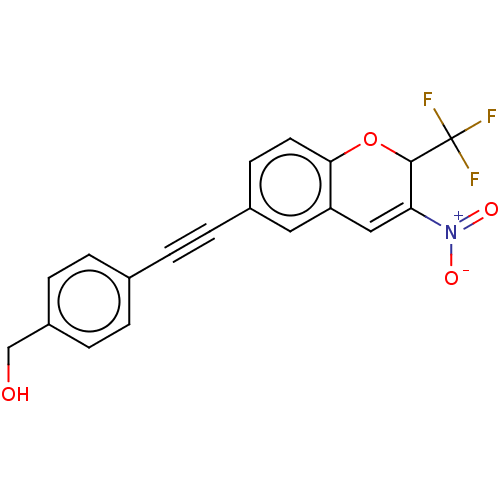
(Homo sapiens (Human)) | BDBM50405423
(CHEMBL5283362)Show SMILES COCCc1cnc([nH]1)-c1ccc(OCC(O)CNCCc2ccc(OC)c(OC)c2)cc1 Show InChI InChI=1S/C25H33N3O5/c1-30-13-11-20-15-27-25(28-20)19-5-7-22(8-6-19)33-17-21(29)16-26-12-10-18-4-9-23(31-2)24(14-18)32-3/h4-9,14-15,21,26,29H,10-13,16-17H2,1-3H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Histamine H2 receptor antagonistic activity on the isolated spontaneously beating guinea pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50545317

(CHEMBL4645283)Show SMILES Nc1nc(NCC2CCN(CC(=O)NCCc3ccc(cc3)S(N)(=O)=O)CC2)nc2sc(nc12)-c1ccco1 Show InChI InChI=1S/C25H30N8O4S2/c26-22-21-24(38-23(30-21)19-2-1-13-37-19)32-25(31-22)29-14-17-8-11-33(12-9-17)15-20(34)28-10-7-16-3-5-18(6-4-16)39(27,35)36/h1-6,13,17H,7-12,14-15H2,(H,28,34)(H2,27,35,36)(H3,26,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127067
BindingDB Entry DOI: 10.7270/Q26M3BFS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537592

(CHEMBL4632881)Show SMILES C1CN(CCN1c1cc(-c2ccncc2)c(nn1)-c1ccccc1)c1ncccn1 Show InChI InChI=1S/C23H21N7/c1-2-5-19(6-3-1)22-20(18-7-11-24-12-8-18)17-21(27-28-22)29-13-15-30(16-14-29)23-25-9-4-10-26-23/h1-12,17H,13-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data