 Found 353 hits with Last Name = 'roche' and Initial = 'j'
Found 353 hits with Last Name = 'roche' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor expressed in CHO cells |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50068669
(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C\c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068669

(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C\c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068669

(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C\c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068669

(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C\c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand at site 1 |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50493249

(CHEMBL2420249)Show SMILES [H][C@@]12CC[C@@](C)(C1)[C@H](NC(=O)c1cc(-c3ccc(Cl)c(C)c3)n(Cc3ccc(C)cc3)c1)C2(C)C |r,TLB:8:7:6:2.3| Show InChI InChI=1S/C30H35ClN2O/c1-19-6-8-21(9-7-19)17-33-18-23(15-26(33)22-10-11-25(31)20(2)14-22)27(34)32-28-29(3,4)24-12-13-30(28,5)16-24/h6-11,14-15,18,24,28H,12-13,16-17H2,1-5H3,(H,32,34)/t24-,28-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50493245

(CHEMBL2420251)Show SMILES [H][C@]12CC[C@](C)([C@@H](C1)NC(=O)c1cc(-c3ccc(Cl)c(C)c3)n(Cc3ccc(C)cc3)n1)C2(C)C |r,TLB:8:6:32:2.3| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)16-24(32-33)27(34)31-26-15-22-12-13-29(26,5)28(22,3)4/h6-11,14,16,22,26H,12-13,15,17H2,1-5H3,(H,31,34)/t22-,26+,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50493248

(CHEMBL2420252)Show SMILES [H][C@@]12CC[C@@](C)(C1)[C@H](NC(=O)c1cc(-c3ccc(Cl)c(C)c3)n(n1)-c1ccccc1)C2(C)C |r,TLB:8:7:3.2:6| Show InChI InChI=1S/C27H30ClN3O/c1-17-14-18(10-11-21(17)28)23-15-22(30-31(23)20-8-6-5-7-9-20)24(32)29-25-26(2,3)19-12-13-27(25,4)16-19/h5-11,14-15,19,25H,12-13,16H2,1-4H3,(H,29,32)/t19-,25-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50068667

(4-(2-{3-[1-Naphthalen-1-yl-meth-(Z)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C\c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068667

(4-(2-{3-[1-Naphthalen-1-yl-meth-(Z)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C\c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50493247

(CHEMBL2420248)Show SMILES [H][C@@]12CC[C@@](C)(C1)[C@H](\C=C\c1cc(-c3ccc(Cl)c(C)c3)n(Cc3ccc(C)cc3)n1)C2(C)C |r,TLB:8:7:6:2.3| Show InChI InChI=1S/C30H35ClN2/c1-20-6-8-22(9-7-20)19-33-27(23-10-12-26(31)21(2)16-23)17-25(32-33)11-13-28-29(3,4)24-14-15-30(28,5)18-24/h6-13,16-17,24,28H,14-15,18-19H2,1-5H3/b13-11+/t24-,28-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 527 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 611 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand at site 2 |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50068668
(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(E)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C/c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 658 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068667

(4-(2-{3-[1-Naphthalen-1-yl-meth-(Z)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C\c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 928 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50493246

(CHEMBL2420250)Show SMILES [H][C@@]12CC[C@@](C)(C1)[C@H](\C=C\c1cc(-c3ccc(Cl)c(C)c3)n(Cc3ccc(C)cc3)c1)C2(C)C |r,TLB:8:7:6:2.3| Show InChI InChI=1S/C31H36ClN/c1-21-6-8-23(9-7-21)19-33-20-24(17-28(33)25-11-12-27(32)22(2)16-25)10-13-29-30(3,4)26-14-15-31(29,5)18-26/h6-13,16-17,20,26,29H,14-15,18-19H2,1-5H3/b13-10+/t26-,29-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068668

(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(E)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C/c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068668

(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(E)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C/c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]SR-141,716A ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM323704
(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM323702
(US10188756, Compound CN89)Show SMILES CN(CCc1ccccc1)S(=O)(=O)c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C18H20N2O4S/c1-20(13-12-15-6-3-2-4-7-15)25(23,24)17-9-5-8-16(14-17)10-11-18(21)19-22/h2-11,14,22H,12-13H2,1H3,(H,19,21)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM323704

(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM323704

(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM323704

(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM323702

(US10188756, Compound CN89)Show SMILES CN(CCc1ccccc1)S(=O)(=O)c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C18H20N2O4S/c1-20(13-12-15-6-3-2-4-7-15)25(23,24)17-9-5-8-16(14-17)10-11-18(21)19-22/h2-11,14,22H,12-13H2,1H3,(H,19,21)/b11-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM22449
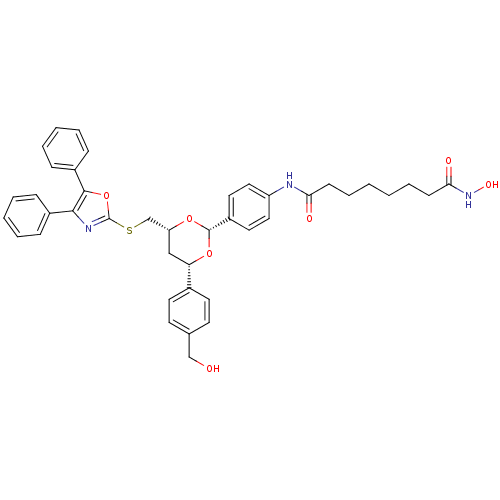
(CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...)Show SMILES OCc1ccc(cc1)[C@@H]1C[C@H](CSc2nc(c(o2)-c2ccccc2)-c2ccccc2)O[C@@H](O1)c1ccc(NC(=O)CCCCCCC(=O)NO)cc1 |r| Show InChI InChI=1S/C41H43N3O7S/c45-26-28-17-19-29(20-18-28)35-25-34(27-52-41-43-38(30-11-5-3-6-12-30)39(51-41)31-13-7-4-8-14-31)49-40(50-35)32-21-23-33(24-22-32)42-36(46)15-9-1-2-10-16-37(47)44-48/h3-8,11-14,17-24,34-35,40,45,48H,1-2,9-10,15-16,25-27H2,(H,42,46)(H,44,47)/t34-,35+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM323702

(US10188756, Compound CN89)Show SMILES CN(CCc1ccccc1)S(=O)(=O)c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C18H20N2O4S/c1-20(13-12-15-6-3-2-4-7-15)25(23,24)17-9-5-8-16(14-17)10-11-18(21)19-22/h2-11,14,22H,12-13H2,1H3,(H,19,21)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM323702

(US10188756, Compound CN89)Show SMILES CN(CCc1ccccc1)S(=O)(=O)c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C18H20N2O4S/c1-20(13-12-15-6-3-2-4-7-15)25(23,24)17-9-5-8-16(14-17)10-11-18(21)19-22/h2-11,14,22H,12-13H2,1H3,(H,19,21)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50015233
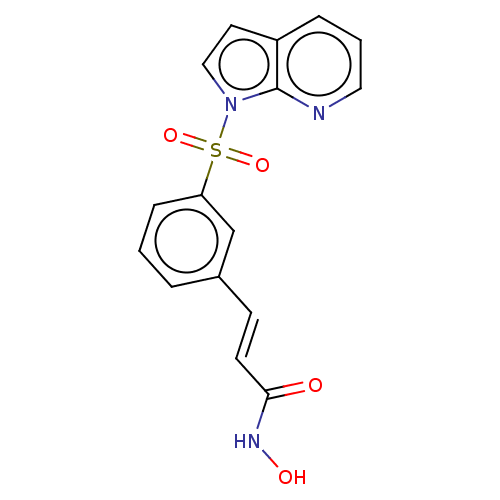
(CHEMBL3262727)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)n1ccc2cccnc12 Show InChI InChI=1S/C16H13N3O4S/c20-15(18-21)7-6-12-3-1-5-14(11-12)24(22,23)19-10-8-13-4-2-9-17-16(13)19/h1-11,21H,(H,18,20)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50613610

(CHEMBL5270345)Show SMILES CC(C)Cc1c(OCCOc2cccc(\C=C\C(=O)NO)c2)ccc2CCC(=O)Oc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613620

(CHEMBL5275736)Show SMILES CC[N+]1=C(\C=C\C=C\C=C2\N(CCCCC(=O)N(CCc3c(C)[nH]c4ccccc34)Cc3ccc(\C=C\C(=O)NO)cc3)c3ccc4c(cc(cc4c3C2(C)C)S(O)(=O)=O)S(O)(=O)=O)C(C)(C)c2c1ccc1c(cc(cc21)S(O)(=O)=O)S([O-])(=O)=O |c:2| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50525406
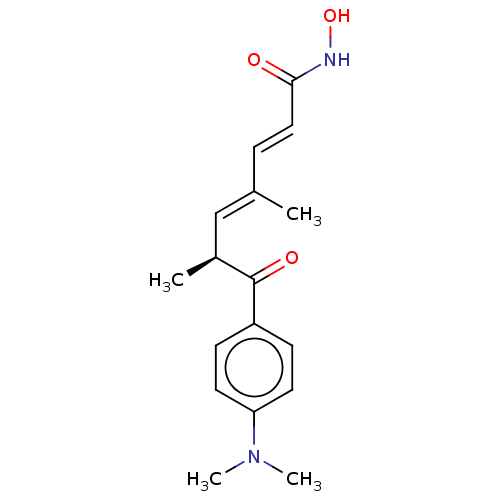
(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using fluorogenic HDAC substrate measured after 10 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613612

(CHEMBL5283887) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50613614

(CHEMBL5276344) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50613612

(CHEMBL5283887) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613615

(CHEMBL5265931)Show SMILES ONC(=O)c1ccc(NC(=O)CCc2ccc(Oc3cccs3)c(O)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50332494
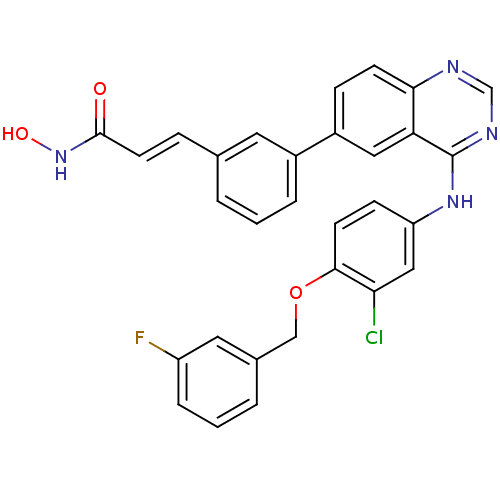
((E)-3-{3-[4-(3-Chloro-4-(3-fluorobenzyloxy)phenyla...)Show SMILES ONC(=O)\C=C\c1cccc(c1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C30H22ClFN4O3/c31-26-16-24(9-11-28(26)39-17-20-4-2-6-23(32)14-20)35-30-25-15-22(8-10-27(25)33-18-34-30)21-5-1-3-19(13-21)7-12-29(37)36-38/h1-16,18,38H,17H2,(H,36,37)(H,33,34,35)/b12-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate measured after 45 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50492703
(CHEMBL2408158)Show InChI InChI=1S/C15H14N4O/c16-9-11-4-5-12(17-10-11)7-15(20)14-8-13-3-1-2-6-19(13)18-14/h4-5,8,10H,1-3,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Addex Therapeutics
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGluR5 receptor expressed in HEK293 cells assessed as intracellular calcium flux after 170 seconds by FLIPR ass... |
Bioorg Med Chem Lett 23: 4523-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.044
BindingDB Entry DOI: 10.7270/Q29S1V02 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM198121
(HPOB)Show InChI InChI=1S/C17H18N2O4/c20-11-10-19(15-4-2-1-3-5-15)16(21)12-13-6-8-14(9-7-13)17(22)18-23/h1-9,20,23H,10-12H2,(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data