 Found 200 hits with Last Name = 'sohn' and Initial = 'j'
Found 200 hits with Last Name = 'sohn' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311254
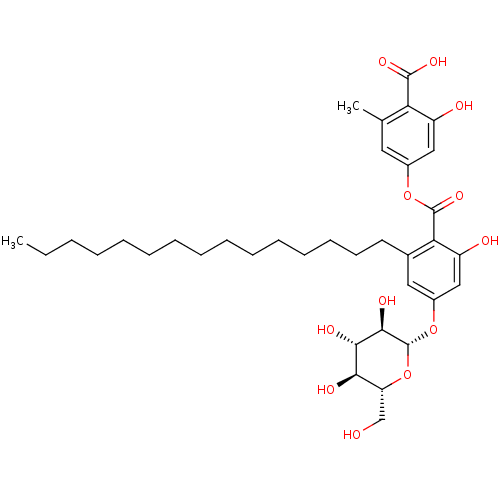
(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Competitive inhibition of PTP1B mediated pNPP hydrolysis by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50129580
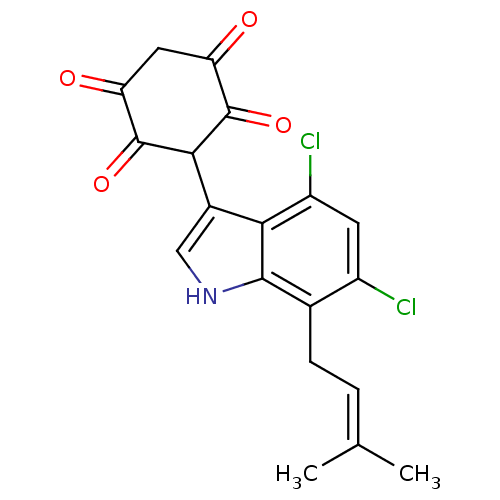
(3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...)Show SMILES CC(C)=CCc1c(Cl)cc(Cl)c2c(c[nH]c12)C1C(=O)C(=O)CC(=O)C1=O |(-2.72,-7.25,;-1.19,-7.49,;-.63,-8.92,;-.23,-6.3,;-.78,-4.85,;.19,-3.66,;-.39,-2.22,;-1.93,-1.99,;.58,-1.03,;2.1,-1.27,;2.87,.07,;2.66,-2.69,;4.09,-3.24,;4.03,-4.76,;2.55,-5.16,;1.71,-3.89,;5.41,-2.45,;5.39,-.89,;4.03,-.12,;6.71,-.12,;6.67,1.42,;8.06,-.87,;8.08,-2.41,;9.44,-3.18,;6.76,-3.2,;6.76,-4.74,)| Show InChI InChI=1S/C19H15Cl2NO4/c1-8(2)3-4-9-11(20)5-12(21)15-10(7-22-17(9)15)16-18(25)13(23)6-14(24)19(16)26/h3,5,7,16,22H,4,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibitory constant of compound against Cell division cycle 25B was determined using mFP as a substrate |
J Med Chem 46: 2580-8 (2003)
Article DOI: 10.1021/jm0300835
BindingDB Entry DOI: 10.7270/Q2RJ4HVW |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50129576
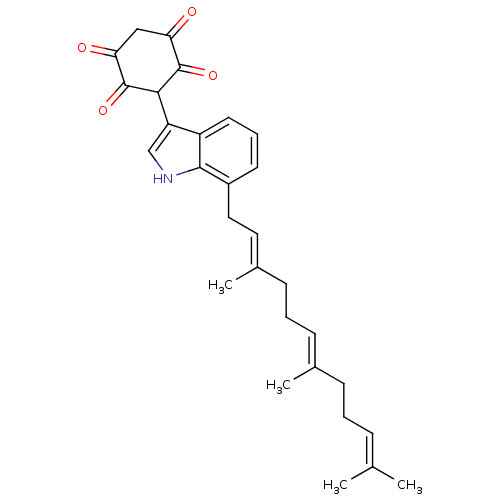
(2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...)Show SMILES CC(C)=CCC\C(C)=C\CC\C(C)=C\Cc1cccc2c(c[nH]c12)C1C(=O)C(=O)CC(=O)C1=O |(-9.11,4.99,;-7.6,4.77,;-6.64,5.99,;-7.04,3.33,;-5.51,3.1,;-4.95,1.67,;-3.42,1.44,;-2.47,2.65,;-2.86,.02,;-1.35,-.22,;-.77,-1.66,;.75,-1.87,;1.31,-3.32,;1.71,-.68,;3.23,-.91,;4.18,.3,;3.62,1.73,;4.58,2.93,;6.11,2.7,;6.67,1.26,;8.1,.72,;8.03,-.82,;6.56,-1.21,;5.72,.06,;9.43,1.51,;9.4,3.06,;8.03,3.83,;10.72,3.84,;10.69,5.38,;12.07,3.1,;12.09,1.54,;13.46,.77,;10.76,.76,;10.79,-.78,)| Show InChI InChI=1S/C29H33NO4/c1-18(2)8-5-9-19(3)10-6-11-20(4)14-15-21-12-7-13-22-23(17-30-27(21)22)26-28(33)24(31)16-25(32)29(26)34/h7-8,10,12-14,17,26,30H,5-6,9,11,15-16H2,1-4H3/b19-10+,20-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibitory constant of compound against Cell division cycle 25 was determined |
J Med Chem 46: 2580-8 (2003)
Article DOI: 10.1021/jm0300835
BindingDB Entry DOI: 10.7270/Q2RJ4HVW |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50129574

(2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...)Show SMILES CC(C)=CCc1cccc2c(c[nH]c12)C1C(=O)C(=O)CC(=O)C1=O |(-1.02,-4.51,;.5,-4.74,;1.06,-6.18,;1.45,-3.53,;2.98,-3.76,;3.94,-2.56,;3.37,-1.13,;4.32,.06,;5.86,-.17,;6.42,-1.59,;7.86,-2.15,;7.79,-3.67,;6.3,-4.06,;5.46,-2.8,;9.17,-1.35,;9.15,.2,;7.79,.97,;10.47,.98,;10.43,2.52,;11.82,.23,;11.84,-1.31,;13.2,-2.08,;10.52,-2.11,;10.52,-3.65,)| Show InChI InChI=1S/C19H17NO4/c1-10(2)6-7-11-4-3-5-12-13(9-20-17(11)12)16-18(23)14(21)8-15(22)19(16)24/h3-6,9,16,20H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibitory constant of compound against Cell division cycle 25B was determined using mFP as a substrate |
J Med Chem 46: 2580-8 (2003)
Article DOI: 10.1021/jm0300835
BindingDB Entry DOI: 10.7270/Q2RJ4HVW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50374277

(CHEMBL403094)Show SMILES Oc1ccc-2c(c1)[C@@H]1Oc3ccccc3[C@@]3(O)CC(=O)c4c(O)cc(O)c-2c4[C@@H]13 Show InChI InChI=1S/C23H16O6/c24-10-5-6-11-12(7-10)22-21-20-18(11)14(25)8-15(26)19(20)16(27)9-23(21,28)13-3-1-2-4-17(13)29-22/h1-8,21-22,24-26,28H,9H2/t21-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B by Lineweaver-Burke plot |
Bioorg Med Chem Lett 18: 772-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.036
BindingDB Entry DOI: 10.7270/Q25B03BB |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50129575
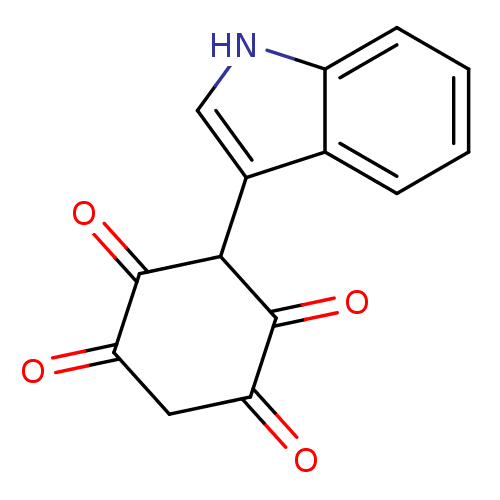
(2,5-Dihydroxy-3-(1H-indol-3-yl)-[1,4]benzoquinone ...)Show InChI InChI=1S/C14H9NO4/c16-10-5-11(17)14(19)12(13(10)18)8-6-15-9-4-2-1-3-7(8)9/h1-4,6,12,15H,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibitory constant of compound against Cdc25B phosphatase was determined using mFP as a substrate |
J Med Chem 46: 2580-8 (2003)
Article DOI: 10.1021/jm0300835
BindingDB Entry DOI: 10.7270/Q2RJ4HVW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM198005

(US9221790, 15)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCNC(Cc2c[nH]c3ccccc23)C1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197995
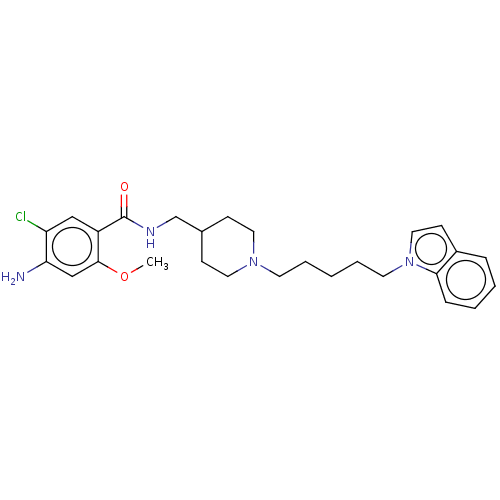
(US9221790, 5)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCCCn2ccc3ccccc23)CC1 Show InChI InChI=1S/C27H35ClN4O2/c1-34-26-18-24(29)23(28)17-22(26)27(33)30-19-20-9-14-31(15-10-20)12-5-2-6-13-32-16-11-21-7-3-4-8-25(21)32/h3-4,7-8,11,16-18,20H,2,5-6,9-10,12-15,19,29H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197993

(US9221790, 3)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCn2ccc3ccccc23)CC1 Show InChI InChI=1S/C25H31ClN4O2/c1-32-24-16-22(27)21(26)15-20(24)25(31)28-17-18-7-12-29(13-8-18)10-4-11-30-14-9-19-5-2-3-6-23(19)30/h2-3,5-6,9,14-16,18H,4,7-8,10-13,17,27H2,1H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197997

(US9221790, 7)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCn2ccnn2)CC1 Show InChI InChI=1S/C19H27ClN6O2/c1-28-18-12-17(21)16(20)11-15(18)19(27)22-13-14-3-8-25(9-4-14)6-2-7-26-10-5-23-24-26/h5,10-12,14H,2-4,6-9,13,21H2,1H3,(H,22,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM198000

(US9221790, 10)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(Cc2cn(C)c3ccccc23)CC1 Show InChI InChI=1S/C24H29ClN4O2/c1-28-14-17(18-5-3-4-6-22(18)28)15-29-9-7-16(8-10-29)13-27-24(30)19-11-20(25)21(26)12-23(19)31-2/h3-6,11-12,14,16H,7-10,13,15,26H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197996
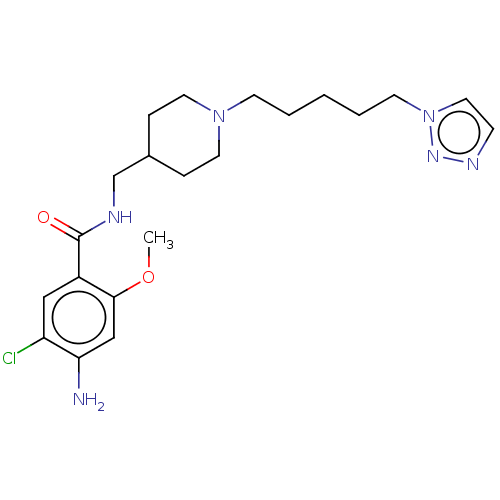
(US9221790, 6)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCCCn2ccnn2)CC1 Show InChI InChI=1S/C21H31ClN6O2/c1-30-20-14-19(23)18(22)13-17(20)21(29)24-15-16-5-10-27(11-6-16)8-3-2-4-9-28-12-7-25-26-28/h7,12-14,16H,2-6,8-11,15,23H2,1H3,(H,24,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM198003

(US9221790, 13)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(Cc2ccc(F)cc2)CC1 Show InChI InChI=1S/C21H25ClFN3O2/c1-28-20-11-19(24)18(22)10-17(20)21(27)25-12-14-6-8-26(9-7-14)13-15-2-4-16(23)5-3-15/h2-5,10-11,14H,6-9,12-13,24H2,1H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197992
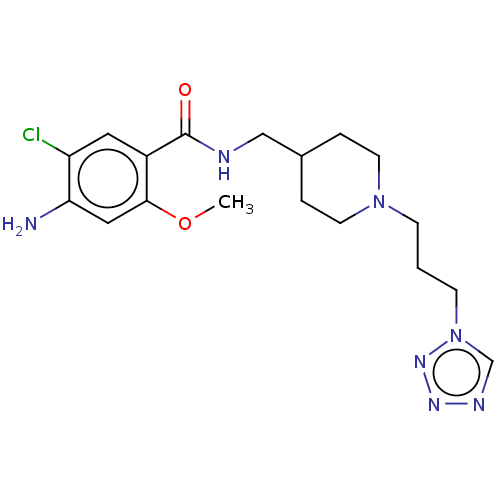
(US9221790, 2)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCn2cnnn2)CC1 Show InChI InChI=1S/C18H26ClN7O2/c1-28-17-10-16(20)15(19)9-14(17)18(27)21-11-13-3-7-25(8-4-13)5-2-6-26-12-22-23-24-26/h9-10,12-13H,2-8,11,20H2,1H3,(H,21,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.164 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197998

(US9221790, 8)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCn2nccn2)CC1 Show InChI InChI=1S/C19H27ClN6O2/c1-28-18-12-17(21)16(20)11-15(18)19(27)22-13-14-3-9-25(10-4-14)7-2-8-26-23-5-6-24-26/h5-6,11-12,14H,2-4,7-10,13,21H2,1H3,(H,22,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197991

(US9221790, 1)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCn2cncn2)CC1 Show InChI InChI=1S/C19H27ClN6O2/c1-28-18-10-17(21)16(20)9-15(18)19(27)23-11-14-3-7-25(8-4-14)5-2-6-26-13-22-12-24-26/h9-10,12-14H,2-8,11,21H2,1H3,(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.226 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197994

(US9221790, 4)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CCCn2ccnc2C)CC1 Show InChI InChI=1S/C21H30ClN5O2/c1-15-24-6-11-27(15)8-3-7-26-9-4-16(5-10-26)14-25-21(28)17-12-18(22)19(23)13-20(17)29-2/h6,11-13,16H,3-5,7-10,14,23H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.229 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM197999
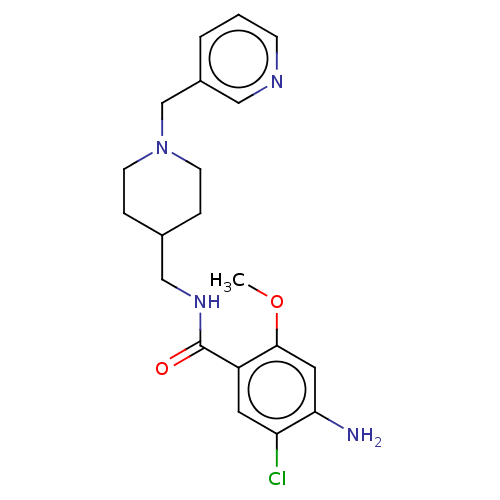
(US9221790, 9)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(Cc2cccnc2)CC1 Show InChI InChI=1S/C20H25ClN4O2/c1-27-19-10-18(22)17(21)9-16(19)20(26)24-12-14-4-7-25(8-5-14)13-15-3-2-6-23-11-15/h2-3,6,9-11,14H,4-5,7-8,12-13,22H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.267 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.362 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM198004
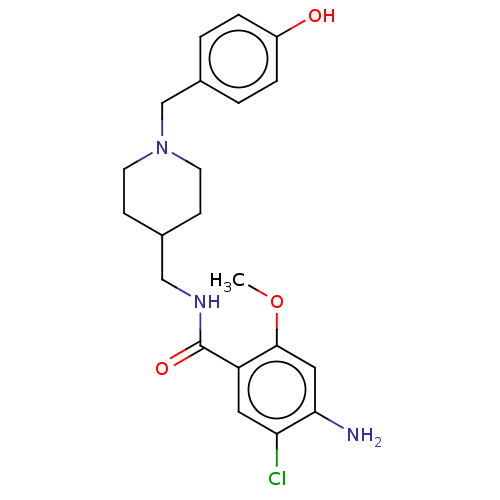
(US9221790, 14)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(Cc2ccc(O)cc2)CC1 Show InChI InChI=1S/C21H26ClN3O3/c1-28-20-11-19(23)18(22)10-17(20)21(27)24-12-14-6-8-25(9-7-14)13-15-2-4-16(26)5-3-15/h2-5,10-11,14,26H,6-9,12-13,23H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.375 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM198002
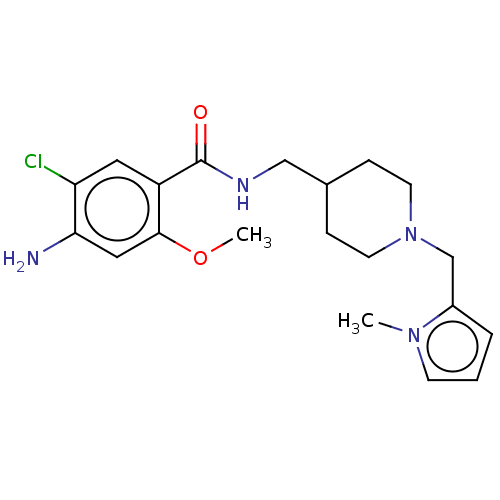
(US9221790, 12)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(Cc2cccn2C)CC1 Show InChI InChI=1S/C20H27ClN4O2/c1-24-7-3-4-15(24)13-25-8-5-14(6-9-25)12-23-20(26)16-10-17(21)18(22)11-19(16)27-2/h3-4,7,10-11,14H,5-6,8-9,12-13,22H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM198001
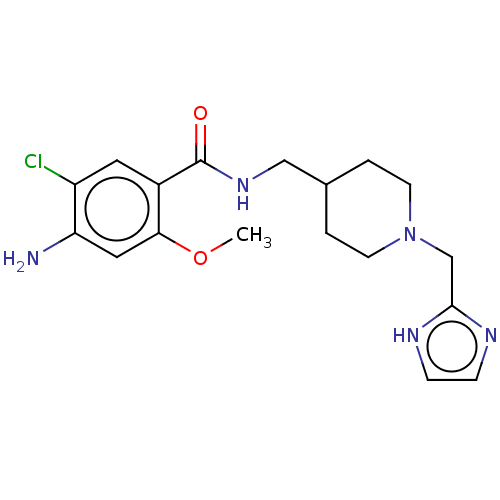
(US9221790, 11)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(Cc2ncc[nH]2)CC1 Show InChI InChI=1S/C18H24ClN5O2/c1-26-16-9-15(20)14(19)8-13(16)18(25)23-10-12-2-6-24(7-3-12)11-17-21-4-5-22-17/h4-5,8-9,12H,2-3,6-7,10-11,20H2,1H3,(H,21,22)(H,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.454 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd.
US Patent
| Assay Description
The binding affinity of the compounds for a human 5-HT4 receptor was assayed according to the method as disclosed in the literature [Wyngaert et al.,... |
US Patent US9221790 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MZP |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus) | BDBM50089194

((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...)Show SMILES CCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CO Show InChI InChI=1S/C19H35N3O5/c1-4-5-6-8-14(11-16(24)21-27)18(25)20-17(13(2)3)19(26)22-10-7-9-15(22)12-23/h13-15,17,23,27H,4-12H2,1-3H3,(H,20,25)(H,21,24)/t14-,15+,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus peptide deformylase using N-formylmethionine-alanine-serine as substrate by spectrophotometry |
J Nat Prod 75: 271-4 (2012)
Article DOI: 10.1021/np200720v
BindingDB Entry DOI: 10.7270/Q24B3299 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477454
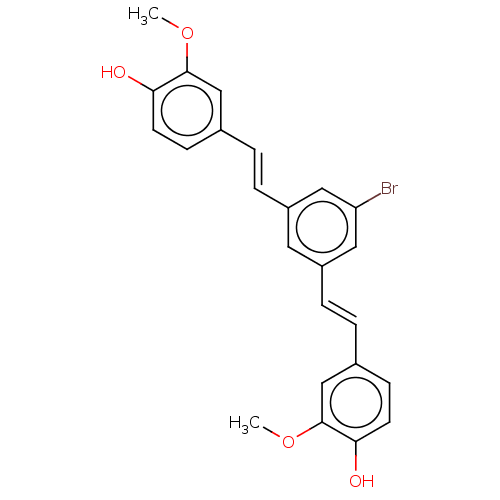
(CHEMBL245688)Show SMILES COc1cc(\C=C\c2cc(Br)cc(\C=C\c3ccc(O)c(OC)c3)c2)ccc1O Show InChI InChI=1S/C24H21BrO4/c1-28-23-14-16(7-9-21(23)26)3-5-18-11-19(13-20(25)12-18)6-4-17-8-10-22(27)24(15-17)29-2/h3-15,26-27H,1-2H3/b5-3+,6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311254

(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311256
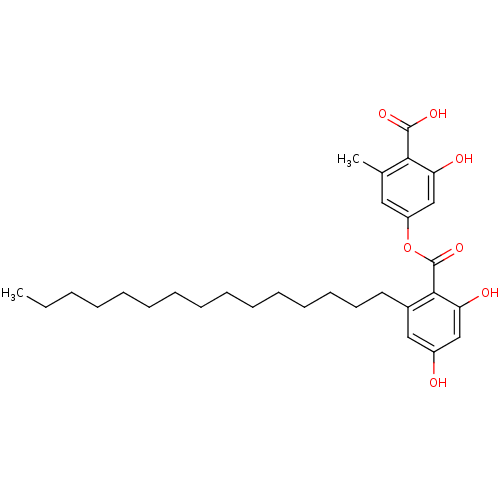
(4-(2,4-dihydroxy-6-pentadecylbenzoyloxy)-2-hydroxy...)Show SMILES CCCCCCCCCCCCCCCc1cc(O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 Show InChI InChI=1S/C30H42O7/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-22-18-23(31)19-25(32)28(22)30(36)37-24-17-21(2)27(29(34)35)26(33)20-24/h17-20,31-33H,3-16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
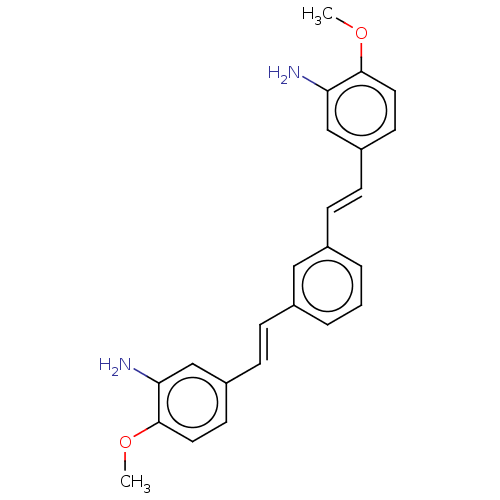
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477429
(CHEMBL248132)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(N)c3)c2)cc1N Show InChI InChI=1S/C24H24N2O2/c1-27-23-12-10-19(15-21(23)25)8-6-17-4-3-5-18(14-17)7-9-20-11-13-24(28-2)22(26)16-20/h3-16H,25-26H2,1-2H3/b8-6+,9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50309551
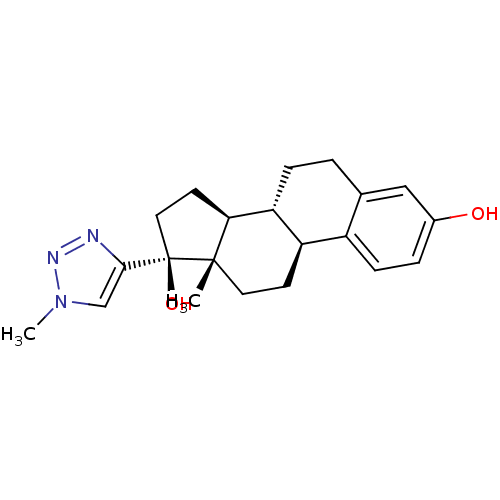
(17alpha-(17-1,2,3-Triazolmethyl)ethynyl-estra-1,3,...)Show SMILES Cn1cc(nn1)[C@]1(O)CC[C@H]2[C@@H]3CCc4cc(O)ccc4[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C21H27N3O2/c1-20-9-7-16-15-6-4-14(25)11-13(15)3-5-17(16)18(20)8-10-21(20,26)19-12-24(2)23-22-19/h4,6,11-12,16-18,25-26H,3,5,7-10H2,1-2H3/t16-,17-,18+,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha by liquid scintillation counting |
Bioorg Med Chem 18: 809-21 (2010)
Article DOI: 10.1016/j.bmc.2009.11.046
BindingDB Entry DOI: 10.7270/Q28K797G |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477447

(CHEMBL248327)Show SMILES CNc1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(NC)c3)n2)ccc1O Show InChI InChI=1S/C23H23N3O2/c1-24-20-14-16(8-12-22(20)27)6-10-18-4-3-5-19(26-18)11-7-17-9-13-23(28)21(15-17)25-2/h3-15,24-25,27-28H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477441

(CHEMBL247674)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(OC)c3)n2)cc1OC Show InChI InChI=1S/C25H27N3O2/c1-26-22-14-10-18(16-24(22)29-3)8-12-20-6-5-7-21(28-20)13-9-19-11-15-23(27-2)25(17-19)30-4/h5-17,26-27H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114333

(3-Methyl-but-2-enoic acid 4-{(S)-2-[(S)-2-((R)-2-a...)Show SMILES [#6]-[#8]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8]-[#6](=O)\[#6]=[#6](\[#6])-[#6])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6](-[#6])-[#6] Show InChI InChI=1S/C23H33N3O6S/c1-13(2)10-19(27)32-16-8-6-15(7-9-16)11-18(23(30)31-5)25-22(29)20(14(3)4)26-21(28)17(24)12-33/h6-10,14,17-18,20,33H,11-12,24H2,1-5H3,(H,25,29)(H,26,28)/t17-,18-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50222222
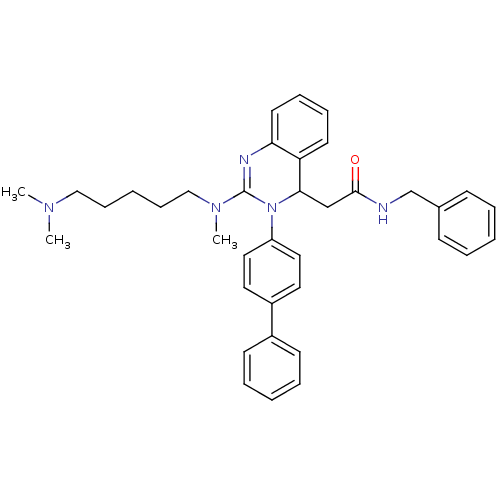
(CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...)Show SMILES CN(C)CCCCCN(C)C1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:10| Show InChI InChI=1S/C37H43N5O/c1-40(2)25-13-6-14-26-41(3)37-39-34-20-12-11-19-33(34)35(27-36(43)38-28-29-15-7-4-8-16-29)42(37)32-23-21-31(22-24-32)30-17-9-5-10-18-30/h4-5,7-12,15-24,35H,6,13-14,25-28H2,1-3H3,(H,38,43) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of T type calcium channel Cav3.1 (unknown origin) expressed in HEK293T cells by patch clamp method relative to control |
Citation and Details
Article DOI: 10.1016/j.bmcl.2015.12.010
BindingDB Entry DOI: 10.7270/Q22J6GJ0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50311254

(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311257

(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5S,6R)-3,4,5-t...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(O)=O |r| Show InChI InChI=1S/C28H46O9/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19-16-20(17-21(30)23(19)27(34)35)36-28-26(33)25(32)24(31)22(18-29)37-28/h16-17,22,24-26,28-33H,2-15,18H2,1H3,(H,34,35)/t22-,24-,25+,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477437
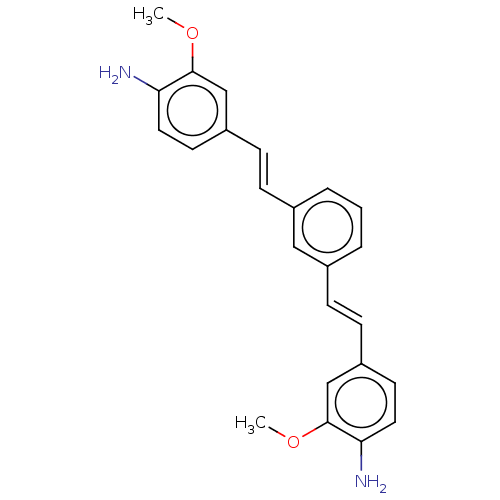
(CHEMBL247930)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N)c(OC)c3)c2)ccc1N Show InChI InChI=1S/C24H24N2O2/c1-27-23-15-19(10-12-21(23)25)8-6-17-4-3-5-18(14-17)7-9-20-11-13-22(26)24(16-20)28-2/h3-16H,25-26H2,1-2H3/b8-6+,9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477456
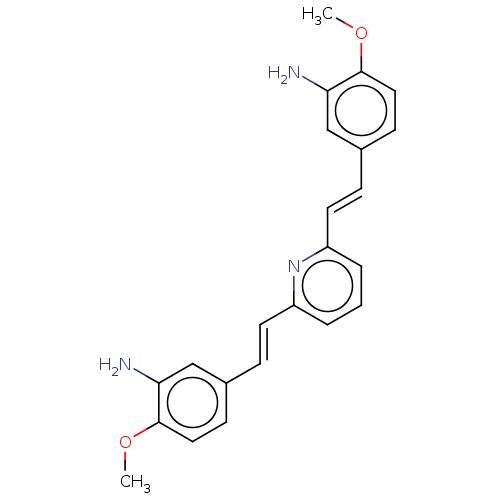
(CHEMBL392878)Show SMILES COc1ccc(\C=C\c2cccc(\C=C\c3ccc(OC)c(N)c3)n2)cc1N Show InChI InChI=1S/C23H23N3O2/c1-27-22-12-8-16(14-20(22)24)6-10-18-4-3-5-19(26-18)11-7-17-9-13-23(28-2)21(25)15-17/h3-15H,24-25H2,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50105462
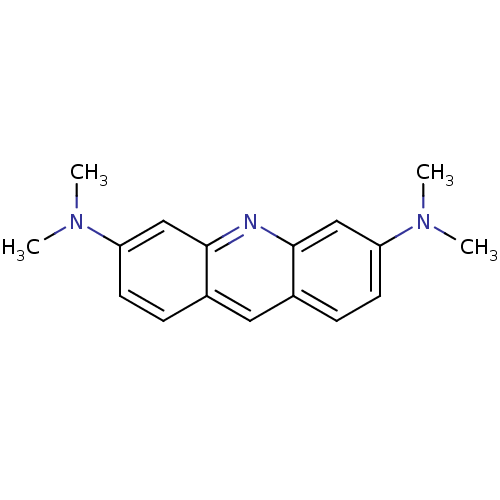
(CHEMBL81880 | N,N,N',N'-Tetramethyl-acridine-3,6-d...)Show InChI InChI=1S/C17H19N3/c1-19(2)14-7-5-12-9-13-6-8-15(20(3)4)11-17(13)18-16(12)10-14/h5-11H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477434

(CHEMBL247931)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(OC)c3)c2)cc1OC Show InChI InChI=1S/C26H28N2O2/c1-27-23-14-12-21(17-25(23)29-3)10-8-19-6-5-7-20(16-19)9-11-22-13-15-24(28-2)26(18-22)30-4/h5-18,27-28H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477428

(CHEMBL248131)Show SMILES CN(C)c1ccc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(O)c3)c2)cc1O Show InChI InChI=1S/C26H28N2O2/c1-27(2)23-14-12-21(17-25(23)29)10-8-19-6-5-7-20(16-19)9-11-22-13-15-24(28(3)4)26(30)18-22/h5-18,29-30H,1-4H3/b10-8+,11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477439

(CHEMBL247524)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N)c(OC)c3)n2)ccc1N Show InChI InChI=1S/C23H23N3O2/c1-27-22-14-16(8-12-20(22)24)6-10-18-4-3-5-19(26-18)11-7-17-9-13-21(25)23(15-17)28-2/h3-15H,24-25H2,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477435
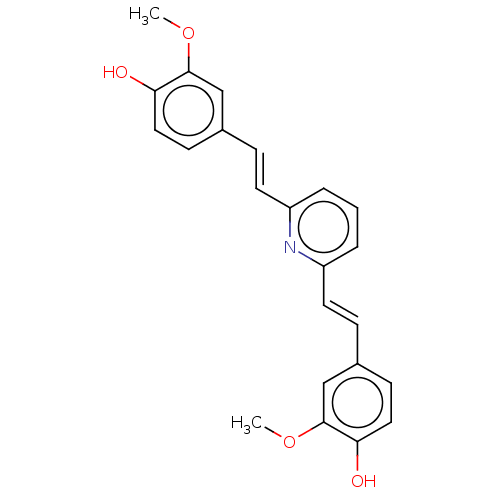
(CHEMBL247328)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(O)c(OC)c3)n2)ccc1O Show InChI InChI=1S/C23H21NO4/c1-27-22-14-16(8-12-20(22)25)6-10-18-4-3-5-19(24-18)11-7-17-9-13-21(26)23(15-17)28-2/h3-15,25-26H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50067040
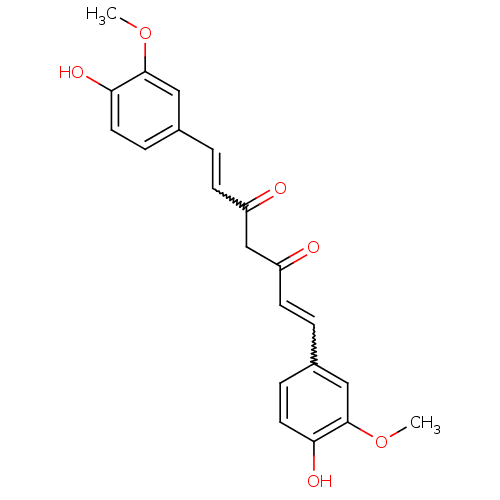
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477426

(CHEMBL391205)Show SMILES COc1cc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(OC)c3)n2)ccc1N(C)C Show InChI InChI=1S/C27H31N3O2/c1-29(2)24-16-12-20(18-26(24)31-5)10-14-22-8-7-9-23(28-22)15-11-21-13-17-25(30(3)4)27(19-21)32-6/h7-19H,1-6H3/b14-10+,15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50114334

(3-Methyl-butyric acid 4-{(S)-2-[(S)-2-((R)-2-amino...)Show SMILES COC(=O)[C@H](Cc1ccc(OC(=O)CC(C)C)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C23H35N3O6S/c1-13(2)10-19(27)32-16-8-6-15(7-9-16)11-18(23(30)31-5)25-22(29)20(14(3)4)26-21(28)17(24)12-33/h6-9,13-14,17-18,20,33H,10-12,24H2,1-5H3,(H,25,29)(H,26,28)/t17-,18-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
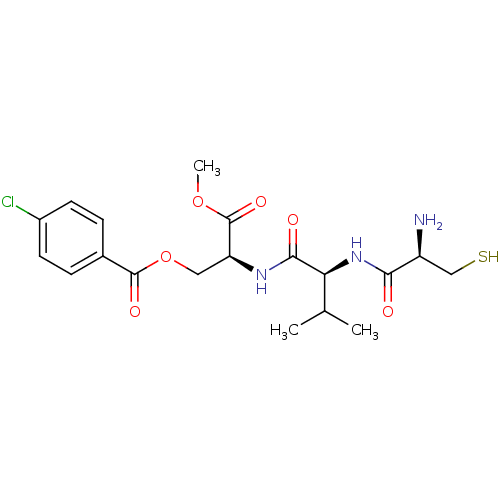
(Bos taurus (bovine)) | BDBM50114335
(4-Chloro-benzoic acid (S)-2-[(S)-2-((R)-2-amino-3-...)Show SMILES COC(=O)[C@H](COC(=O)c1ccc(Cl)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C Show InChI InChI=1S/C19H26ClN3O6S/c1-10(2)15(23-16(24)13(21)9-30)17(25)22-14(19(27)28-3)8-29-18(26)11-4-6-12(20)7-5-11/h4-7,10,13-15,30H,8-9,21H2,1-3H3,(H,22,25)(H,23,24)/t13-,14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry and School of Molecular Science(BK21)
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine Farnesyltrasferase. |
Bioorg Med Chem Lett 12: 1599-602 (2002)
BindingDB Entry DOI: 10.7270/Q24B30MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50294526
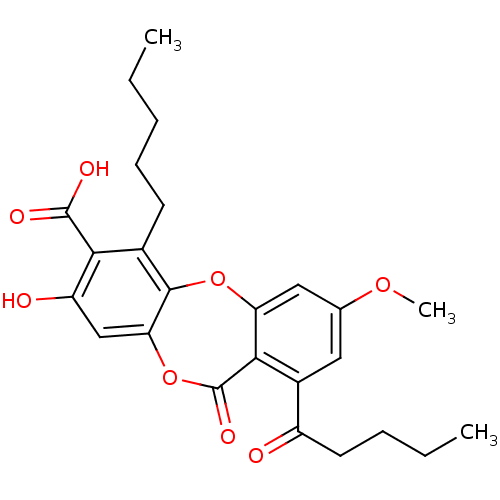
(CHEMBL551842 | cid_73157 | lobaric acid)Show SMILES CCCCCc1c2Oc3cc(OC)cc(C(=O)CCCC)c3C(=O)Oc2cc(O)c1C(O)=O Show InChI InChI=1S/C25H28O8/c1-4-6-8-9-15-21(24(28)29)18(27)13-20-23(15)32-19-12-14(31-3)11-16(17(26)10-7-5-2)22(19)25(30)33-20/h11-13,27H,4-10H2,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 19: 2801-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.108
BindingDB Entry DOI: 10.7270/Q20P10ZF |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
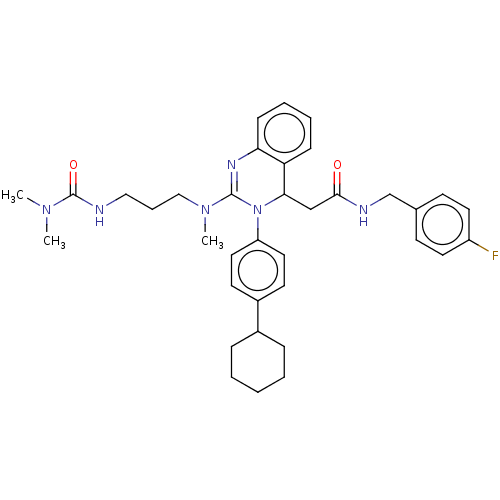
(Homo sapiens (Human)) | BDBM50558021
(CHEMBL4779125)Show SMILES CN(C)C(=O)NCCCN(C)C1=Nc2ccccc2C(CC(=O)NCc2ccc(F)cc2)N1c1ccc(cc1)C1CCCCC1 |t:11| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of T type calcium channel Cav3.1 (unknown origin) expressed in HEK293T cells by patch clamp method relative to control |
Citation and Details
Article DOI: 10.1016/j.bmcl.2015.12.010
BindingDB Entry DOI: 10.7270/Q22J6GJ0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477450
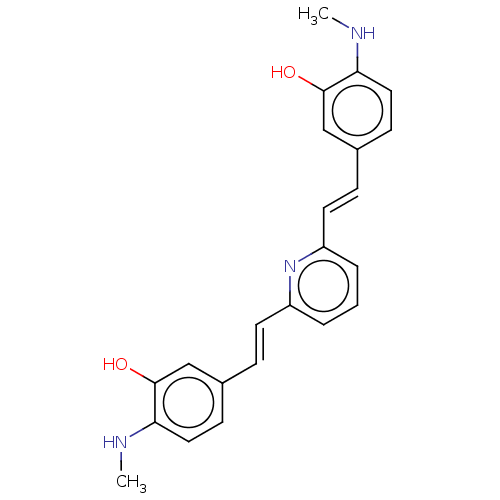
(CHEMBL247725)Show SMILES CNc1ccc(\C=C\c2cccc(\C=C\c3ccc(NC)c(O)c3)n2)cc1O Show InChI InChI=1S/C23H23N3O2/c1-24-20-12-8-16(14-22(20)27)6-10-18-4-3-5-19(26-18)11-7-17-9-13-21(25-2)23(28)15-17/h3-15,24-25,27-28H,1-2H3/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50477444
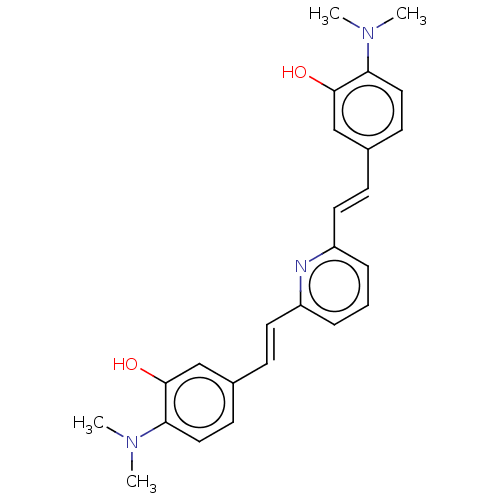
(CHEMBL247927)Show SMILES CN(C)c1ccc(\C=C\c2cccc(\C=C\c3ccc(N(C)C)c(O)c3)n2)cc1O Show InChI InChI=1S/C25H27N3O2/c1-27(2)22-14-10-18(16-24(22)29)8-12-20-6-5-7-21(26-20)13-9-19-11-15-23(28(3)4)25(30)17-19/h5-17,29-30H,1-4H3/b12-8+,13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta 42 fibril formation by thioflavin T assay |
Bioorg Med Chem Lett 17: 1466-70 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.090
BindingDB Entry DOI: 10.7270/Q2PV6P4S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data