 Found 346 hits with Last Name = 'tillett' and Initial = 'j'
Found 346 hits with Last Name = 'tillett' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098668
(4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...)Show SMILES CC(C)(C)NC[C@H](O)COc1cccc2[nH]c(=O)[nH]c12 Show InChI InChI=1S/C14H21N3O3/c1-14(2,3)15-7-9(18)8-20-11-6-4-5-10-12(11)17-13(19)16-10/h4-6,9,15,18H,7-8H2,1-3H3,(H2,16,17,19)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098668

(4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...)Show SMILES CC(C)(C)NC[C@H](O)COc1cccc2[nH]c(=O)[nH]c12 Show InChI InChI=1S/C14H21N3O3/c1-14(2,3)15-7-9(18)8-20-11-6-4-5-10-12(11)17-13(19)16-10/h4-6,9,15,18H,7-8H2,1-3H3,(H2,16,17,19)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50099151

(CHEMBL177442 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(2-oxo...)Show SMILES CN(C)C(=N)NC1=NC(=O)C(S1)=Cc1ccc(cc1)N1CCC(CC1)NC[C@H](O)COc1cccc2[nH]c(=O)[nH]c12 |w:12.13,t:6| Show InChI InChI=1S/C28H34N8O4S/c1-35(2)26(29)34-28-33-25(38)23(41-28)14-17-6-8-19(9-7-17)36-12-10-18(11-13-36)30-15-20(37)16-40-22-5-3-4-21-24(22)32-27(39)31-21/h3-9,14,18,20,30,37H,10-13,15-16H2,1-2H3,(H2,31,32,39)(H2,29,33,34,38)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor |
Bioorg Med Chem Lett 11: 981-4 (2001)
BindingDB Entry DOI: 10.7270/Q2125RZ5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50099151

(CHEMBL177442 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(2-oxo...)Show SMILES CN(C)C(=N)NC1=NC(=O)C(S1)=Cc1ccc(cc1)N1CCC(CC1)NC[C@H](O)COc1cccc2[nH]c(=O)[nH]c12 |w:12.13,t:6| Show InChI InChI=1S/C28H34N8O4S/c1-35(2)26(29)34-28-33-25(38)23(41-28)14-17-6-8-19(9-7-17)36-12-10-18(11-13-36)30-15-20(37)16-40-22-5-3-4-21-24(22)32-27(39)31-21/h3-9,14,18,20,30,37H,10-13,15-16H2,1-2H3,(H2,31,32,39)(H2,29,33,34,38)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor |
Bioorg Med Chem Lett 11: 981-4 (2001)
BindingDB Entry DOI: 10.7270/Q2125RZ5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50070156
((S)-4-(3-hexylureido)-N-(4-(2-(1-hydroxy-2-(4-hydr...)Show SMILES CCCCCCNC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(CCNC[C@H](O)COc2ccc(O)cc2)cc1 Show InChI InChI=1S/C30H40N4O6S/c1-2-3-4-5-19-32-30(37)33-24-10-16-29(17-11-24)41(38,39)34-25-8-6-23(7-9-25)18-20-31-21-27(36)22-40-28-14-12-26(35)13-15-28/h6-17,27,31,34-36H,2-5,18-22H2,1H3,(H2,32,33,37)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098662
(2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C30H36N4O7S/c1-42(40,41)33-25-18-22(9-12-27(25)35)28(36)19-31-23-13-15-34(16-14-23)24-10-7-21(8-11-24)29(37)32-26(30(38)39)17-20-5-3-2-4-6-20/h2-12,18,23,26,28,31,33,35-36H,13-17,19H2,1H3,(H,32,37)(H,38,39)/t26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50070156

((S)-4-(3-hexylureido)-N-(4-(2-(1-hydroxy-2-(4-hydr...)Show SMILES CCCCCCNC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(CCNC[C@H](O)COc2ccc(O)cc2)cc1 Show InChI InChI=1S/C30H40N4O6S/c1-2-3-4-5-19-32-30(37)33-24-10-16-29(17-11-24)41(38,39)34-25-8-6-23(7-9-25)18-20-31-21-27(36)22-40-28-14-12-26(35)13-15-28/h6-17,27,31,34-36H,2-5,18-22H2,1H3,(H2,32,33,37)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50099160

(CHEMBL354906 | [5-(4-{4-[(R)-2-Hydroxy-2-(4-hydrox...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(Cc2sc(=O)n(CC(O)=O)c2O)cc1 Show InChI InChI=1S/C26H32N4O8S2/c1-40(37,38)28-20-13-17(4-7-21(20)31)22(32)14-27-18-8-10-29(11-9-18)19-5-2-16(3-6-19)12-23-25(35)30(15-24(33)34)26(36)39-23/h2-7,13,18,22,27-28,31-32,35H,8-12,14-15H2,1H3,(H,33,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor |
Bioorg Med Chem Lett 11: 981-4 (2001)
BindingDB Entry DOI: 10.7270/Q2125RZ5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098659
(2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N1CCC(CC1)NC[C@H](O)c1ccc(O)c(NS(C)(=O)=O)c1)C(O)=O Show InChI InChI=1S/C27H38N4O7S/c1-17(2)14-23(27(35)36)29-26(34)18-4-7-21(8-5-18)31-12-10-20(11-13-31)28-16-25(33)19-6-9-24(32)22(15-19)30-39(3,37)38/h4-9,15,17,20,23,25,28,30,32-33H,10-14,16H2,1-3H3,(H,29,34)(H,35,36)/t23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098661
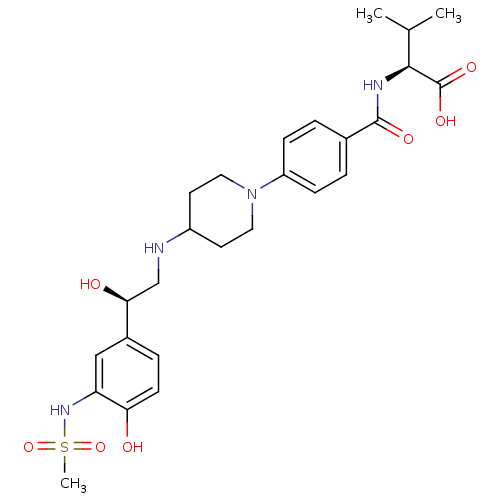
(2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)N1CCC(CC1)NC[C@H](O)c1ccc(O)c(NS(C)(=O)=O)c1)C(O)=O Show InChI InChI=1S/C26H36N4O7S/c1-16(2)24(26(34)35)28-25(33)17-4-7-20(8-5-17)30-12-10-19(11-13-30)27-15-23(32)18-6-9-22(31)21(14-18)29-38(3,36)37/h4-9,14,16,19,23-24,27,29,31-32H,10-13,15H2,1-3H3,(H,28,33)(H,34,35)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50099160

(CHEMBL354906 | [5-(4-{4-[(R)-2-Hydroxy-2-(4-hydrox...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(Cc2sc(=O)n(CC(O)=O)c2O)cc1 Show InChI InChI=1S/C26H32N4O8S2/c1-40(37,38)28-20-13-17(4-7-21(20)31)22(32)14-27-18-8-10-29(11-9-18)19-5-2-16(3-6-19)12-23-25(35)30(15-24(33)34)26(36)39-23/h2-7,13,18,22,27-28,31-32,35H,8-12,14-15H2,1H3,(H,33,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor |
Bioorg Med Chem Lett 11: 981-4 (2001)
BindingDB Entry DOI: 10.7270/Q2125RZ5 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50099150
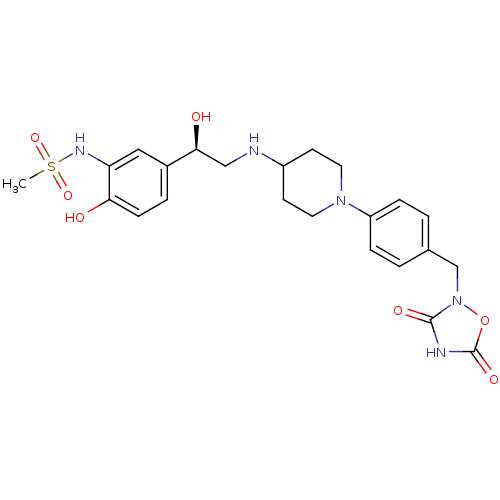
(CHEMBL368584 | N-[5-(2-{1-[4-((R)-3,5-Dioxo-[1,2,4...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(Cn2oc(=O)[nH]c2=O)cc1 Show InChI InChI=1S/C23H29N5O7S/c1-36(33,34)26-19-12-16(4-7-20(19)29)21(30)13-24-17-8-10-27(11-9-17)18-5-2-15(3-6-18)14-28-22(31)25-23(32)35-28/h2-7,12,17,21,24,26,29-30H,8-11,13-14H2,1H3,(H,25,31,32)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 9.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor |
Bioorg Med Chem Lett 11: 981-4 (2001)
BindingDB Entry DOI: 10.7270/Q2125RZ5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098654

((4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonylami...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(cc1)C(=O)NCC(O)=O Show InChI InChI=1S/C23H30N4O7S/c1-35(33,34)26-19-12-16(4-7-20(19)28)21(29)13-24-17-8-10-27(11-9-17)18-5-2-15(3-6-18)23(32)25-14-22(30)31/h2-7,12,17,21,24,26,28-29H,8-11,13-14H2,1H3,(H,25,32)(H,30,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50099150

(CHEMBL368584 | N-[5-(2-{1-[4-((R)-3,5-Dioxo-[1,2,4...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(Cn2oc(=O)[nH]c2=O)cc1 Show InChI InChI=1S/C23H29N5O7S/c1-36(33,34)26-19-12-16(4-7-20(19)29)21(30)13-24-17-8-10-27(11-9-17)18-5-2-15(3-6-18)14-28-22(31)25-23(32)35-28/h2-7,12,17,21,24,26,29-30H,8-11,13-14H2,1H3,(H,25,31,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor |
Bioorg Med Chem Lett 11: 981-4 (2001)
BindingDB Entry DOI: 10.7270/Q2125RZ5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002134
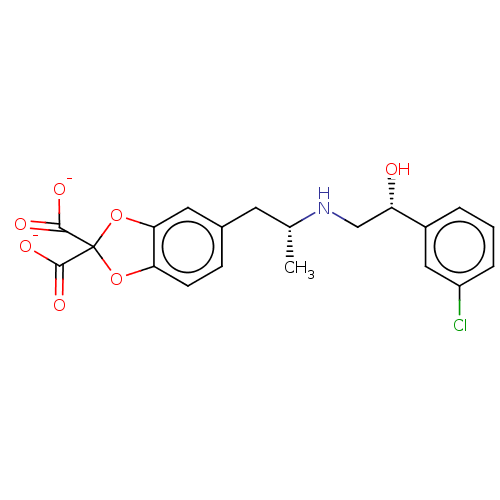
(5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...)Show SMILES C[C@H](Cc1ccc2OC(Oc2c1)(C([O-])=O)C([O-])=O)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C20H20ClNO7/c1-11(22-10-15(23)13-3-2-4-14(21)9-13)7-12-5-6-16-17(8-12)29-20(28-16,18(24)25)19(26)27/h2-6,8-9,11,15,22-23H,7,10H2,1H3,(H,24,25)(H,26,27)/p-2/t11-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098659

(2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N1CCC(CC1)NC[C@H](O)c1ccc(O)c(NS(C)(=O)=O)c1)C(O)=O Show InChI InChI=1S/C27H38N4O7S/c1-17(2)14-23(27(35)36)29-26(34)18-4-7-21(8-5-18)31-12-10-20(11-13-31)28-16-25(33)19-6-9-24(32)22(15-19)30-39(3,37)38/h4-9,15,17,20,23,25,28,30,32-33H,10-14,16H2,1-3H3,(H,29,34)(H,35,36)/t23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098654

((4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonylami...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(cc1)C(=O)NCC(O)=O Show InChI InChI=1S/C23H30N4O7S/c1-35(33,34)26-19-12-16(4-7-20(19)28)21(29)13-24-17-8-10-27(11-9-17)18-5-2-15(3-6-18)23(32)25-14-22(30)31/h2-7,12,17,21,24,26,28-29H,8-11,13-14H2,1H3,(H,25,32)(H,30,31)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098662

(2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...)Show SMILES CS(=O)(=O)Nc1cc(ccc1O)[C@@H](O)CNC1CCN(CC1)c1ccc(cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C30H36N4O7S/c1-42(40,41)33-25-18-22(9-12-27(25)35)28(36)19-31-23-13-15-34(16-14-23)24-10-7-21(8-11-24)29(37)32-26(30(38)39)17-20-5-3-2-4-6-20/h2-12,18,23,26,28,31,33,35-36H,13-17,19H2,1H3,(H,32,37)(H,38,39)/t26-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002134

(5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...)Show SMILES C[C@H](Cc1ccc2OC(Oc2c1)(C([O-])=O)C([O-])=O)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C20H20ClNO7/c1-11(22-10-15(23)13-3-2-4-14(21)9-13)7-12-5-6-16-17(8-12)29-20(28-16,18(24)25)19(26)27/h2-6,8-9,11,15,22-23H,7,10H2,1H3,(H,24,25)(H,26,27)/p-2/t11-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098658
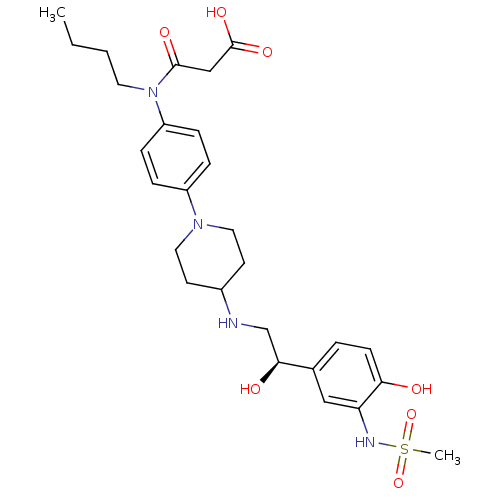
(CHEMBL418600 | N-Butyl-N-(4-{4-[2-hydroxy-2-(4-hyd...)Show SMILES CCCCN(C(=O)CC(O)=O)c1ccc(cc1)N1CCC(CC1)NC[C@H](O)c1ccc(O)c(NS(C)(=O)=O)c1 Show InChI InChI=1S/C27H38N4O7S/c1-3-4-13-31(26(34)17-27(35)36)22-8-6-21(7-9-22)30-14-11-20(12-15-30)28-18-25(33)19-5-10-24(32)23(16-19)29-39(2,37)38/h5-10,16,20,25,28-29,32-33H,3-4,11-15,17-18H2,1-2H3,(H,35,36)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098661

(2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)N1CCC(CC1)NC[C@H](O)c1ccc(O)c(NS(C)(=O)=O)c1)C(O)=O Show InChI InChI=1S/C26H36N4O7S/c1-16(2)24(26(34)35)28-25(33)17-4-7-20(8-5-17)30-12-10-19(11-13-30)27-15-23(32)18-6-9-22(31)21(14-18)29-38(3,36)37/h4-9,14,16,19,23-24,27,29,31-32H,10-13,15H2,1-3H3,(H,28,33)(H,34,35)/t23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098658

(CHEMBL418600 | N-Butyl-N-(4-{4-[2-hydroxy-2-(4-hyd...)Show SMILES CCCCN(C(=O)CC(O)=O)c1ccc(cc1)N1CCC(CC1)NC[C@H](O)c1ccc(O)c(NS(C)(=O)=O)c1 Show InChI InChI=1S/C27H38N4O7S/c1-3-4-13-31(26(34)17-27(35)36)22-8-6-21(7-9-22)30-14-11-20(12-15-30)28-18-25(33)19-5-10-24(32)23(16-19)29-39(2,37)38/h5-10,16,20,25,28-29,32-33H,3-4,11-15,17-18H2,1-2H3,(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128643
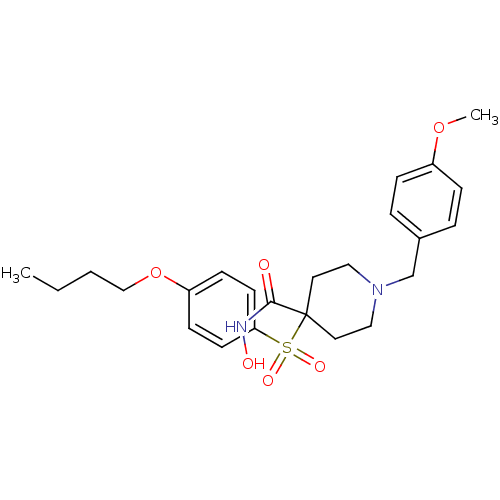
(1-(4-methoxybenzyl)-4-(4-butoxyphenylsulfonyl)-N-h...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2ccc(OC)cc2)CC1)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-3-4-17-32-21-9-11-22(12-10-21)33(29,30)24(23(27)25-28)13-15-26(16-14-24)18-19-5-7-20(31-2)8-6-19/h5-12,28H,3-4,13-18H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128649
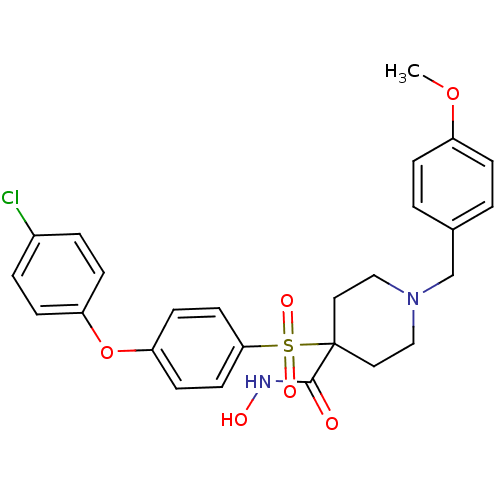
(1-(4-methoxybenzyl)-4-(4-(4-chlorophenoxy)phenylsu...)Show SMILES COc1ccc(CN2CCC(CC2)(C(=O)NO)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)cc1 Show InChI InChI=1S/C26H27ClN2O6S/c1-34-21-6-2-19(3-7-21)18-29-16-14-26(15-17-29,25(30)28-31)36(32,33)24-12-10-23(11-13-24)35-22-8-4-20(27)5-9-22/h2-13,31H,14-18H2,1H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-9. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128645
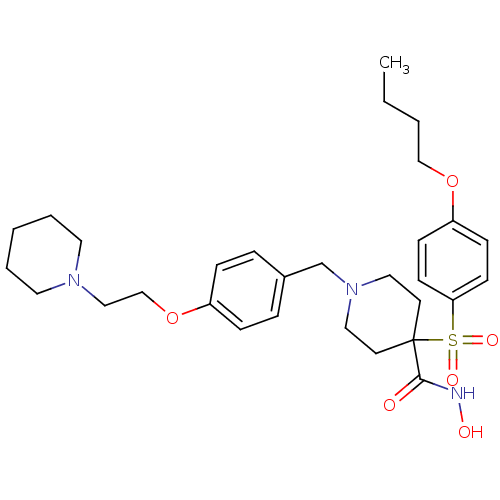
(1-(4-(2-(piperidin-1-yl)ethoxy)benzyl)-4-(4-butoxy...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2ccc(OCCN3CCCCC3)cc2)CC1)C(=O)NO Show InChI InChI=1S/C30H43N3O6S/c1-2-3-22-38-27-11-13-28(14-12-27)40(36,37)30(29(34)31-35)15-19-33(20-16-30)24-25-7-9-26(10-8-25)39-23-21-32-17-5-4-6-18-32/h7-14,35H,2-6,15-24H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128649

(1-(4-methoxybenzyl)-4-(4-(4-chlorophenoxy)phenylsu...)Show SMILES COc1ccc(CN2CCC(CC2)(C(=O)NO)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)cc1 Show InChI InChI=1S/C26H27ClN2O6S/c1-34-21-6-2-19(3-7-21)18-29-16-14-26(15-17-29,25(30)28-31)36(32,33)24-12-10-23(11-13-24)35-22-8-4-20(27)5-9-22/h2-13,31H,14-18H2,1H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128673
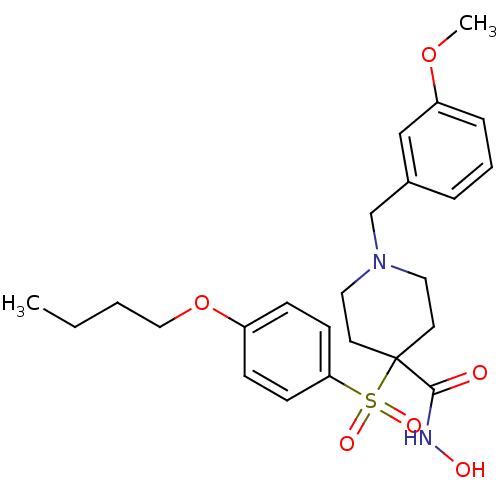
(1-(3-methoxybenzyl)-4-(4-butoxyphenylsulfonyl)-N-h...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2cccc(OC)c2)CC1)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-3-4-16-32-20-8-10-22(11-9-20)33(29,30)24(23(27)25-28)12-14-26(15-13-24)18-19-6-5-7-21(17-19)31-2/h5-11,17,28H,3-4,12-16,18H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128668
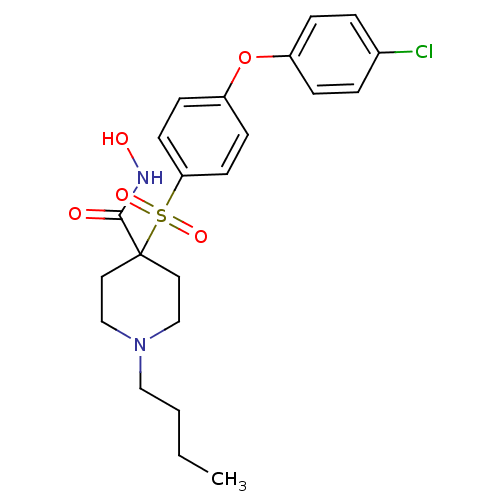
(1-Butyl-4-[4-(4-chloro-phenoxy)-benzenesulfonyl]-p...)Show SMILES CCCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H27ClN2O5S/c1-2-3-14-25-15-12-22(13-16-25,21(26)24-27)31(28,29)20-10-8-19(9-11-20)30-18-6-4-17(23)5-7-18/h4-11,27H,2-3,12-16H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-9. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128667
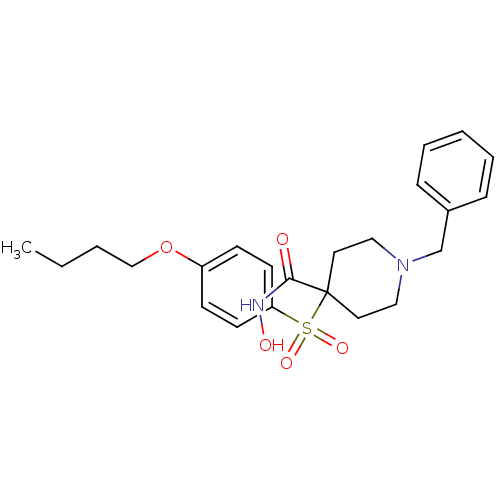
(1-Benzyl-4-(4-butoxy-benzenesulfonyl)-piperidine-4...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2ccccc2)CC1)C(=O)NO Show InChI InChI=1S/C23H30N2O5S/c1-2-3-17-30-20-9-11-21(12-10-20)31(28,29)23(22(26)24-27)13-15-25(16-14-23)18-19-7-5-4-6-8-19/h4-12,27H,2-3,13-18H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128648
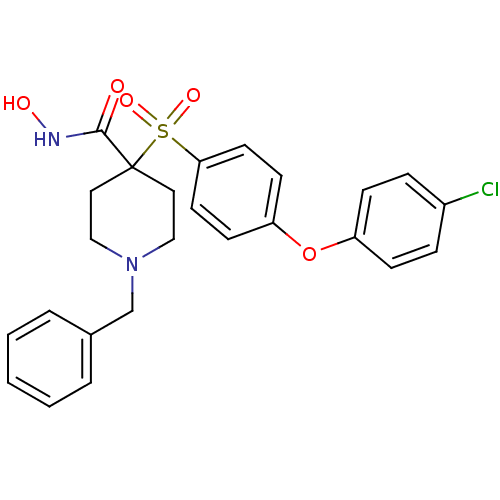
(1-Benzyl-4-[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES ONC(=O)C1(CCN(Cc2ccccc2)CC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H25ClN2O5S/c26-20-6-8-21(9-7-20)33-22-10-12-23(13-11-22)34(31,32)25(24(29)27-30)14-16-28(17-15-25)18-19-4-2-1-3-5-19/h1-13,30H,14-18H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-9. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50128648

(1-Benzyl-4-[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES ONC(=O)C1(CCN(Cc2ccccc2)CC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H25ClN2O5S/c26-20-6-8-21(9-7-20)33-22-10-12-23(13-11-22)34(31,32)25(24(29)27-30)14-16-28(17-15-25)18-19-4-2-1-3-5-19/h1-13,30H,14-18H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-8 |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128668

(1-Butyl-4-[4-(4-chloro-phenoxy)-benzenesulfonyl]-p...)Show SMILES CCCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H27ClN2O5S/c1-2-3-14-25-15-12-22(13-16-25,21(26)24-27)31(28,29)20-10-8-19(9-11-20)30-18-6-4-17(23)5-7-18/h4-11,27H,2-3,12-16H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128654
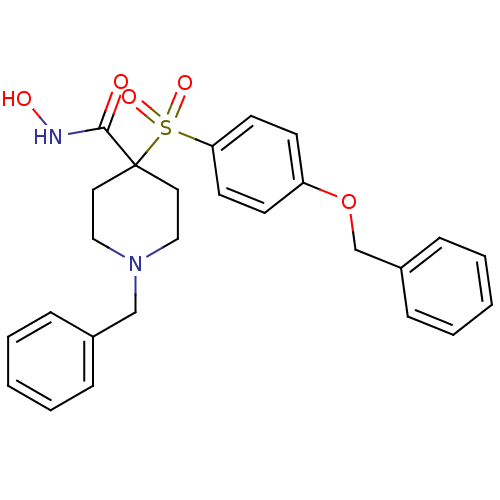
(1-Benzyl-4-(4-benzyloxy-benzenesulfonyl)-piperidin...)Show SMILES ONC(=O)C1(CCN(Cc2ccccc2)CC1)S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C26H28N2O5S/c29-25(27-30)26(15-17-28(18-16-26)19-21-7-3-1-4-8-21)34(31,32)24-13-11-23(12-14-24)33-20-22-9-5-2-6-10-22/h1-14,30H,15-20H2,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128631
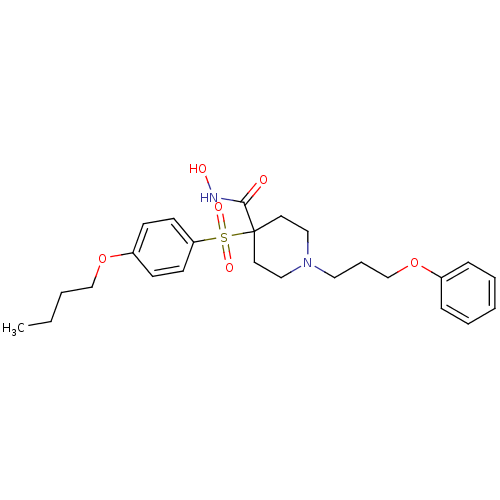
(4-(4-Butoxy-benzenesulfonyl)-1-(3-phenoxy-propyl)-...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(CCCOc2ccccc2)CC1)C(=O)NO Show InChI InChI=1S/C25H34N2O6S/c1-2-3-19-32-22-10-12-23(13-11-22)34(30,31)25(24(28)26-29)14-17-27(18-15-25)16-7-20-33-21-8-5-4-6-9-21/h4-6,8-13,29H,2-3,7,14-20H2,1H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128658
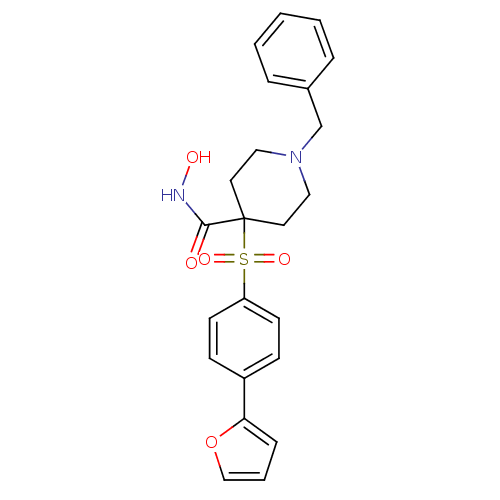
(1-Benzyl-4-(4-furan-2-yl-benzenesulfonyl)-piperidi...)Show SMILES ONC(=O)C1(CCN(Cc2ccccc2)CC1)S(=O)(=O)c1ccc(cc1)-c1ccco1 Show InChI InChI=1S/C23H24N2O5S/c26-22(24-27)23(12-14-25(15-13-23)17-18-5-2-1-3-6-18)31(28,29)20-10-8-19(9-11-20)21-7-4-16-30-21/h1-11,16,27H,12-15,17H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128651
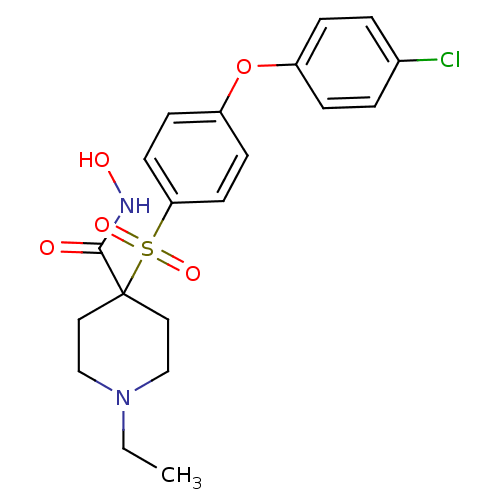
(4-(4-(4-chlorophenoxy)phenylsulfonyl)-1-ethyl-N-hy...)Show SMILES CCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C20H23ClN2O5S/c1-2-23-13-11-20(12-14-23,19(24)22-25)29(26,27)18-9-7-17(8-10-18)28-16-5-3-15(21)4-6-16/h3-10,25H,2,11-14H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-9. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128648

(1-Benzyl-4-[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES ONC(=O)C1(CCN(Cc2ccccc2)CC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H25ClN2O5S/c26-20-6-8-21(9-7-20)33-22-10-12-23(13-11-22)34(31,32)25(24(29)27-30)14-16-28(17-15-25)18-19-4-2-1-3-5-19/h1-13,30H,14-18H2,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50128648

(1-Benzyl-4-[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES ONC(=O)C1(CCN(Cc2ccccc2)CC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H25ClN2O5S/c26-20-6-8-21(9-7-20)33-22-10-12-23(13-11-22)34(31,32)25(24(29)27-30)14-16-28(17-15-25)18-19-4-2-1-3-5-19/h1-13,30H,14-18H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-2 |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128597
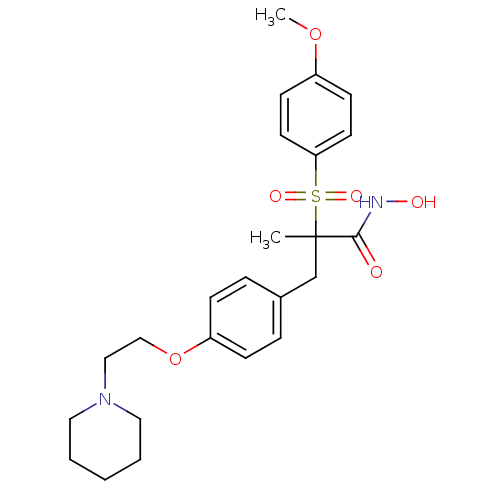
((R)-N-hydroxy-2-(4-methoxyphenylsulfonyl)-2-methyl...)Show SMILES COc1ccc(cc1)S(=O)(=O)C(C)(Cc1ccc(OCCN2CCCCC2)cc1)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-24(23(27)25-28,33(29,30)22-12-10-20(31-2)11-13-22)18-19-6-8-21(9-7-19)32-17-16-26-14-4-3-5-15-26/h6-13,28H,3-5,14-18H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128654

(1-Benzyl-4-(4-benzyloxy-benzenesulfonyl)-piperidin...)Show SMILES ONC(=O)C1(CCN(Cc2ccccc2)CC1)S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C26H28N2O5S/c29-25(27-30)26(15-17-28(18-16-26)19-21-7-3-1-4-8-21)34(31,32)24-13-11-23(12-14-24)33-20-22-9-5-2-6-10-22/h1-14,30H,15-20H2,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128651

(4-(4-(4-chlorophenoxy)phenylsulfonyl)-1-ethyl-N-hy...)Show SMILES CCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C20H23ClN2O5S/c1-2-23-13-11-20(12-14-23,19(24)22-25)29(26,27)18-9-7-17(8-10-18)28-16-5-3-15(21)4-6-16/h3-10,25H,2,11-14H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128659
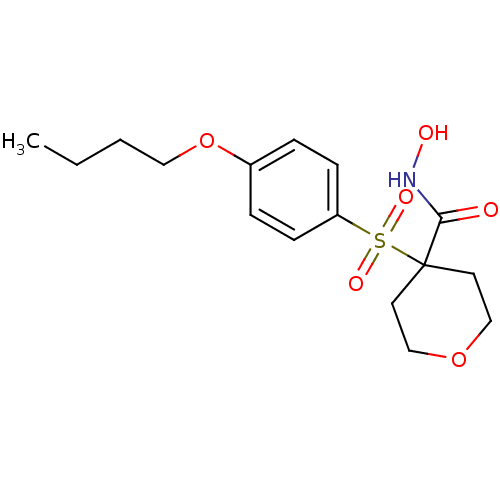
(4-(4-Butoxy-benzenesulfonyl)-tetrahydro-pyran-4-ca...)Show InChI InChI=1S/C16H23NO6S/c1-2-3-10-23-13-4-6-14(7-5-13)24(20,21)16(15(18)17-19)8-11-22-12-9-16/h4-7,19H,2-3,8-12H2,1H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128640
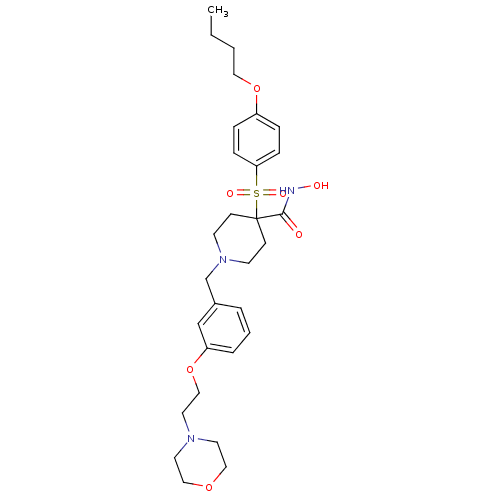
(4-(4-Butoxy-benzenesulfonyl)-1-[3-(2-morpholin-4-y...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2cccc(OCCN3CCOCC3)c2)CC1)C(=O)NO Show InChI InChI=1S/C29H41N3O7S/c1-2-3-18-38-25-7-9-27(10-8-25)40(35,36)29(28(33)30-34)11-13-32(14-12-29)23-24-5-4-6-26(22-24)39-21-17-31-15-19-37-20-16-31/h4-10,22,34H,2-3,11-21,23H2,1H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128645

(1-(4-(2-(piperidin-1-yl)ethoxy)benzyl)-4-(4-butoxy...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2ccc(OCCN3CCCCC3)cc2)CC1)C(=O)NO Show InChI InChI=1S/C30H43N3O6S/c1-2-3-22-38-27-11-13-28(14-12-27)40(36,37)30(29(34)31-35)15-19-33(20-16-30)24-25-7-9-26(10-8-25)39-23-21-32-17-5-4-6-18-32/h7-14,35H,2-6,15-24H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-9. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128656
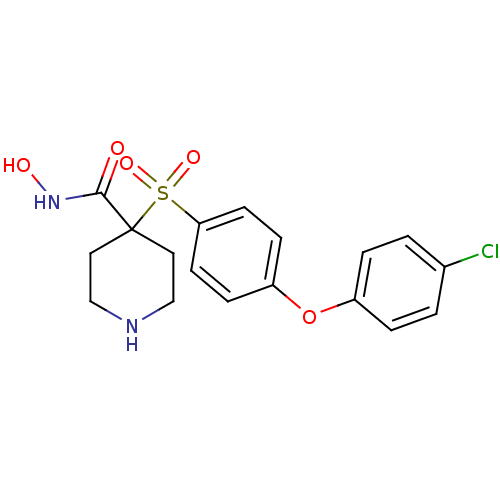
(4-(4-(4-chlorophenoxy)phenylsulfonyl)-N-hydroxypip...)Show SMILES ONC(=O)C1(CCNCC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H19ClN2O5S/c19-13-1-3-14(4-2-13)26-15-5-7-16(8-6-15)27(24,25)18(17(22)21-23)9-11-20-12-10-18/h1-8,20,23H,9-12H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-9. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128656

(4-(4-(4-chlorophenoxy)phenylsulfonyl)-N-hydroxypip...)Show SMILES ONC(=O)C1(CCNCC1)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H19ClN2O5S/c19-13-1-3-14(4-2-13)26-15-5-7-16(8-6-15)27(24,25)18(17(22)21-23)9-11-20-12-10-18/h1-8,20,23H,9-12H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128640

(4-(4-Butoxy-benzenesulfonyl)-1-[3-(2-morpholin-4-y...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2cccc(OCCN3CCOCC3)c2)CC1)C(=O)NO Show InChI InChI=1S/C29H41N3O7S/c1-2-3-18-38-25-7-9-27(10-8-25)40(35,36)29(28(33)30-34)11-13-32(14-12-29)23-24-5-4-6-26(22-24)39-21-17-31-15-19-37-20-16-31/h4-10,22,34H,2-3,11-21,23H2,1H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-9. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128657
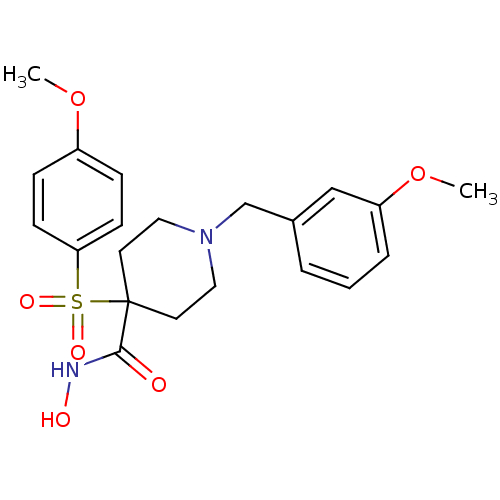
(1-(3-methoxybenzyl)-N-hydroxy-4-(4-methoxyphenylsu...)Show SMILES COc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2cccc(OC)c2)CC1)C(=O)NO Show InChI InChI=1S/C21H26N2O6S/c1-28-17-6-8-19(9-7-17)30(26,27)21(20(24)22-25)10-12-23(13-11-21)15-16-4-3-5-18(14-16)29-2/h3-9,14,25H,10-13,15H2,1-2H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128630
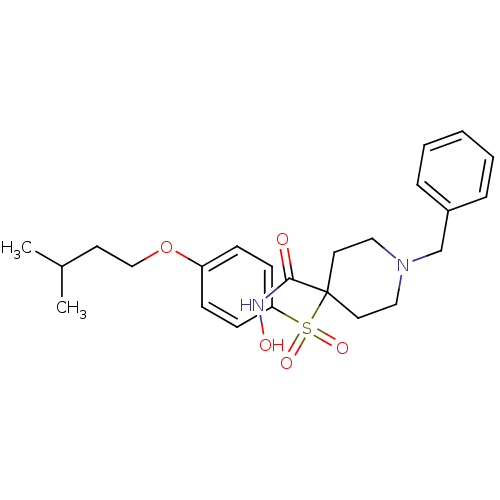
(1-Benzyl-4-[4-(3-methyl-butoxy)-benzenesulfonyl]-p...)Show SMILES CC(C)CCOc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2ccccc2)CC1)C(=O)NO Show InChI InChI=1S/C24H32N2O5S/c1-19(2)12-17-31-21-8-10-22(11-9-21)32(29,30)24(23(27)25-28)13-15-26(16-14-24)18-20-6-4-3-5-7-20/h3-11,19,28H,12-18H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50128637
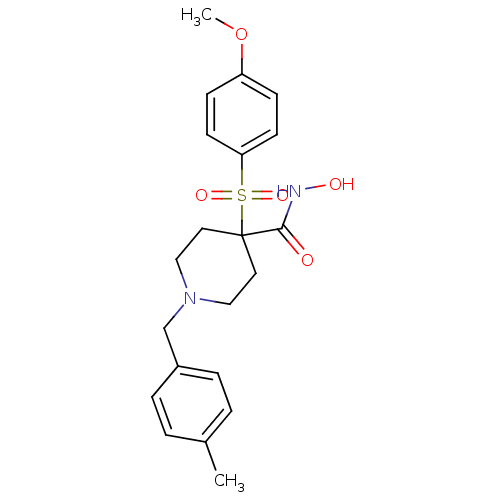
(4-(4-Methoxy-benzenesulfonyl)-1-(4-methyl-benzyl)-...)Show SMILES COc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2ccc(C)cc2)CC1)C(=O)NO Show InChI InChI=1S/C21H26N2O5S/c1-16-3-5-17(6-4-16)15-23-13-11-21(12-14-23,20(24)22-25)29(26,27)19-9-7-18(28-2)8-10-19/h3-10,25H,11-15H2,1-2H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13. |
J Med Chem 46: 2376-96 (2003)
Article DOI: 10.1021/jm0205550
BindingDB Entry DOI: 10.7270/Q2N0178C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data