 Found 21740 hits with Last Name = 'tu' and Initial = 'j'
Found 21740 hits with Last Name = 'tu' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548
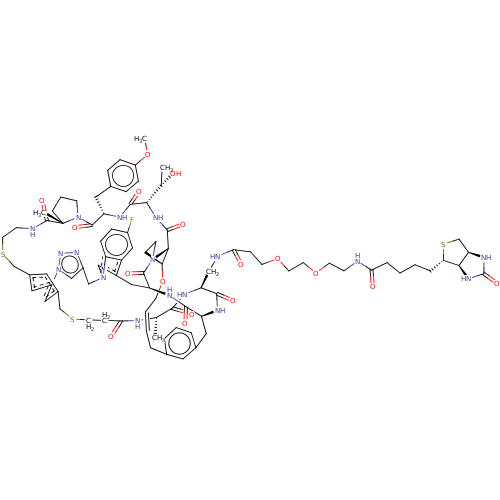
(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547
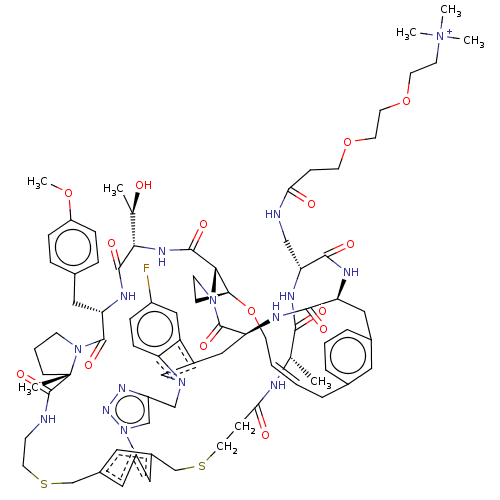
(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546
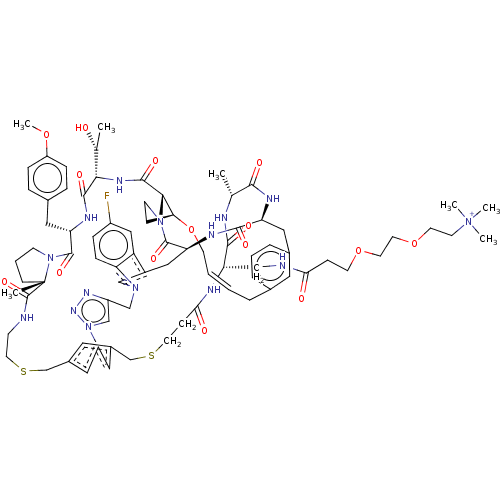
(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50056769
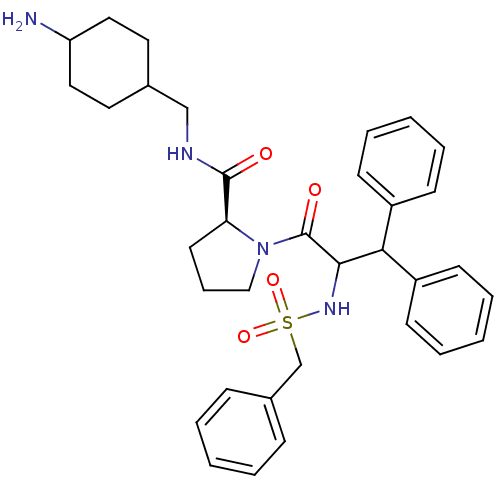
((S)-1-(3,3-Diphenyl-2-phenylmethanesulfonylamino-p...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)C(NS(=O)(=O)Cc2ccccc2)C(c2ccccc2)c2ccccc2)CC1 |wU:9.8,(16.05,-4.09,;16.82,-5.42,;16.81,-7.08,;17.34,-8.54,;16.12,-9.36,;16.57,-10.83,;15.52,-11.95,;14.02,-11.6,;12.97,-12.72,;13.56,-10.13,;14.5,-8.9,;13.63,-7.64,;12.11,-8.1,;12.12,-9.64,;10.86,-10.51,;10.97,-12.05,;9.46,-9.85,;8.2,-10.74,;6.87,-11.5,;6.09,-10.16,;7.64,-12.86,;5.51,-12.28,;4.18,-11.51,;4.18,-9.97,;2.85,-9.2,;1.52,-9.97,;1.52,-11.53,;2.85,-12.28,;9.33,-8.33,;7.93,-7.68,;7.8,-6.14,;6.4,-5.49,;5.14,-6.38,;5.28,-7.92,;6.68,-8.56,;10.59,-7.44,;9.81,-6.11,;10.59,-4.77,;12.13,-4.77,;12.89,-6.11,;12.13,-7.44,;16.29,-7.89,;15.73,-6.52,)| Show InChI InChI=1S/C34H42N4O4S/c35-29-20-18-25(19-21-29)23-36-33(39)30-17-10-22-38(30)34(40)32(37-43(41,42)24-26-11-4-1-5-12-26)31(27-13-6-2-7-14-27)28-15-8-3-9-16-28/h1-9,11-16,25,29-32,37H,10,17-24,35H2,(H,36,39)/t25?,29?,30-,32?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin |
J Med Chem 40: 830-2 (1997)
Article DOI: 10.1021/jm960762y
BindingDB Entry DOI: 10.7270/Q25H7GXW |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545
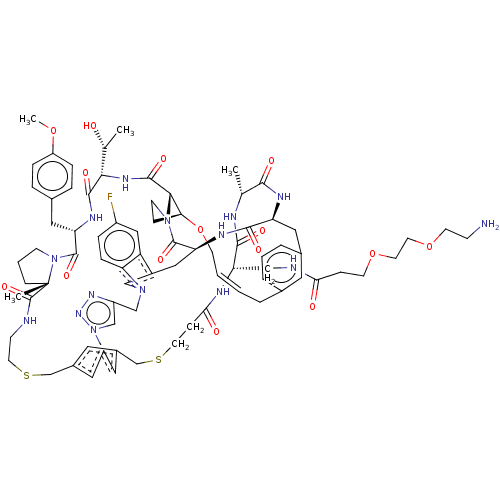
(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544
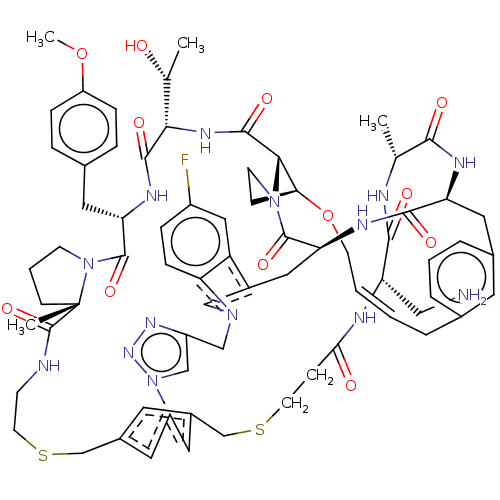
(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266
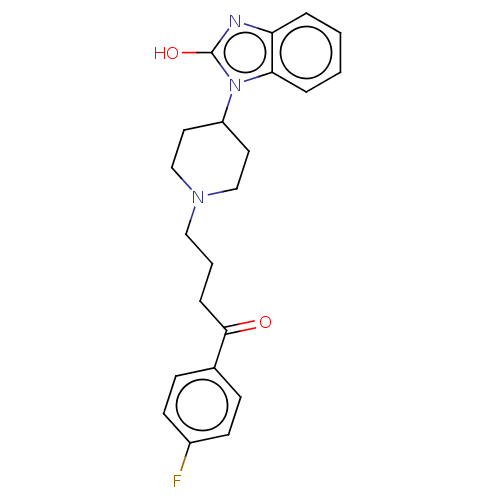
(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2 receptor by radioligand displacement assay |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592
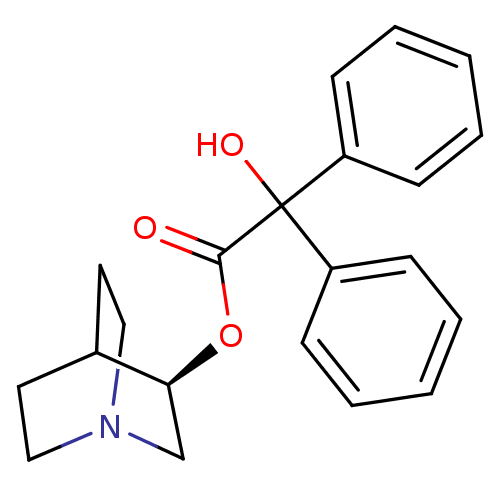
(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-quinuclidinyl benzilate(QNB) from muscarinic (M2) receptor in rat heart homogenates |
J Med Chem 34: 2984-9 (1991)
BindingDB Entry DOI: 10.7270/Q27H1K5M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM430

(3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...)Show SMILES CC(C)C1(CCc2ccc(O)cc2)CC(=O)C(Sc2cc(C)c(O)cc2C(C)(C)C)C(=O)O1 Show InChI InChI=1S/C27H34O5S/c1-16(2)27(12-11-18-7-9-19(28)10-8-18)15-22(30)24(25(31)32-27)33-23-13-17(3)21(29)14-20(23)26(4,5)6/h7-10,13-14,16,24,28-29H,11-12,15H2,1-6H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research
| Assay Description
For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... |
Bioorg Med Chem 7: 2775-800 (1999)
Article DOI: 10.1016/s0968-0896(99)00215-1
BindingDB Entry DOI: 10.7270/Q21C1V2B |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM430

(3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...)Show SMILES CC(C)C1(CCc2ccc(O)cc2)CC(=O)C(Sc2cc(C)c(O)cc2C(C)(C)C)C(=O)O1 Show InChI InChI=1S/C27H34O5S/c1-16(2)27(12-11-18-7-9-19(28)10-8-18)15-22(30)24(25(31)32-27)33-23-13-17(3)21(29)14-20(23)26(4,5)6/h7-10,13-14,16,24,28-29H,11-12,15H2,1-6H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 43: 843-58 (2000)
Article DOI: 10.1021/jm990281p
BindingDB Entry DOI: 10.7270/Q21N7Z9R |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM472

(5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...)Show SMILES CC(C)[C@]1(CCc2ccc(O)cc2)CC(=O)C(Sc2cc(C)c(OS(C)(=O)=O)cc2C(C)(C)C)C(=O)O1 |r| Show InChI InChI=1S/C28H36O7S2/c1-17(2)28(13-12-19-8-10-20(29)11-9-19)16-22(30)25(26(31)34-28)36-24-14-18(3)23(35-37(7,32)33)15-21(24)27(4,5)6/h8-11,14-15,17,25,29H,12-13,16H2,1-7H3/t25?,28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 43: 843-58 (2000)
Article DOI: 10.1021/jm990281p
BindingDB Entry DOI: 10.7270/Q21N7Z9R |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50409174

(CHEMBL169119)Show SMILES COC1=C(Sc2cc(C)c(O)cc2C(C)(C)C)C(=O)OC(CCc2ccc(O)cc2)(C1)C(C)C |c:2| Show InChI InChI=1S/C28H36O5S/c1-17(2)28(13-12-19-8-10-20(29)11-9-19)16-23(32-7)25(26(31)33-28)34-24-14-18(3)22(30)15-21(24)27(4,5)6/h8-11,14-15,17,29-30H,12-13,16H2,1-7H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
Tested for binding affinity against HIV protease |
J Med Chem 43: 843-58 (2000)
Article DOI: 10.1021/jm990281p
BindingDB Entry DOI: 10.7270/Q21N7Z9R |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(BtCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| WIPO WO2021205298
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q232001P |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(BtCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| WIPO WO2021205298
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q232001P |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM472

(5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...)Show SMILES CC(C)[C@]1(CCc2ccc(O)cc2)CC(=O)C(Sc2cc(C)c(OS(C)(=O)=O)cc2C(C)(C)C)C(=O)O1 |r| Show InChI InChI=1S/C28H36O7S2/c1-17(2)28(13-12-19-8-10-20(29)11-9-19)16-22(30)25(26(31)34-28)36-24-14-18(3)23(35-37(7,32)33)15-21(24)27(4,5)6/h8-11,14-15,17,25,29H,12-13,16H2,1-7H3/t25?,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
Tested for binding affinity against HIV protease |
J Med Chem 43: 843-58 (2000)
Article DOI: 10.1021/jm990281p
BindingDB Entry DOI: 10.7270/Q21N7Z9R |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071565
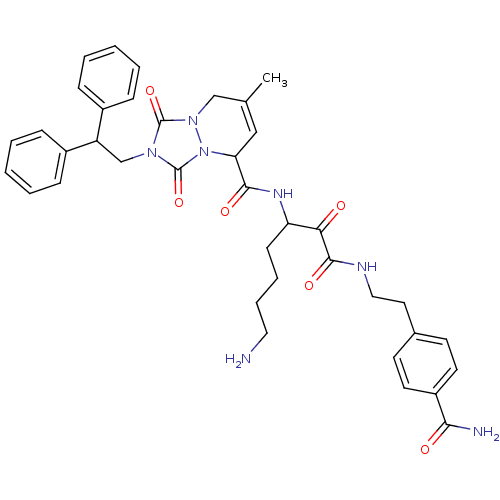
(2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...)Show SMILES CC1=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc2ccc(cc2)C(N)=O)n2n(C1)c(=O)n(CC(c1ccccc1)c1ccccc1)c2=O |t:1| Show InChI InChI=1S/C38H43N7O6/c1-25-22-32(35(48)42-31(14-8-9-20-39)33(46)36(49)41-21-19-26-15-17-29(18-16-26)34(40)47)45-38(51)43(37(50)44(45)23-25)24-30(27-10-4-2-5-11-27)28-12-6-3-7-13-28/h2-7,10-13,15-18,22,30-32H,8-9,14,19-21,23-24,39H2,1H3,(H2,40,47)(H,41,49)(H,42,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581549
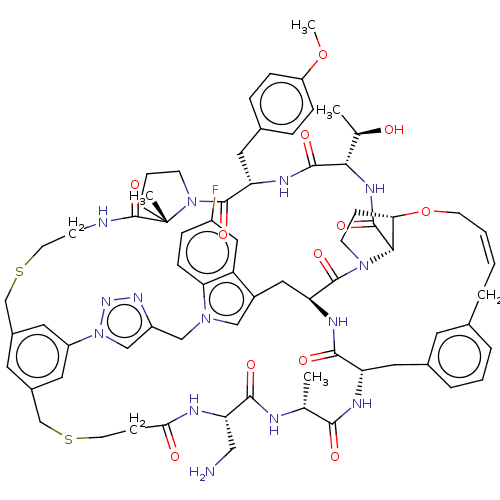
(CHEMBL5082483)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C/CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,c:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM221048
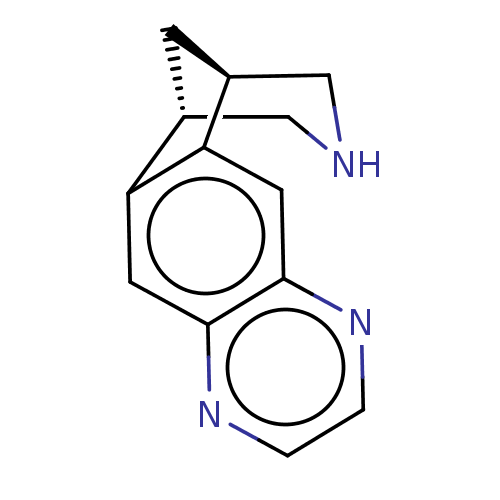
(US9284322, varenicline | US9303017, Varenicline)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2/t8-,9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method |
J Med Chem 63: 2833-2853 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00790
BindingDB Entry DOI: 10.7270/Q29027BJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519706
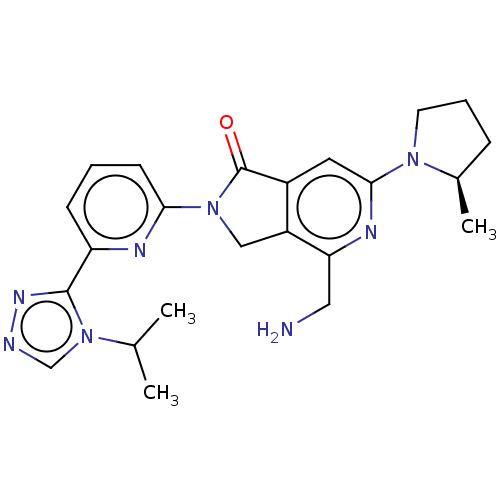
(US11142525, Example 107)Show SMILES CC(C)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CN)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606126
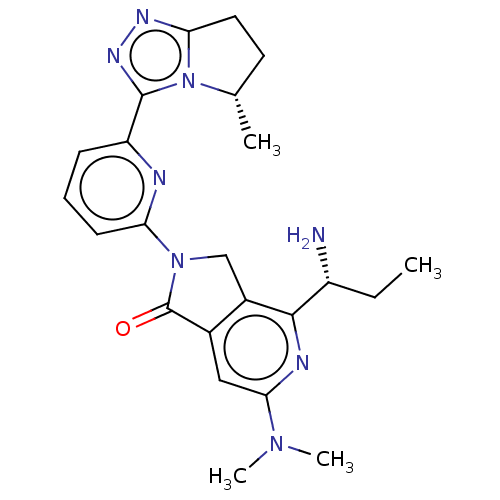
(4-[(1R)-1- aminopropyl]- 6-(dimethyl- amino)-2-{6-...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519590

(US11142525, Example 6 | US11142525, Example 79)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1C(C)C)N(C)C(C)C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606205

(2-{6-[(5S,7S)- 5,7-dimethyl-6,7- dihydro-5H- pyrro...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2[C@@H](C)C[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606115

(4-(2- aminopropan- 2-yl)-2-{6- [(5S)-5-methyl- 6,7...)Show SMILES C[C@@H]1CCCN1c1cc2C(=O)N(Cc2c(n1)C(C)(C)N)c1cccc(n1)-c1nnc2CC[C@H](C)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606114

(4-[(1S)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606118

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606123

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606098
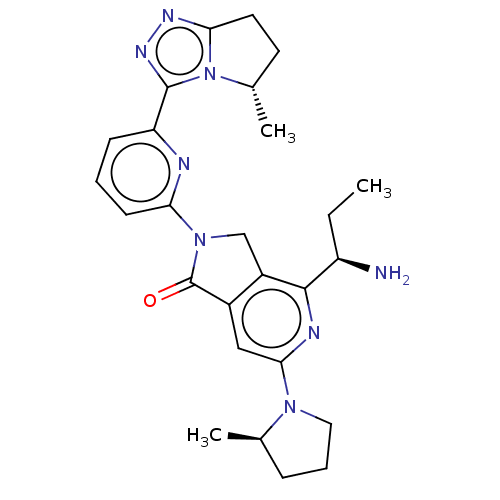
(US11684616, Example 1)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606099

(US11684616, Example 2)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606100
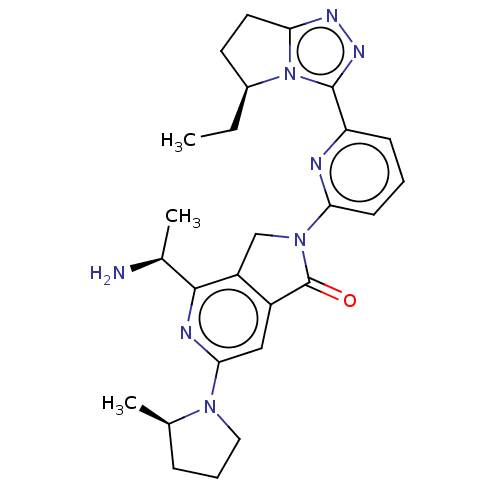
(US11684616, Example 3)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606101

(US11684616, Example 4)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606102

(US11684616, Example 5)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606125

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519699

(US11142525, Example 100)Show SMILES CC(C)N(C)c1cc2C(=O)N(Cc2c(CN)n1)c1cccc(n1)N1[C@@H](C)COC1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606128
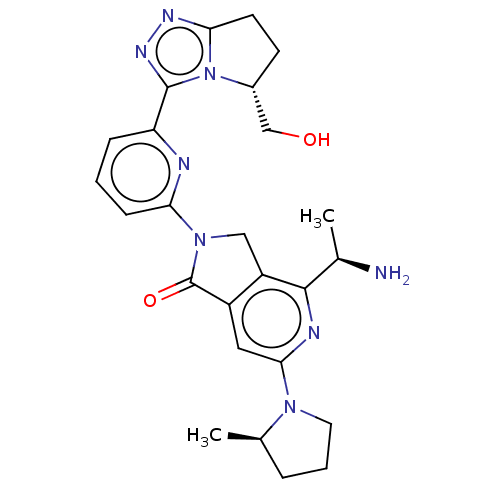
(4-[(1R)-1- aminoethyl]-2- {6-[(5R)-5- (hydroxy- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606129

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519601

(US11142525, Example 17)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1[C@@H](C)CC(F)(F)F)N(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519602

(US11142525, Example 18)Show SMILES CC[C@@H](CC(F)(F)F)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N(C)C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606135
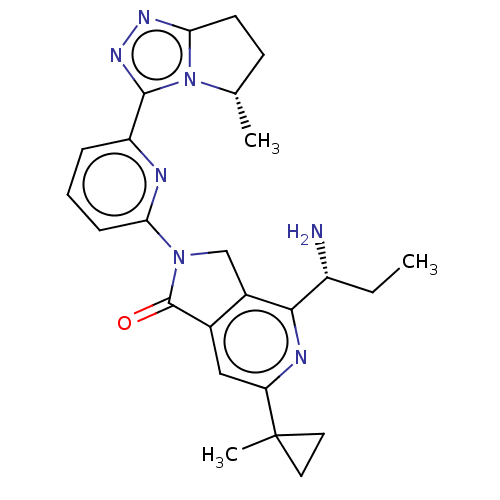
(4-[(1$#958;)-1- aminopropyl]- 6-(1- methylcyclo- p...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606137
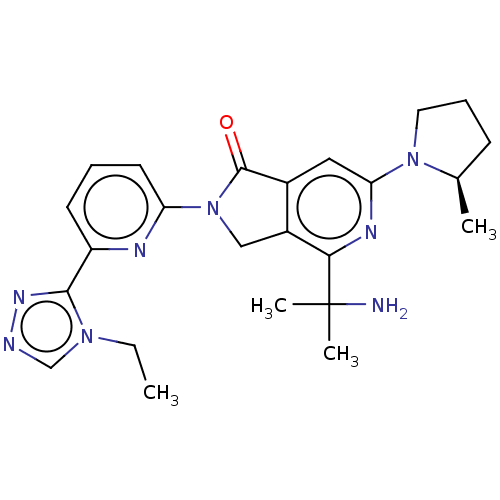
(US11684616, Example 100)Show SMILES CCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2C(C)(C)N)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606194

(US11684616, Example 200)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606195

(US11684616, Example 201)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606199

(2-{6-[(5R)-5- (fluoromethyl)- 6,7-dihydro-5H- pyrr...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM519743

(US11142525, Example 144)Show SMILES CCC(CC)n1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2CNC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HPK1 enzyme inhibition was measured using a microfluidic mobility shift assay (MSA). The reactions were conducted in 50 μL volumes in 96-well pl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21C211B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606204

(4-[(methyl- amino)methyl]- 2-{6-[(5S)- 5-methyl-6,...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606130
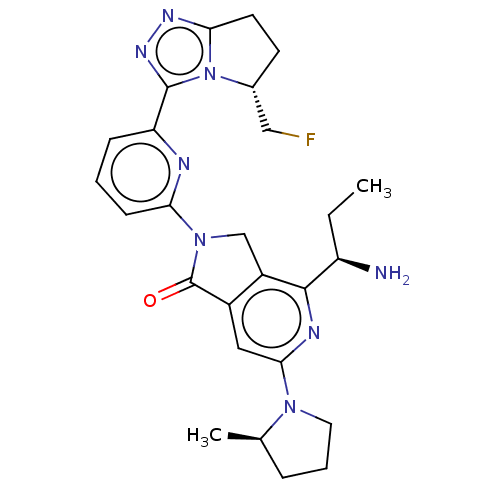
(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (fluorometh...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data